Translate this page into:
A mechanistic insight into cytokines and growth factors in saudi pomegranate peel extract (Punica granatum L.) mediated diabetic wound healing in alloxan induced diabetic rats
⁎Corresponding author at: Dr. Shahid Karim, Building 7, Ground floor, Department of Clinical Pharmacology, Faculty of Medicine, King Abdulaziz University, Jeddah 21589, Saudi Arabia. skaled@kau.edu.sa (Shahid Karim),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Introduction
Present study was designed to understand the role of inflammatory markers and growth factors in later stage of wound healing after treatment with SPPE on wound in alloxan induced diabetic rats.
Methodology
The study was performed by inducing diabetes in wistar rats using alloxan at 150 mg/kg, subcutaneously. The animals were allocated in three groups: Group 1- diabetic excision wound (DEW) without any treatment, Group 2- DEW treated with gel vehicle only, Group 3- DEW treated with 5 %SPPE containing gel. During the treatment period of twenty-one days animals were monitored based on clinical signs, morbidity, and mortality. Percent wound contraction, Growth factors (VEGF, and EGF), proinflammatory markers TNF- α, IL-1β, MCP-1, IL-4, and IL-17a, anti-inflammatory marker IL-10 in wound lysates, and histology of skin were all considered as indicators of effectiveness.
Results
The vehicle control when compared with the SPPE in gel (5.0 g extract per 100 g of gel) presented significant [p < 0.001] wound healing activity at day 14 and 21 of treatment. Throughout the treatment period the animals were normal and showed no comparable difference in terms of body weight both in group 1 and treatment groups. Both growth factors VEGF & EGF levels were significantly increased in SPPE gel treated wounds on day 14 and 21 compared to Group 2. Other inflammatory markers like TNF- α, IL-1 β and IL-17a were also significantly decreased in SPPE treated wounds as compared to other groups.
Conclusions
This study concludes that topical application of SPPE gel to alloxan-induced diabetic wounds decreases pro-inflammation markers and increases growth factors, hence promoting wound healing.
Keywords
Alloxan
Diabetes
Pomegranate peel extract
Topical gel
Wound healing
1 Introduction
Skin is largest organ of the human body and provides physical barrier against all pathogens (Ragab et al., 2019). As skin loses its integrity, suffer lesions, it disturbs the cellular and anatomic structures that also alters its function (Broughton et al., 2006). Acute wounds generally go through the stages of hemostasis, inflammation, proliferation, and remodelling. The pro-inflammatory macrophages destroy germs and eat up dead cells, preparing the affected part to heal. Angiogenesis, formation of granulation tissue, deposition of collagen, and re-epithelialization are all aided by prowound-healing macrophages present during proliferation. Pro-resolving macrophages allows collagen to mature and the newly regenerated skin to be strengthened by destroying the granulation tissue during remodelling (Krzyszczyk et al., 2018; Thangarajoo et al., 2023).
Since new and better therapies aren't being discovered as quickly as required, many chronic wounds don't heal properly. Patients with diabetes run the risk of experiencing medical issues that could make their rehabilitation more difficult (Ashcroft et al., 2003). Due to the delayed healing of wounds in diabetes, treatment with medicines and occasionally even amputation or excision may be necessary (Chen and Wen, 2011). A clinical consequence of a poor wound healing process is the development of a chronic wound, which raises the patient's risk and costs of care. Death rates are relatively high for diabetics with chronic and persistent wounds (Nayak et al., 2013).
Pomegranate, scientifically known as Punica granatum, is a fruit that has been used for centuries in traditional medicine due to its various health benefits (Reddy et al., 2007; Yuan et al., 2015). It contains wide range of phytoconstituents, which are natural chemical compounds found in plants. Some of the main phytoconstituents found in pomegranate and their medicinal properties are as follows: polyohenols, flavonoids, tannins, vitamin C, punicalagins and phytosterols (Bahadoram et al., 2022; Hayouni et al., 2011a).
Pomegranate's anti-inflammatory capabilities, as well as its antibacterial, antioxidant, and antiallergenic effects, are attributed to polyphenols (like ellagitannins) (Yuniarti et al., 2018). Results from previous studies demonstrated superior wound healing with the pomegranate peel extract-based formulations (Ismail et al., 2012; Karim et al., 2021; Mastrogiovanni et al., 2020; Pirbalouti et al., 2010; Yuniarti et al., 2018).
The inflammation response to injury aids in the clearance of damaged cells and the stimulation of vasoconstriction (Mastrogiovanni et al., 2020). The growth factors and cytokines (CKs) that are generated by these cells are essential for neovascularization and wound healing. Additionally, inflammation in the subcutaneous tissue, particularly in people with diabetes and obesity, may impede the healing process. Pomegranate extracts' anti-inflammatory benefits have also been studied extensively, and punicalagin has been found to play a pivotal role in various studies (El-Missiry et al., 2015; Houston et al., 2017; Larrosa et al., 2010).
In our previous study, after 14 and 21 days of treatment, we measured the degree of inflammatory cells infiltration in the skin of rats treated with SPPE gel. The inflitration of proinflammatory cells was decreased in all treated groups, including the Control and vehicle groups, while the SPPE group exhibited the most pronounced reduction (Karim et al., 2021). The histopathological data supports the earlier fibroplasia induction result, suggesting that the pomegranate formulations in these groups decreased the inflammatory response process after a week’s treatment. Finally, all the groups showed very low levels of inflammatory cell infiltration following the 14-day treatment period. Topical application of pomegranate rind extracts (PRE) to ex-vivo skin significantly decreased cyclooxygenase-2 (COX-2) expression 6 h after application and maintained this reduction for up to 24 h(Lukiswanto et al., 2019; Yuniarti et al., 2018). Surprisingly, in another study in mice treated with Pomegranate formulation, in addition to the findings for the inflammatory infiltrate, a rise in Myeloperoxidase levels was seen, indicating that this therapy may have caused proinflammatory rather than an anti-inflammatory condition. In literature reports demonstrates the production of proinflammatory cytokines (CKs). The wound's macrophages can release strong chemoattractant, which can increase monocyte recruitment and inflammation. TNF- α, MCP1, and IL-1β expression levels rise as a result of the activation of p38-MAPK/NFkB pathway According to some reports, pomegranate suppresses the p38-MAPK/ NFkB pathway. (Larrosa et al., 2010; Mengshol, 2001).
For this reason, we tested the CK and chemokine activity of a control, vehicle, and SPPE gel in alloxan-induced diabetic rats to learn more about the role these inflammatory markers play during the late stage of wound healing in vivo.
2 Materials and methods
2.1 Materials
Pomegranate fruit grown in the Taif were bought from a supermarket (Hyperpanda) in Jeddah, KSA, and authenticated at King Saud University's College of Pharmacy's Pharmacognosy Department in Riyadh, KSA. The peel was peeled by hand and sliced into small pieces. Only analytical-grade chemicals/solvents were utilized for extraction and formulation techniques.
2.2 Preparation of extract
With a few minor modifications, SPPE was made using the technique outlined by Shiban et al., (2012) (Shiban et al., 2012). The fruits were peeled manually, cleaned, and peel was dried under shade for a week before methanolic (90 %) extraction. Four batches of Fifty grams each of finely crushed peels were blended by using Waring blender for 2 min in 3000 cc of methanol (90 %). After that, the combination was kept at room temperature for twenty-four h while being kept in the dark. Yields (in %age) per initial material were calculated by drying the concentrated extract under reduced pressure at 40 degrees Celsius.
After being frozen at −70 °C ± 10 °C in an ultra-freezer (CL 600–80;ColdLab) for 24 h, the lyophilized (Terroni, modelLS3000) for 26 h thereafter SPPE was stored at 20 °C in a container that was hermetically sealed and protected from light (Zago et al., 2020).
2.3 Preparation of SPPE gel
We made vehicle/base gel as suggested by Murthy et al., (2004). In short, the SPPE gel was made in two phases. Vehicle phase- a vehicle base constituted by following composition: five grams of carbopol 934, 51.5 mL of propane-1,2-diol, 0.5 mL of propylparaben, 1.6 mL of triethanolamine, and enough distilled water to make it 100 g. Which was followed by SPPE Phase- five grams of SPPE was added for every 100 g of vehicle base (Murthy et al., 2004). This gel was placed in collapsible tubes for the tests and kept in a cool, dry place.
2.4 Animal handling and induction of diabetes
Female Sprague Dawley (180–200 g) were obtained from Theraindex Lifesciences' animal facility in Bangalore, India. The Institutional Animal Ethics Committee of Theraindex Lifesciences Pvt Ltd, located in Bangalore, India, conducted a thorough evaluation and granted its approval for the protocol with reference number IAEC/15/2020/187. All animal related experimental methods and protocols were carried out in conformity with the CPCSEA standards (2003) for animal care and use.
The animals were housed in IVC systems with an access to unlimited food and water as well as alternate luminosity cycles every 12 h in a pathogen-free, humidity- and temperature-controlled environment.
Diabetes was induced in rats after an overnight fasting by intraperitoneally administering Alloxan monohydrate dissolved in normal saline (Dose:150 mg/kg BW). To overcome alloxan-induced hypoglycemia, animals were given a 5 % glucose solution to drink overnight. The fasting blood glucose levels of each animal were measured after 72 h using a commercial glucometer (Onetouch ultra, Lifescan, USA) to verify diabetes induction (Blood glucose level of ≥ 250 mg/dL). Rats that had effectively been induced with diabetes were selected and pooled for future study (Karim et al., 2021).
2.5 Diabetic excision wound (DEW) creation
Five days following the introduction of diabetes, rats were shaved using a pet trimmer on the dorsal skin. The rats were anaesthetized with an injection containing xyalzine (10 mg/kg) + ketamine (70 mg/kg) injected intraperitonially. A predetermined area of each rat's skin, measuring roughly 2 cm by 2 cm in its full thickness, was removed using autoclaved surgical tools and anaesthesia. Ketoprofen (5 mL/kg) was administered subcutaneously every day for two days following excision to reduce discomfort and anxiety. All rats received species-specific enrichment after wound formation. The borders of the wounds were traced onto a clear piece of paper to measure the size of the wound areas.
2.6 Experimental design and treatments
For twenty-one days, animals received topical application according to allocated treatment group twice daily as illustrated in Fig. 1.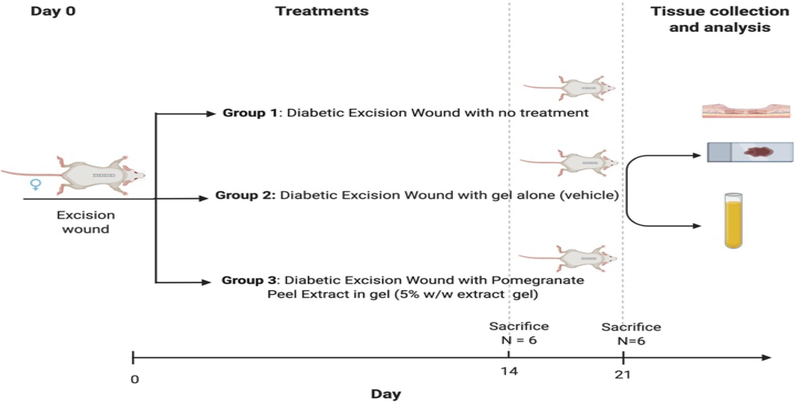
Experimental design and treatments.
2.7 Wound contraction measurement
On days 14 and 21, wounds were measured and their growth was observed planimetrically. The final wound size was measured by tracing it on translucent paper and then plotting it on graph paper. The difference between the original wound size (200 mm2) and the end wound size, divided by the initial wound size, was used to calculate the %age of wound closure. Blinding was followed for all analyses.
2.8 Estimation of wound tissue cytokine and growth factors
On days 14 and 21 following injury, as well as throughout therapy, six rats/group were sacrificed after an excess of carbon dioxide. The wound tissue was swiftly removed via the least healed area, down to the fascia, and separated into three parts. In order to homogenise the wound tissue samples for CK measurement were processed as described by Cox et al., 2015, the samples were homogenized to a final protein concentration of 8 μg/mL using homogenizing buffer(Cox et al., 2015).
Before being sent to Eve Technologies (https://www.evetechnologies.com) on dry ice and running undiluted on the CK/chemokine 27-plex discovery assay normalised samples were frozen at −80 °C. Eve Technologies Corp. used the LuminexTM 200 system (Luminex, Austin, TX, USA) to perform the multiplexing analysis (Calgary, Alberta). Following the manufacturer's instructions, 27 markers were assessed in the samples at once using Eve Technologies' Rat Cytokine 27-Plex Discovery Assay® (MilliporeSigma, Burlington, Massachusetts, USA). For the cytokine 27-plex, all markers' assay sensitivity ranges from 0.3 to 30.7 pg/mL. The MilliporeSigma MILLIPLEX® MAP procedure provides individual analyte sensitivity data.
2.9 Semi-quantitative histopathological evaluation of inflammatory Cells, Neovascularization, fibroblast formation and granulation tissue
Following euthanasia, on day 14 and day 21 skin samples were processed to make paraffin tissue blocks. Using a rotating microtome, each tissue block was sectioned into 3–5 µm portions and placed on glass slides. After being stained with H&E, the slides were viewed under a light microscope. Inflammatory cells, fibroblast development, neovascularization, and granulation tissue were all assessed on a 4-point scale for each slide: 0 meant there were none, 1 meant they were uncommon or minimal, 2 meant they were moderate, 3 meant they were numerous, and 4 meant they were severe or marked. Three randomly chosen sections from each specimen were used for the histopathological (HP) analysis. Blinding was used for all analyses.
2.10 Statistical analysis
Every value is displayed as the mean ± SEM. At p < 0.05, differences were considered significant. Differences between day 14 and day 21 were identified using one-way analysis of variance(ANOVA). The differences between Group 1 and the treatment groups were analysed using an independent sample t-test. Semi-quantitative HP ratings were analysed using the Kruskal-Wallis test. Graphpad Prism (version 5.0) was used for the statistical analysis.
3 Results
3.1 Extraction yield
The extraction process resulted in a methanolic extract yield of 14.5 % w/w from pomegranate peel.
3.2 Clinical outcomes
The experiment resulted in no animal deaths. Throughout the experiment, none of the animals in the groups' weights decreased noticeably (Fig. 2). Additionally, none of the animals displayed any clinical indications other than minor discomfort due to excision wounds within the first seven days.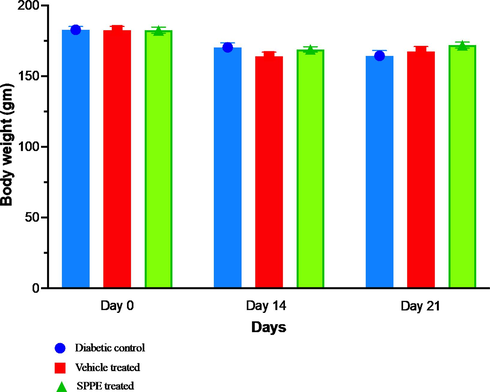
Effect of treatments on body weight in diabetic rats during the study at Day 0, 14 and 21. Data presented as mean and SEM (n = 6).
3.3 Wound contraction
The SPPE gel-treated wounds (group 3) in terms of wound contraction had marked effect than in group 1(diabetic wound) at days 14 and 21. By day 21, SPPE gel-treated wounds had increased wound closure rates that were two folds higher compared with both groups 1 and 2 (vehicle) (Fig. 3). On the other hand, there was no statistically significant difference between groups 2 and 1 in terms of wound closure, with both groups exhibiting patterns of similar wound closure on days 14 and 21.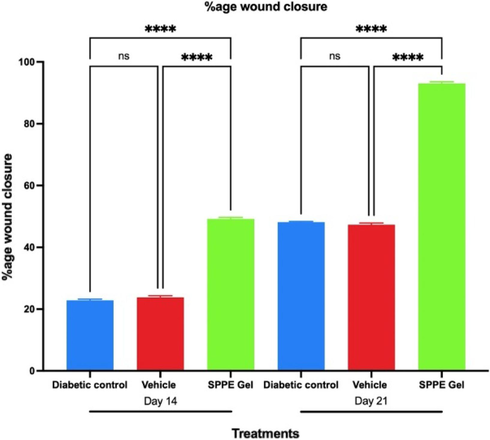
Time-dependent analysis of the effect of SPPE gel on wound closure rates in diabetic rats. The %age wound contraction was assessed on 14 and 21 days after the wound was created. Data expressed as mean and SEM (n = 6), ns indicates non-significant, **** indicates p < 0.001.
3.4 Histopathological observations
Histopathological grading was used to determine the extent of fibroblast infiltration, collagen formation, neovascularization, and re-epithelialization in the DEW. Rats' wounds at Day 0 stage had very little granulation tissue, and the incisional area had few arteries, fibroblasts, and collagen-forming cells (Fig. 4A). The rats in the Group 3 received treatment with SPPE gel, which significantly enhanced the diabetic wound healing, as evidenced by the histology scores (Fig. 4H). On day 14 after wound creation, collagen regeneration and vascularization were higher in the SPPE gel treated group (Fig. 3D) compared to the Diabetic (Fig. 4C) and Vehicle control (Fig. 4B) groups. On day21, epithelialization and granulation were likewise considerably higher in SPPE gel treated groups (Fig. 4G) than in Diabetic control and Vehicle treated groups (Fig. 4E and 4F respectively).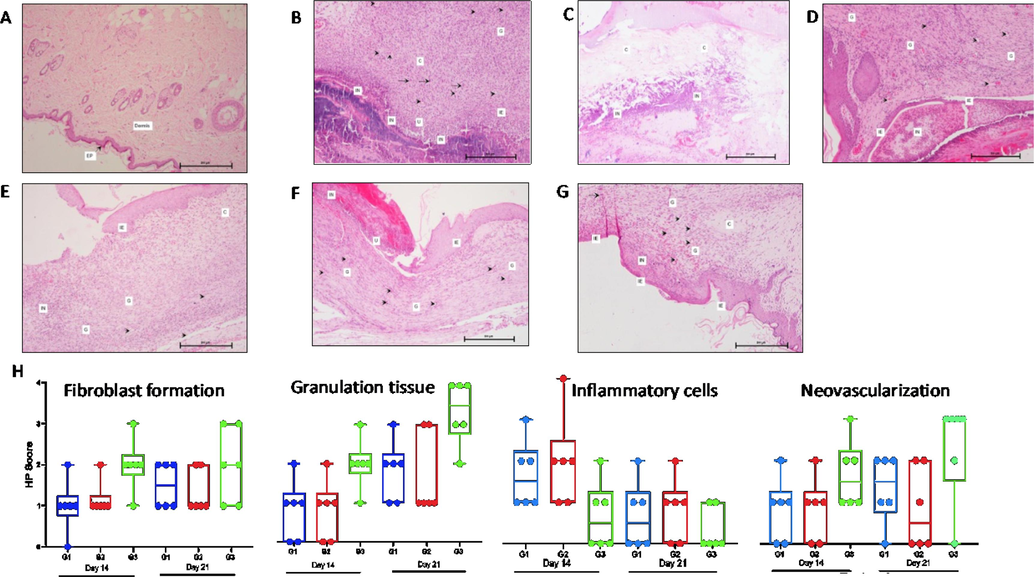
Histopathology with scoring of skin at days 0, 14 & 21 stained with H&E (100 × ). Day 0 - (A) Diabetic Control rat showing normal skin with epidermis (EP) and dermis. Day 14 - (B, C & D) Skin of Diabetic control, Vehicle control & SPPE treated rats respectively showing inflammatory cells, Collagen and granulation tissue within dermis (c), neovascularization (→), immature epidermis (IE), ulceration (U) and fibroblast proliferation (→) respectively. Day 21- (E, F & G) Diabetic control, Vehicle control & SPPE treated rat skins respectively showing inflammatory cells, collagen and granulation tissues in dermis, fibroblast formation with neovascularization and immature epidermis. (H) Box plots showing histopathology scores for skin parameters across all treatments. Median is represented by the horizontal lines, and minimum and maximum by the error bars. *Significant (p < 0.05). A score of 0 indicates none, 1-Minimal/Rare, 2-Moderate, 3-Abundant, and 4-Severe/Marked.
3.5 Effect of SPPE on growth factor
The GFs such as (EGF and VEGF) are responsible in all phases of wound healing. Both Epidermal growth factor (EGF) and VEGF were assessed in the DEW lysate.
When compared to the diabetic wound control, EGF levels were observed to be more than three times higher (p < 0.0001) in Group 3 on day 21, and statistically significant (p < 0.001) rise in EGF was observed on day 14. Although there was a substantial rise in EGF levels in the vehicle treated group compared to the diabetic wound on Day 21, the difference in EGF levels in both the control and vehicle treated groups on Day 14 was not significant. A highly significant increase in EGF levels was observed on Day 14 and 21 in SPPE gel treated wounds as compared to Vehicle treated wounds (Fig. 5A).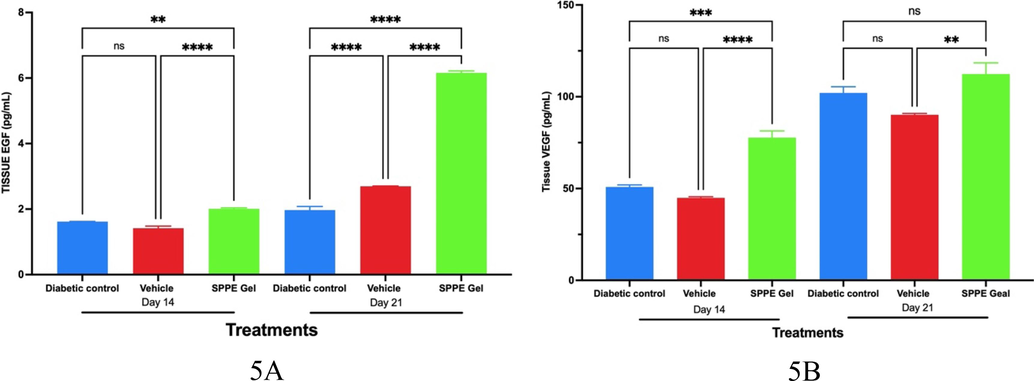
Effect of study treatments on growth factors in wound lysates on days 14 and 21. A) Epidermal Growth Factor (EGF), and B) Vascular Endothelial Growth Factor (VEGF) in Wound Lysates on day 14 and 21 following study treatments. Error bars represent Mean and SEM (n = 6).
On both Day 14 and Day 21, the VEGF levels in SPPE gel-treated wounds were not significantly different from the diabetic control groups. On Day14, the VEGF levels in vehicle treated wounds were significantly elevated than those in diabetic and SPPE gel treated wounds. However, on Day 21, the VEGF levels were not significantly different between diabetic and vehicle treated wounds; but, a significant increase in wound VEGF levels in vehicle treated wounds was observed when compared to SPPE gel treated wounds (Fig. 5B).
3.6 Effect of SPPE gel on wound tissue inflammatory markers
Proinflammatory markers TNF α, IL-1 β, MCP-1, IL-4 and IL-17A were assessed in the tissue lysate. As compared to the vehicle control, TNF α levels were lower in SPPE gel treated wounds on day 14 and day 21. TNF α levels in diabetic wounds and SPPE treated wounds were not statistically significant on days 14 and 21. (Fig. 6A). IL-1β levels were significantly (p < 0.0001) lower in SPPE treated wounds on day 14 and 21 when compared to the diabetic control and vehicle treated wounds (Fig. 6B). While on Day 21 observed MCP-1 levels in the wounds treated with SPPE gel was significantly lower as compared to both treatments diabetic and vehicle (Fig. 6C). IL-4 levels were markedly higher (p < 0.05) in SPPE gel on day 14 when compared with the vehicle & diabetic control groups. But on 21st day there was an insignificant change in IL-4 levels between the SPPE gel and other treatment groups (Fig. 6D). A significant decline in IL-17A levels (p < 0.0001) was observed in both vehicle and SPPE gel treated wounds as compared to Diabetic wound on Days 14 and 21 (Fig. 6E). IL-10 (anti-inflammatory marker) levels in wounds at day 21 was significantly (p < 0.05) raised in diabetic control group as compared to both SPPE and vehicle groups but on day 14 these levels in both SPPE and vehicle groups were higher than the diabetic control group though this difference was insignificant (Fig. 6F).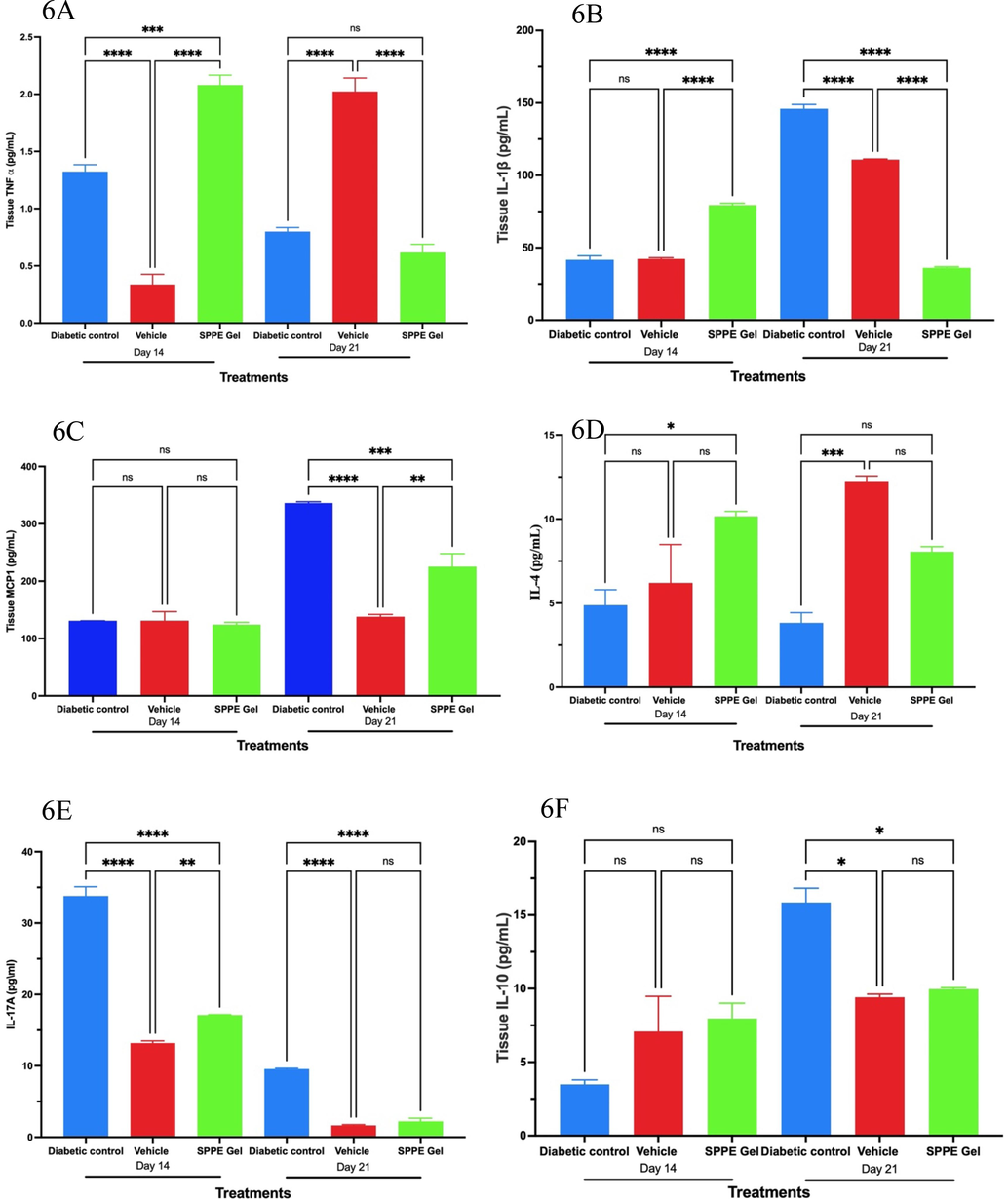
Effects of SPPE gel on TNF-α (A), IL-1β (B), MCP-1 (C), IL-4 (D), IL-17A (E), and IL-10 (F) production at Day 14 and Day 21 after treatment (n = 6). Data are expressed as mean and SEM (n = 8), ****p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05.
4 Discussion
In mice, rabbits, rats, guinea pigs, and humans, pomegranate peel extract-based wound healing formulations have been demonstrated to be successful (George et al., 2019; Hashemi Poor et al., 2022; Stefanou et al., 2021; Yan et al., 2013). According to several studies formulations containing pomegranate peel extract have been demonstrated to hasten the healing of wounds, causing the production of granulation tissue early on and enhancing re-epithelialization (Elzayat et al., 2018; Yan et al., 2013). The molecular mechanisms underlying these rapid wound healing are not yet understood. Despite being shown to occur both in vitro and in vivo, the angiogenetic impact is mostly brought on by the production of EGF and VEGF. Pomegranate peel extract impacts various well-known immune modulators (IL-6,10, IDO or inducible NOS11, and PGE212) that have been involved in modulating the inflammatory milieu in addition to Ellagic acid derivatives and flavonoids. These preparations aid in the phenotypic conversion of inflammatory macrophages (M1) to a reparative type of phenotype (M2) linked to increased IL-10 production and decreased TNF α, which accelerates the process of wound healing. It is believed that soluble immunomodulatory chemicals and cellular contacts mediate this pathway(Arango Duque and Descoteaux, 2014; Koh and DiPietro, 2011). In case of cellular interaction or for promoting macrophage recruitment between macrophages and pomegranate preparations that enhances the process, several CKs are actively involved. In wound healing and correlation of its therapeutic potential more advanced knowledge and appropriate skill for administration of such formulation may benefit in treatment (Koh and DiPietro, 2011).
A diabetic patient is more likely to become infected after an injury and to develop chronic sores. These wounds are characterised by a high degree of inflammation; this process helps wounded cells to be expelled from the area and leads to vasoconstriction (Edwards and Harding, 2004). In addition, the release of proinflammatory mediators is another consequence of immune cell activation (e.g., CKs and ILs). When prolonged inflammatory episodes turn into chronic ones, the inflammatory response frequently takes place in an environment that is balanced, which is detrimental to the process of wound-healing (Mosser and Edwards, 2008).
The proximity of macrophages to neovascularization thereby assisting in their stability and fusion, it also play a crucial role in vascularization. The temporary extracellular matrix is destroyed by macrophage-produced matrix metalloproteinases (MMPs) at the beginning of the final remodelling phase, which then causes the skin to mature to its uninjured, normal state. It is believed that the persistence of pro-inflammatory macrophages in chronic wounds without converting to an anti-inflammatory phenotype causes tissue repair to be unsuccessful (Ferrante and Leibovich, 2012; Sánchez-Zamora and Rodriguez-Sosa, 2014). In our work, diabetic excision wounds inflicted on rats were examined to determine the impact of SPPE gel treatment on various inflammatory chemokines and CKs. As observed during the study the amount of several pro-inflammatory phenotypes significantly decreased. These results support earlier in vitro research that showed macrophage migrationinhibitory factor (MIF) levels increase in medium preconditioned by THP-1 cells under diabetic circumstances. Diabetes-related conditions are associated with lower levels of VEGF-AMCP-2, IL-6, & M−CSF. Furthermore, Dai and co-workers demonstrated that in THP-1 cellconditioned media, under pre-diabetic conditions there was a marked lowering in levels of CKs linked with an anti-inflammatory response, such as IL-5, IL-10, and TGFs.(Dai et al., 2021; Linnemann et al., 2021).
Therefore, we focused at how SPPE gels affected IL-1 β in diabetic wounds. On Day 21, IL-1 β levels in wounds treated with SPPE gel were lower than those in the vehicle control (p < 0.0001). According to Dai et al., (2021) hypothesis's IL-1 β increases ECM breakdown in diabetics by increasing matrix metalloproteinases (MMP) synthesis and decreasing collagenase and tissue inhibitor of metalloproteinases (TIMP) production(Dai et al., 2021).
Healing cells release GFs and CKs, which are essential for neovascularization and wound healing. While VEGF increases angiogenesis, which speeds up wound healing, it also probably encourages collagen deposition and epithelialization (Constantino Rosa Santos et al., 2007). In our study, we found that on days 14 and 21, both EGF and VEGF levels were significantly higher in wounds treated with SPPE compared to wounds treated with a vehicle (Fig. 4A&B). This finding was reflected in the higher histopathological scores of neovascularization’s in wounds treated with SPPE (Fig. 3H). These findings are in corroboration with our earlier study and similar studies with pomegranate peel extract formulations(Karim et al., 2021).
The average time required for significant diabetic wound closure is 14 days and beyond, despite the fact that different formulations containing pomegranate extracts in different concentrations were used in a number of in vivo wound models (Chidambara Murthy et al., 2004; Hayouni et al., 2011b; Yan et al., 2013). Even though all of the previous studies looked at how well different pomegranate-based extracts helped wounds heal, they did so using different wound models and different animal species, which may skew the comparison. These findings demonstrated the value of pomegranate-based topical formulations for the treatment of diabetic wounds that are slow to heal.
Our results emphasise the pleiotropic properties of SPPE gel in the diabetic wound healing process and imply that the previously described rapid anti-inflammatory signals imparted by SPPE may be a factor in the quicker wound healing (Dai et al., 2021; Linnemann et al., 2021). Therefore, by utilising SPPE gel, we intend to move the gel's extremely promising therapeutic potential into clinical investigations, ultimately enabling the creation of a safe and efficient treatment for people who have diabetic wounds.
5 Conclusions
Applying SPPE to diabetic wounds has been shown to increase growth factors and neovascularization while decreasing pro-inflammatory CKs, suggesting the wounds may heal more quickly than they would otherwise.
Our findings further add to the growing body of evidence that SPPE or compounds produced from SPPE may have therapeutic potential in ameliorating inflammation and treating inflammatory diseases.
Authors' contributions
SK, MIK and AA performed the experiments. SK and MIK designed the experiments. AA and SK analyzed the data. MIK, and SK wrote the manuscript. All authors have read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved. SK and MIK confirm the authenticity of all the raw data.
Funding
This Project was funded by the DSR at King Abdulaziz University, Jeddah, KSA under grant no. G-427–140-1442. The authors hereby, acknowledge with thanks DSR for technical and financial support.
Institutional animal ethical committee statement
The Institutional Animal Ethics Committee of Theraindex Lifesciences Pvt Ltd, located in Bangalore, India, conducted a thorough evaluation and granted its approval for the protocol with reference number IAEC/15/2020/187. All animal related experimental methods and protocols were carried out in conformity with the CPCSEA standards (2003) for animal care and use.
Patient consent for publication
Not applicable.
Data availability
The data generated in the present study are included in the figures of this article.
Acknowledgments
We would like to thank Yahya al Bishr for technical support during manuscript preparation and Theraindex Lifesciences Pvt Ltd, Bangalore, India.for the animal work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol.. 2014;5
- [CrossRef] [Google Scholar]
- Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig.. 2003;111:1309-1318.
- [CrossRef] [Google Scholar]
- Effects of Pomegranate on Wound Repair and Regeneration. World J Plast Surg. 2022;11:157-159.
- [CrossRef] [Google Scholar]
- The Basic Science of Wound Healing. Plast. Reconstr. Surg.. 2006;117:12S-34S.
- [CrossRef] [Google Scholar]
- The role of bacterial biofilm in persistent infections and control strategies. Int. J. Oral Sci.. 2011;3:66-73.
- [CrossRef] [Google Scholar]
- Study on Wound Healing Activity of Punica granatum Peel. J. Med. Food. 2004;7:256-259.
- [CrossRef] [Google Scholar]
- Constantino Rosa Santos, S., Miguel, C., Domingues, I., Calado, A., Zhu, Z., Wu, Y., Dias, S., 2007. VEGF and VEGFR-2 (KDR) internalization is required for endothelial recovery during wound healing. Exp Cell Res 313, 1561–1574. https://doi.org/10.1016/j.yexcr.2007.02.020.
- Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue. J. Neurotrauma. 2015;32:1413-1421.
- [CrossRef] [Google Scholar]
- IL-1β Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway. Mediators Inflamm.. 2021;2021:1-10.
- [CrossRef] [Google Scholar]
- Cardioameliorative effect of punicalagin against streptozotocin-induced apoptosis, redox imbalance, metabolic changes and inflammation. Egyptian Journal of Basic and Applied Sciences. 2015;2:247-260.
- [CrossRef] [Google Scholar]
- Evaluation of wound healing activity of henna, pomegranate and myrrh herbal ointment blend. Saudi Pharmaceutical Journal. 2018;26:733-738.
- [CrossRef] [Google Scholar]
- Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care (new Rochelle). 2012;1:10-16.
- [CrossRef] [Google Scholar]
- Pomegranate peel extract alters the microbiome in mice and dysbiosis caused by Citrobacter rodentium infection. Food Sci. Nutr.. 2019;7:2565-2576.
- [CrossRef] [Google Scholar]
- Wound healing potential of pomegranate peel extract in human dermal fibroblasts through regulating the expression of FN1 gene. S. Afr. J. Bot.. 2022;146:222-229.
- [CrossRef] [Google Scholar]
- Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18
- [CrossRef] [Google Scholar]
- Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine. 2011;18:976-984.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm.. 2017;112:30-37.
- [CrossRef] [Google Scholar]
- Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol.. 2012;143:397-405.
- [CrossRef] [Google Scholar]
- Effects of Methanolic Extract Based-Gel From Saudi Pomegranate Peels With Enhanced Healing Potential on Excision Wounds in Diabetic Rats. Front. Pharmacol.. 2021;12
- [CrossRef] [Google Scholar]
- Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med.. 2011;13:e23.
- [Google Scholar]
- The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol.. 2018;9
- [CrossRef] [Google Scholar]
- Larrosa, M., González-Sarrías, A., Yáñez-Gascón, M.J., Selma, M. v., Azorín-Ortuño, M., Toti, S., Tomás-Barberán, F., Dolara, P., Espín, J.C., 2010. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism☆. J Nutr Biochem 21, 717–725. https://doi.org/10.1016/j.jnutbio.2009.04.012.
- Altered Secretome of Diabetic Monocytes Could Negatively Influence Fracture Healing—An In Vitro Study. Int. J. Mol. Sci.. 2021;22:9212.
- [CrossRef] [Google Scholar]
- Evaluation of wound healing potential of pomegranate (Punica granatum) whole fruit extract on skin burn wound in rats (Rattus norvegicus) J Adv Vet Anim Res. 2019;6:202.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory effects of pomegranate peel extracts on bovine mammary epithelial cells BME-UV1. Nat. Prod. Res.. 2020;34:1465-1469.
- [CrossRef] [Google Scholar]
- IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res.. 2001;29:4361-4372.
- [CrossRef] [Google Scholar]
- Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol.. 2008;8:958-969.
- [CrossRef] [Google Scholar]
- Wound Healing Activity of the Fruit Skin of Punica granatum. J. Med. Food. 2013;16:857-861.
- [CrossRef] [Google Scholar]
- Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Pol. Pharm.. 2010;67:511-516.
- [Google Scholar]
- Soft hydrogel based on modified chitosan containing P. granatum peel extract and its nano-forms: Multiparticulate study on chronic wounds treatment. Int. J. Biol. Macromol.. 2019;135:407-421.
- [CrossRef] [Google Scholar]
- The Role of MIF in Type 1 and Type 2 Diabetes Mellitus. J. Diabetes Res.. 2014;2014:1-6.
- [CrossRef] [Google Scholar]
- Antioxidant Activity of Pomegranate (<i>Punica granatum</i> L.) Fruit Peels. Food Nutr. Sci.. 2012;03:991-996.
- [CrossRef] [Google Scholar]
- Wound Healing Properties of Pomegranate. Archives of. Microbiol. Immunol.. 2021;05
- [CrossRef] [Google Scholar]
- A stimuli-responsive in situ spray hydrogel co-loaded with naringenin and gentamicin for chronic wounds. Open Chem.. 2023;21
- [CrossRef] [Google Scholar]
- Effect of pomegranate peel polyphenol gel on cutaneous wound healing in alloxan-induced diabetic rats. Chin Med J (Engl). 2013;126:1700-1706.
- [Google Scholar]
- The activity of pomegranate extract standardized 40% ellagic acid during the healing process of incision wounds in albino rats (Rattus norvegicus) Vet World. 2018;11:321-326.
- [CrossRef] [Google Scholar]
- Zago, G.R., Gottardo, F.M., Bilibio, D., Freitas, C.P., Bertol, C.D., Dickel, E.L., Santos, L.R. dos, 2020. Pomegranate (Punica granatum L.) peel lyophilized extract delays lipid oxidation in tuscan sausages. Ciência Rural 50. https://doi.org/10.1590/0103-8478cr20190689.







