Translate this page into:
A contrastive study of cerebellum development in very premature and full-term infants
⁎Corresponding authors. liuruike2020@sina.com (Ruike Liu), drjoan2008@sina.com (Guifang Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
To compare the difference between full-term infants and very low birth weight (VLBW) premature infants at term equivalent for cerebellum development by cranial ultrasound in order to provide reference for monitoring the extrauterine development of cerebellum in VLBW premature infants.
Methods
With a study of 88 cases which were VLBW premature infants with gestational age less than 32 weeks were examined by cranial ultrasound at 40 weeks of corrected gestational age and 65 full-term infants at 40 weeks of gestational age were examined by cranial ultrasound at the day of birth. Comparing the difference between the two groups for transverse cerebellar diameter, cerebellar vermis height, anteroposterior vermis diameter, cerebellar vermis circumference and cerebellar vermis area . The difference of the above cerebellar indexes between 32 extremely premature infants with gestational age of less than 28 weeks and 56 premature infants with gestational age of greater than or equal to 28 weeks were compared as well.
Results
Compared to the premature infants at term equivalent age group and full-term infants group, the transverse cerebellar diameter in the premature infants at term equivalent age group was smaller than that in the full-term infants group (P < 0.05). There was no statistically significant difference between the two groups in the cerebellar vermis height, anteroposterior vermis diameter, cerebellar vermis circumference and cerebellar vermis area (P > 0.05). The transverse cerebellar diameter in the extremely premature infants group was smaller than that in VLBW premature infants group (P < 0.05). The difference between the two groups was also not statistically significant in the cerebellar vermis height, anteroposterior vermis diameter, cerebellar vermis circumference and cerebellar vermis area (P > 0.05).
Conclusion
The growth of midline structure of the cerebellum was preserved in the premature infants at term equivalent age without supratentorial brain injury probably, and the growth of lateral structure of the cerebellum will be decreased, which is most vulnerable to insult following the factor of premature birth.
Keywords
Cerebellum
Cranial ultrasound
Premature infants
Gestational age
1 Introduction
1.1 Premature
infants, especially those with very low birth weight,(VLBW) with immature brain development, will facing some potential risk factors before, during and after the delivery, such as asphyxia, ischemia, infection, malnutrition and so on. These risk factors have adverse effects on the brain development for premature infants. The long-term incidence of adverse effects in surviving premature infants is still common, especially neurodevelopmental damage. 5% to 10% of premature infants will develop cerebral palsy, and 25% to 50% will show cognitive, behavioral or language defects (Shao, 2017:). Especially in premature infants less than 32 weeks (MazouriA and KhalesiN, 2019). In the third trimester of pregnancy, the cerebellum is the fastest-growing part of brain development. In addition to controlling posture, gait, tone and coordinated motor function, the cerebellum is also involved in more advanced functions of the human brain such as cognitive function, motor function, learning function, language function and memory function (Gano and Barkovich, 2019). The extrauterine growth environment is not conducive to the growth and development of the cerebellum for premature infants. The detection of infratentorial lesions by bedside craniocerebral ultrasound can predict nervous system developmental disorders of premature infants (Sancak et al., 2017). When the doctor S Sancak S’s group were studying the cerebellum develops of premature infants to the full-term infants of same age, there is no difference in the development of cerebellar transverse diameter between premature infants group and full-term infants group of the same age (Sancak et al., 2016). At present, there is no such report on the comparative study of cerebellar development between premature infants and full-term infants of the same age in China. In this study, brain ultrasound was used to evaluate the growth and development of the cerebellum in preterm infants without supratentorial lesions and compared with full-term infants of the same age, in order to understand whether pathological factors such as premature delivery affect the growth and development of the cerebellum of premature infants.
2 Materials and methods
2.1 Studying objects
The inclusion criteria were as follows: (1) neonates who were hospitalized and followed up in the Department of Neonatology, Changzhou people's Hospital from January 2016 to December 2018; (2) very low birth weight (VLBW) infants with gestational age < 32 weeks and birth weight < 1500 g, full-term infants with gestational age of 40 weeks; (3) infants of suitable gestational age (Chen and Newborn, 2019), no congenital malformations and central nervous system abnormalities; 5. The clinical data of neonates are completed. 6. The craniocerebral ultrasound images were completed and clear, and there was no brain injury in premature infants (Neonatal et al., 2012), which meeted the requirements of measurement. Those craniocerebral ultrasound images following the inclusion criteria were collected, in which 88 cases were corrected when the gestational age was 40 weeks in the premature group and 65 cases were corrected on the day of birth in the full-term group. This study was approved by the Medical Ethics Committee of the hospital and the informed consent of the guardian.
2.2 Instruments and methods
The body weight and head circumference were measured and recorded by two neonatal pediatricians respectively. The data of body weight and head circumference were tested for repeatability between groups and within groups, and the average values of the two records were taken. All the subjects were in a quiet state and underwent bedside craniocerebral ultrasound examinations. Using Philips iuElite ultrasonic diagnostic apparatus and C8-5 neonatal brain special probe with a frequency of 5 MHz to 8 MHz, the anterior fontanelle was used as an acoustic window with the supine position. Make a sagittal scan, adjust the probe to obtain the standard median sagittal section, display the cerebellar vermis completely, do the coronal scan, and adjusted the probe to obtain the standard transverse section of the cerebellum. The best image was frozen and stored, and all pictures and text materials were input into the PACS system. The collected images were sketched and measured by two doctors familiar with neonatal ultrasound diagnosis, and the transverse diameter of the cerebellum was measured. The coronal section measured the maximum distance from the outer edge of the transverse diameter of the cerebellum to the outer edge of the transverse diameter of the cerebellum as shown in Fig. 1A. As the height of the cerebellar vermis, the maximum distance between the highest point of the superior cerebellar vermis and the lowest point of the inferior vermis as shown in Fig. 1B. As the anterior and posterior diameter of the cerebellar vermis, the distance from the apex of the fourth ventricle to the posterior edge of the cerebellar vermis as shown in Fig. 1C .As perimeter of the cerebellar vermis and the area of the cerebellar vermis , the outline of the vermis was drawn by hand in the median sagittal section of the cerebellar vermis(Fig. 1D). Each physician measured twice at different times, tested the repeatability of all the measurement results between groups and within groups, using the average value of the two effective measurements. All measurements were processed after the image was frozen and magnified.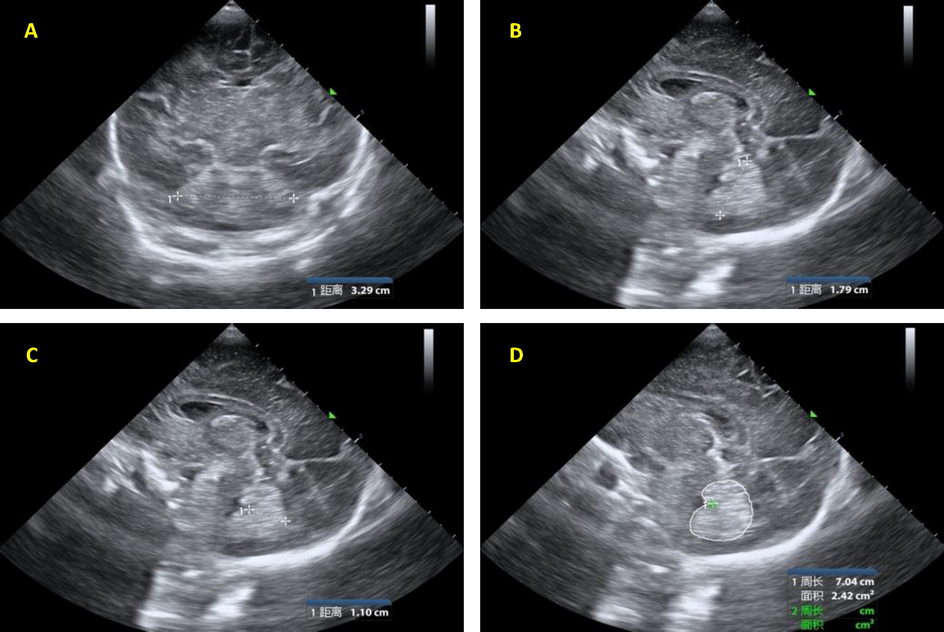
1A: Measurement of the transverse diameter of the cerebellum;1B: Measurement of the height of cerebellar vermis .1C: Measurement of the anteroposterior diameter of the cerebellar vermis.1D: Measurement of perimeter and area of cerebellar vermis.Noted(距离 means distance 周长 means Perimeter 面积means area).
2.3 Head circumference correction
When premature infants reach the full-term age, there is a significant difference in the shape of the head between the premature infants and the full-term neonates (Graca et al., 2013). To eliminate the potential influence of different neonatal head shape differences on cerebellar measurement results when studying the differences of cerebellar indexes between premature and full-term infants, the method of covariance (Thompson et al., 2011) was used in this study. the transverse diameter of the cerebellum and the anterior and posterior diameter of cerebellar vermis were corrected by using neonatal head circumference as a covariable. The correction formula is cD = mD-g × (mHC-aveHC), in which cD represent the corrected cerebellar index, MD is the actual measured cerebellar index, g is the regression line gradient between the actual measured cerebellar index and neonatal head circumference (slope), mHC is the actual measured neonatal head circumference, and aveHC is the actual measured average head circumference of all neonates (Agyemang et al., 2017).
2.4 Statistical analysis
SPSS22.0 and Microsoft Excel 2017 were used for statistical analysis and processing, and all the measured data were expressed as mean standard deviation (). It was statistically significant when P < 0.05. A paired sample t-test was used for inter-group and intra-group repetition test, and independent sample t-test was used to compare the differences of cerebellar indexes between premature infants and full-term infants of the same age.
3 Results
3.1 Inter-group and intra-group repeatability test
The paired sample t-test was used for inter-group and intra-group repeatability test, the results showed that the P values were all greater than 0.05, and there was no significant difference between the two neonatal pediatricians and between the two neonatal pediatricians. There was also no significant difference between the two ultrasound doctors’ measurements and between the two groups of dates which were their own measurement.
3.2 Measurement results of premature and full-term infants
When we the corrected gestational age approached to 40 weeks for the premature groups, the transverse diameter of cerebellum in premature group was 4.17 ± 0.39 cm, which was significantly smaller than that in term group and has statistically differences. (4.36 ± 0.38 cm, P < 0.05), and the anteroposterior diameter of cerebellar vermis in premature group was 1.53 ± 0.18 cm, which was larger than that in term group (1.46 ± 0.18 cm) (P < 0.05). The height of cerebellar vermis in premature infants was 2.49 ± 0.20 cm, which was 2.44 ± 0.22 cm higher than that in term infants. The perimeter of the cerebellar vermis in premature infants was 9.88 ± 0.70 cm, which was larger than that in term infants 9.80 ± 0.76 cm. The area of the median sagittal position of the cerebellar vermis in premature infants was 5.07 ± 0.59 cm ∼ 2, which was larger than 4.92 ± 0.59 cm ∼ 2 in term infants. There was no significant difference in the height of cerebellar vermis, the perimeter of the cerebellar vermis and the median sagittal area of cerebellar vermis between the two groups (P > 0.05) (Table 1).
premature infants
full-term infants
P
gestational age(weeks)
28.45 ± 2.12
40.43 ± 0.33
<0.001
Weight(g)
1136 ± 258
3457 ± 355
<0.001
weight when ultrasound exam(g)
3190 ± 363
3457 ± 355
<0.001
Head circumference (cm)
34.2 ± 1.37
34.8 ± 1.29
0.47
Transverse diameter of the cerebellum (cm)
4.17 ± 0.39
4.36 ± 0.38
0.003
Height of cerebellar vermis (cm)
2.49 ± 0.20
2.44 ± 0.22
0.169
Anterior and posterior diameter of the cerebellar vermis (cm)
1.53 ± 0.18
1.46 ± 0.18
0.019
Perimeter of cerebellar vermis (cm)
9.88 ± 0.70
9.80 ± 0.76
0.532
Area of the median sagittal position of cerebellar vermis (cm2)
5.07 ± 0.59
4.92 ± 0.59
0.151
3.3 Results of head circumference correction in premature and full-term infants
The transverse diameter of the cerebellum and the anterior and posterior diameter of cerebellar vermis were corrected by the covariance method (Thompson et al., 2011). It was found that the transverse diameter of the cerebellum in the premature group was 4.21 ± 0.28 cm, which was smaller than that in the full-term group (4.30 ± 0.29 cm). The difference was statistically significant (P < 0.05). The anterior and posterior diameter of the cerebellar vermis in the premature group was 1.51 ± 0.15 cm, which was larger than 1.48 ± 0.16 cm in the full-term group. The difference was not statistically significant (P greater than 0.05).
3.4 The results of cerebellar indexes in super preterm infants with gestational age < 28 weeks and extremely preterm infants with gestational age ≥ 28 weeks
The transverse diameter of the cerebellum in the premature group was 4.03 ± 0.41 cm, which was smaller than that in the premature group (4.24 ± 0.36 cm). There was a significant difference between the two groups (P < 0.05). The height of cerebellar vermis, the anterior and posterior diameter of the cerebellar vermis, the perimeter of cerebellar vermis and the area of the median sagittal position of cerebellar vermis in the super premature group were lower than those in a very premature group, but the differences were not statistically significant (P > 0.05) (Table 2).
<28 weeks premature infants
≥28 weeks premature infants
P
gestational age(weeks)
26.42 ± 0.79
30.09 ± 1.24
P<0.001
Weight(g)
923 ± 212
1309 ± 186
P<0.001
weight when ultrasound exam(g)
3180 ± 385
3220 ± 450
0.49
Head circumference (cm)
33.9 ± 1.36
34.3 ± 1.38
0.48
Transverse diameter of the cerebellum (cm)
4.03 ± 0.41
4.24 ± 0.36
0.013
Height of cerebellar vermis (cm)
2.47 ± 0.18
2.50 ± 0.22
0.645
Anterior and posterior diameter of the cerebellar vermis (cm)
1.49 ± 0.19
1.54 ± 0.17
0.504
Perimeter of cerebellar vermis (cm)
9.77 ± 0.71
9.95 ± 0.69
0.242
Area of the median sagittal position of cerebellar vermis (cm2)
5.01 ± 0.65
5.12 ± 0.54
0.389
4 Discussion
Previous studies suggested that nervous system damage in premature infants is mainly caused by white matter pathological changes (LimperopoulosC and Sullivan, 2014), but recent studies have found that neurological dysfunction in premature infants is also related to cerebellar abnormalities. The cerebellum is located in the posterior fossa, the posterior lower part of the brain, the dorsal side of the brain stem, and forms the fourth ventricle with the medulla oblongata and pons, connecting the whole brain, especially the cerebral cortex. It plays a important role in controlling motor functions such as coordination, balance, posture, and learning, and also plays a key role in non-motor functions such as cognition and emotion. cerebellar developmental defects are also associated with a wide range of diseases. Examples include ataxia, dystonia tremor, schizophrenia, dyslexia, and autism spectrum disorders (Beckinghausen and Sillitoe, 2018; Adamaszek et al., 2017; Baumann et al., 2015).
At 12 weeks, the two lateral sides of the cerebellar plate dilated to form the cerebellar hemisphere, and the central part of the cerebellar plate became thinner, forming the cerebellar vermis (Chen and Newborn, 2019). The bilateral cerebellar hemispheres and the middle cerebellar vermis constitute the lateral structure and midline structure of the cerebellum respectively. The growth of cerebellum is not parallel to other brain development levels, the development of cerebellum mainly occurred in the third trimester of pregnancy (Botellero et al., 2016). From gestational age 28 weeks to 40 weeks, the volume of cerebellum increases nearly 5 times, and the surface area of cortex increases by more than 30 times (Adre, et al., 2018). In this study, premature infants with gestational age less than 32 weeks were selected as subjects to observe the development level of cerebellum at full-term age of 40 weeks. The reason is that the cerebellum grows rapidly in this period, which is a sensitive period of cerebellar development. Long-term follow-up is prone to detect some abnormal neuromotor developments. According to the projection law between the piriform neurons and the cerebellar nucleus in the cerebellar cortex, it is divided into three longitudinal regions from the inside to the outside, namely, the medial area, the intermediate area and the lateral area (Jufang, 2018) .The brain ultrasound technique was used to quantitatively analyze and compare whether there were statistical differences in the indexes of lateral and medial areas between the two groups, that is, the full-term group and the full-term group of the same age, to completely evaluate the extrauterine growth and development of cerebellum in premature infants. The transverse diameter of cerebellum, the height of cerebellar vermis, the anterior and posterior diameter of cerebellar vermis, the perimeter of cerebellar vermis and the area of median sagittal position of cerebellar vermis are all indicators for quantitative analysis of the development of lateral and medial cerebellar regions in newborns. This study did not include the relevant measurement indicators of the intermediate area, because the recognition of the cerebellar intermediate region in neonatal craniocerebral ultrasound images is poor and difficult to identify.
Through the comparative study of VLBW premature infants with gestational age less than 32 weeks to 40 weeks and full-term infants of the same gestational age, it was found that the transverse diameter of cerebellum in the premature group was smaller than that in the full-term group, while the height of cerebellar vermis, the anterior and posterior diameter of cerebellar vermis, the perimeter of cerebellar vermis and the median sagittal area of cerebellar vermis in the premature group were larger than those in the full-term group. After statistical analysis, it was found that there were statistical differences in the transverse diameter of cerebellum and the anterior and posterior diameter of cerebellar vermis. Because there is a significant difference in head shape between premature infants and full-term neonates when they reach full-term age (Graca et al., 2013), to eliminate the influence of neonatal head shape on the results of the study, in this study, the method of covariance (Thompson et al., 2011) was used to correct the head circumference of the transverse diameter of cerebellum and the anterior and posterior diameter of cerebellar vermis, and the differences of the above two indexes between the two groups were compared again after correction. The results showed that the size relationship between the two indexes remained unchanged, however the difference decreased. After fathermore statistical analysis, it was found that there was no statistically significant difference in the anterior and posterior diameter of the cerebellar vermis between the two groups, but there was still a statistically significant difference in the transverse diameter of the cerebellum. This conclusion further shows that when premature infants reach full-term age, compared with full-term infants of the same age, there is a difference in the shape of the head of neonates indeed, which verifies the claim of Dr Graca AM’s group. (Graca et al., 2013). In addition, according to the conclusion of this study, it is speculated that the factors of premature delivery have different effects on the growth and development of the lateral and medial cerebellar areas, while the lateral areas are more affected than the medial cerebellar areas. The reason may be that the cortical area of the lateral cerebellar area is larger than that of the medial cerebellar area, and the number of cerebellar granule cells is more than that of the medial cerebellar cortex. the granule cells are excitatory neurons which derived by glutamate energy (Jufang, 2018) . It is more sensitive to the pathological factor of preterm delivery which indicate it could be damaged more easily. The maturity of extrauterine growth and development of premature infants is lower than that of full-term infants, and the growth and development of cerebellum is lower than that of full-term infants in varying degrees. The result of this study shows that the growth and development of lateral cerebellar area lags obviously, while the medial area is equal to the level of full-term infants. Dr Sancak S’s (Sancak et al., 2016)group measured the transverse diameter of cerebellum and the height of cerebellar vermis in 38 premature infants with a gestational age of 27 weeks to 32 weeks and compared with 40 cases of full-term infants, which indicated that the development of transverse diameter of cerebellum in premature infants could catch up with that in full-term infants before full-term age, and the difference was not statistically significant, but the difference in the height of cerebellar vermis was statistically significant. This is not consistent with the results of this study, and the reason for the analysis may be due to the number and individual differences of the subjects included in their respective studies, as well as whether or not to correct the head circumference. In this study, 88 premature infants were included, all of which were suitable for gestational age. the average gestational age was 29.03 ± 2.18 weeks and the body weight was 1136 ± 258 g. In order to eliminate the effect of head shape on the results, the head circumference of cerebellar transverse diameter and anterior and posterior diameter of cerebellar vermis were corrected and statistically analyzed again. In order to further verify the above conclusions, the premature infants with gestational age<32 weeks VLBW were re-divided into two groups according to the boundary of 28 weeks, that is, the super premature infants with gestational age<28 weeks and the very premature infants with gestational age from 28 weeks to 31 + 6 weeks. The indexes of lateral and medial cerebellar areas were compared between the two groups when they developed to 40 weeks of full-term age. The results showed that the transverse diameter of cerebellum, the height of cerebellar vermis, the anterior and posterior diameter of cerebellar vermis, the perimeter of cerebellar vermis and the area of median sagittal position of cerebellar vermis in the super premature group were smaller than those in the very premature group, but only the transverse diameter of the cerebellum had statistical difference. This further indicates that the shorter the time for neonates to grow and develop in the uterus, the more vulnerable the lateral cerebellar area is.
As an important regulatory center of subcortical sensation and movement, the cerebellum functions mainly to maintain body balance, regulate muscle tension and regulate the random and fine movement of skeletal muscle (Jufang, 2018) . The lateral cerebellar area develops synchronously with the cerebral cortex and forms a fiber connection loop with the cerebral cortex, which mainly receives the afferent fibers from the cortical pontine tract through the middle foot of the cerebellum after the pontine nucleus relay, and regulates the random and fine movement of skeletal muscle (Allin et al., 2005) . Compared with the medial cerebellar area, the lateral area is more related to cognitive function (Allin et al., 2005). Cerebellar diseases can lead to eye movement disorders, dysarthria, language disorders, physical ataxia, postural and gait ataxia, as well as cognitive and behavioral disorders. The purpose of this study was to compare the differences in the growth and development of the lateral and medial cerebellar regions between the full-term infants with a gestational age of<32 weeks and the full-term infants with a gestational age of 40 weeks, to understand the development of the extrauterine cerebellum of VLBW premature infants. It is expected to measure the indexes of the lateral and medial cerebellar regions by cranial ultrasound and find its predictive value for the long-term prognosis of premature infants. The lateral cerebellar area is more easily affected by pathological factors such as premature delivery during the development of premature infants.
This study is still in the preliminary stage, and the results are preliminary conclusions. Premature infants with gestational age less than 32 weeks need to be followed up for a long time to further confirm the research results. The further research direction should be to evaluate whether there is a correlation between cerebellar measurements and the outcome of neuromotor development in premature infants. In addition, this study also has some limitations, such as premature infants whose supratentorial lesions are not included in the study. Dr Srinivasan’s group (Srinivasan et al., 2006) have shown that cerebellar volume reduction in premature infants is related to supratentorial lesions, such as cerebral hemorrhage, cerebral infarction, intraventricular hemorrhage with dilatation and periventricular leukomalacia. In future studies, these premature infants need to be measured and evaluated separately. To evaluate the growth and development of the cerebellum. In addition, in the aspect of data processing, when using the covariance method to correct the cerebellar data, only the neonatal head circumference is used as the covariable to correct, but the head circumference is not enough to represent the whole difference of the whole head shape of the newborn. The next step is to introduce more covariance variables for data correction and statistical analysis, to make the corrected cerebellar index parameters more accurate.
Acknowledgement
The project supported by the Foundation of Health Commission of Hebei Province,(Correlation between cerebellar development and neuromotor development in premature infants) (Grant No. 20200173) China.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Xiaomei Shao., Brain development in preterm infants and brain damage in preterm infants [M] Xiaomei Shao, Wenhao Zhou. Fetal and neonatal brain injury., 2017: 51.
- Investigation of the relationship between umbilical cord pH and intraventricular hemorrhage of infants delivered preterm. J. Brazilian Medical Assoc.. 2019;65(5):647-656.
- [Google Scholar]
- Cerebellar hypoplasia of prematurity: Causes and consequences. Handbook of Clin. Neurol.. 2019;162:201-216.
- [Google Scholar]
- Effect of intraventricular hemorrhage on cerebellar growth in preterm neonates. Cerebellum. 2017;16(1):89-94.
- [Google Scholar]
- classification; Congle Zhou. Nervous system embryo development [M]. Xiaomei Shao, Hongyi Ye, Xiaocan Qiu. Practical (neonatology(5th edition).). Beijing: People's Medical Publishing House; 2019.
- Expert consensus on diagnosis and prevention of brain injury in premature infants. China J. Contemporary Pediatrics. 2012;12:883-884.
- [Google Scholar]
- Cerebral volume at term age: Comparison between preterm and term-born infants using cranial ultrasound. Early Hum Dev. 2013;89(9):643-648.
- [Google Scholar]
- Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55(2):479-490.
- [Google Scholar]
- Cerebellar exposure to cell-free hemoglobin following preterm intraventricular hemorrhage: causal in cerebellar damage? Translational Stroke Research 2017
- [Google Scholar]
- Injury to the Premature Cerebellum: Outcome is Related to Remote Cortical Development[J] Cereb. Cortex. 2014;24(3):728-736.
- [Google Scholar]
- Insights into cerebellar development and connectivity[J] Neurosci. Lett.. 2018;S0304394018303409
- [Google Scholar]
- Consensus Paper: The Role of the Cerebellum in Perceptual Processes[J] The Cerebellum. 2015;14(2):197-220.
- [Google Scholar]
- Mental health and cerebellar volume during adolescence in very-low-birth-weight infants: a longitudinal study[J] Child and Adolescent Psychiatry and Mental Health. 2016;10(1):6.
- [Google Scholar]
- Adre J, du Plessis, Limperopoulos C, et al. Chapter 4-Cerebellar Development[J]. Volpes Neurology of the Newborn, 2018:73-99.
- Huang Jufang. Cerebellum [M] .Wen Long Ding, Xue Zheng Liu. System Anatomy(9th Edition). Beijing: People's Medical Publishing House, 2018.
- Vermis and lateral lobes of the cerebellum in adolescents born very preterm[J] NeuroReport. 2005;16(16):1821-1824.
- [Google Scholar]
- Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. Ajnr Am. J. Neuroradiol.. 2006;27(3):573-579.
- [Google Scholar]







