Translate this page into:
A computational approach for screening of phytochemicals from Oxalis corniculata as promising anti-cancer candidates
⁎Corresponding author at: IMTECH Centre for Animal Resources & Experimentation (iCARE), Council of Scientific and Industrial Research-Institute of Microbial Technology (CSIR-IMTECH), Sector 39-A, Chandigarh 160036, India. neeraj@imtech.res.in (Neeraj Khatri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives.
In silico techniques are a highly efficient, cost-effective, and rapid approach to identify potent herbal lead compounds with therapeutic potential against cancer. Phytochemicals are abundant in plants and may represent a promising cancer prevention and treatment approach. The plant species Oxalis corniculata has long been employed in traditional medicine to treat various diseases. Indeed, previous studies have demonstrated the anti-cancer properties of the crude extract obtained from this plant. However, further research is essential to elucidate precisely the underlying molecular mechanisms responsible for these activities.
Methods.
The study investigated the potential of bioactive compounds from O. corniculata to bind with different targets of anti-cancer drugs. Employing molecular docking and drug-likeness, 19 bioactive compounds from the plant were tested as potential anti-cancer leads. Compounds that exhibit both favorable drug-likeness and binding energies comparable to those of standard drugs like curcumin, doxorubicin, and paclitaxel were selected for further evaluation of their pharmacokinetic properties.
Results and Conclusions.
Among the 19 selected phytoconstituents of O. corniculata, compounds 15 and 16 exhibited the most effective binding energy (−8.68, −8.22 and −8.70, −8.22, −8.52 kcal/mol, respectively) with key cancer targets including Programmed death-ligand 1 (PDB: 7bea), B-Raf proto-oncogene, serine/threonine kinase (PDB: 8c7y) and poly (ADP-ribose) polymerase (PDB: 7kk4). Pharmacokinetic and toxicity analysis provided additional insights into the potential of these compounds as anti-cancer drugs. The computational analysis holds promise in accelerating the development of novel drug therapies aimed at treating various cancer types.
Keywords
Oxalis corniculata
Phytochemical constituents
Anti-cancer drug candidates
Medicinal plants
Anti-cancer activity
In silico assessment
- ADME/T
-
Absorption, Distribution, Metabolism, Excretion, and Toxicity
- ADP
-
Adenosine Diphosphate
- BBB
-
Blood-Brain Barrier
- BRAF
-
Serine/Threonine-Protein Kinase B-Raf
- CDK4
-
Cyclin-Dependent Kinase 4
- CDK6
-
Cyclin-dependent kinase 6
- CDK9
-
Cyclin-Dependent Kinase 9
- CNS
-
Central Nervous System
- COX-2
-
Cyclooxygenase-2
- DNA
-
Deoxyribonucleic Acid
- EGFR
-
Epidermal Growth Factor Receptor
- HBA
-
Hydrogen Bond Acceptor
- HBD
-
Hydrogen Bond Donor
- HER2
-
Human Epidermal Growth Factor Receptor 2
- MAPK
-
Mitogen-activated protein Kinase
- mTOR
-
Mammalian Target of Rapamycin
- MW
-
Molecular Weight
- NF-Kb
-
Nuclear factor kappa B
- OCT2
-
Organic Cation Transporter 2
- PAK1
-
p21-Activated Kinase 1
- PARP
-
Poly (ADP-ribose) Polymerase
- PD1
-
Programmed Cell Death Protein 1
- PDB
-
Protein Data Bank
- PD-L1
-
Programmed death-ligand 1
- RB
-
Rotatable Bond
- RCSB
-
Research Collaboratory for Structural Bioinformatics
- SMILES
-
Simplified Molecular-Input Line-Entry System
- TPSA
-
Topological Polar Surface Area
Abbreviations
1 Introduction
Plants have emerged as a rich reservoir of medicinal compounds that have played a crucial role in developing many modern medicines. The medicinal potency of plants stems from their intricate chemical composition, which includes a wide array of phytochemicals such as terpenoids, alkaloids, flavonoids, phenols, glycosides, and various secondary constituents (Zahra et al., 2023; Shah et al., 2023; Riaz et al., 2023). natural alkaloids like berberine, evodiamine, and matrine are among the phytochemicals identified for their medicinal properties (Gaikwad & Srivastava, 2021). Medicinal plants contain bioactive compounds with potential anti-cancer properties, targeting cancer through diverse mechanisms (Newman & Cragg, 2020). However, challenges in standardization and clinical validation persist, necessitating interdisciplinary research efforts to fully unlock their potential in cancer treatment.Phytochemicals effectively treat anaplastic thyroid, breast, and other cancers by modulating tumor aggressiveness and acting as potent anti-cancer agents.Oxalis corniculata, a creeping wood sorrel native to Europe, has attracted attention for its potential pharmacological activities, including anti-cancer properties (Groom et al., 2019). Studies show that an ethanol extract from the leaves of this plant induces apoptosis in human MCF-7 breast cancer cells (Gholipour et al., 2022). This plant shows promising pharmacological activities and has potential as an anti-cancer agent due to its bioactive compounds, including flavonoids and phenolic compounds, which have antioxidant and anti-inflammatory properties.
Computational methods like virtual screening and molecular docking have been used to identify potential anti-cancer lead molecules and pharmaceutical targets, such as p21-activated kinase 1 (PAK1) (Yao et al., 2020), and analysis of large phytochemical datasets using computer programs can reliably identify active compounds for use as drugs. Cancer continues to be a major global health challenge, affecting millions of people. Targeted therapies have proven to be promising interventions to combat various types of cancer. Inhibitors of programmed death-ligand 1 (PDL-1), poly (ADP-ribose) polymerase (PARP), and B-Raf proto-oncogene serine/threonine kinase (BRAF) can revolutionize cancer treatment by unleashing anti-tumor immune responses. PDL-1 inhibitors are effective in metastatic breast cancer, liver cancer, and colorectal Cancer (Reck et al., 2016), while PARP inhibitors exploit DNA repair vulnerabilities in cancer cells (Knelson, 2021). BRAF inhibitors target aberrant MAPK signaling in cancer cells, with some patients responding to them in certain subtypes (Arora et al., 2021). Challenges in treatment resistance, biomarker identification, and patient stratification necessitate ongoing research and clinical trials to advance precision medicine in cancer treatment (Al-Nour et al., 2021).
In the present study, we sought to investigate the potent anti-cancer properties of phytocompounds extracted from O. corniculata against PDL-1, PARP, BRAF, and other receptors associated with various cancer types (Table 1) using in silico methods. We examined the binding affinities, drug-likeness, ADME/T, and molecular interactions of the selected phytochemicals for specific biological receptors from five different cancer types, which play a crucial role in cell growth, cell cycle, and DNA replication.
Cancer Types
Drug Targets
References
Cervical CancerPD-L1
(Huang et al., 2022)
HER2
(Taja-Chayeb et al., 2020)
EGFR
(Krishna et al., 2023))
mTOR
(Kim et al., 2010)
PARP
(Tomao et al., 2020)
Liver CancerAnnexin A3
(Pan et al., 2015)
CDK9
(Yao, 2021)
EGFR
(Fang et al., 2017)
PD1
(Zeng et al., 2023)
PD-L1
(Zeng et al., 2023)
Colorectal CancerEGFR
(Chan et al., 2017)
mTOR
(Kim & Eng, 2012)
PD1
(Yang et al., 2022)
PD-L1
(Yang et al., 2022)
BRAF
(Grassi et al., 2021
Breast CancerAkt
(Martorana et al., 2021)
CDK4
(Dean et al., 2010)
CDK6
(Dean et al., 2010)
COX-2
(Sahu et al., 2023)
EGFR
(Li et al., 2022)
mTOR
(Ortega et al., 2020)
NF-Kb
(Sampepajung et al., 2021)
p53
(Marvalim et al., 2023)
PARP
(Tung & Garber, 2022)
PD-L1
(Vranic et al., 2021)
Lung CancerBRAF
(Yan et al., 2022)
EGFR
(Tian et al., 2022)
PD-1
(Yang et al., 2020)
PD-L1
(Yang et al., 2020)
mTOR
(Sui et al., 2021)
2 Materials and methods
The methodological approach consists of five discrete steps: (i) Phytochemical ligand library construction, (ii) Druggable cancer target selection, (iii) Molecular docking, (iv) Physicochemical analysis, and (v) ADME/T profiling. The overall data processing and analysis workflow is illustrated in Fig. 1, and detailed descriptions of the different steps are provided in the sections below. This methodological approach employs a systematic and rigorous process to identify potential anti-cancer compounds from a traditional plant source, ensuring the accuracy and reliability of results throughout the process.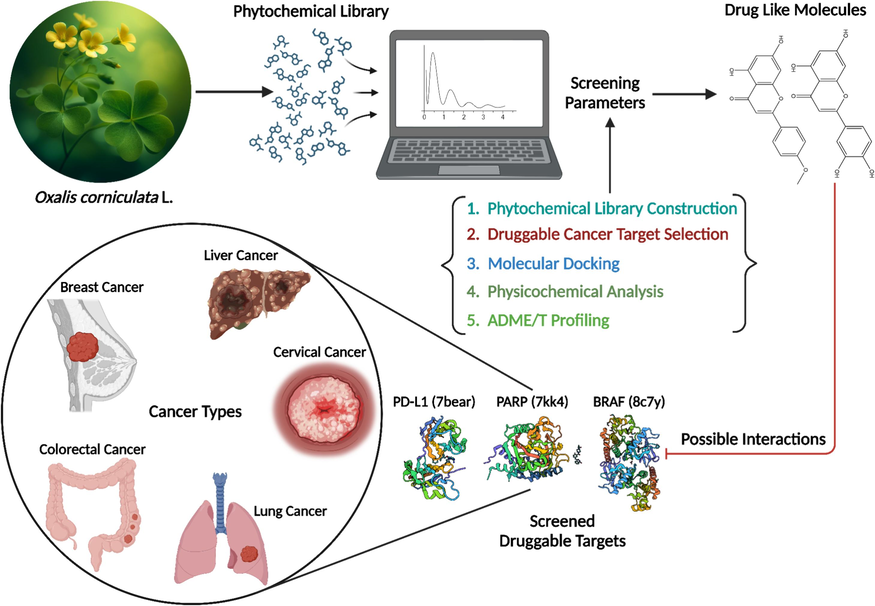
Schematic illustration of steps involved in the computational screening of phytoconstituents against druggable targets of various cancer types.
2.1 Phytochemical ligand library construction
The chemical ligand classes are based on diverse scaffolds of Oxalis corniculata, which are traditionally used for treating cancer and related ailments (Khan, 2014). A herbal-based ligand library was constructed, comprising 19 ligands belonging to the above chemical classes (Table S2 − S6). The two-dimensional structure of each ligand was created using ChemDraw 9.0 software using their canonical SMILES from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), followed by their energy minimization with the MM2 force field of the Chem3D tool to generate their three-dimensional shape for computational evaluation (Naveed et al., 2024; Zahra et al., 2023).
2.2 Druggable cancer target selection
Cancer types and their potential drug targets were selected for molecular docking, and their structural models for the selected macromolecular targets were taken from the Protein Data Bank (https://www.rcsb.org/) and converted into monomeric nascent receptors by deleting other chains and complex ligands. The resulting monomeric receptor was prepared for docking analysis by adding polar hydrogens and assigning a uniform distribution of Gasteiger charge to the macromolecular residues.
2.3 Molecular docking studies
All targets included in the current docking analysis were redocked with the Autodock software using individual reference ligands (Gupta et al., 2022). A focused grid box was created for molecular docking of all target receptors considered in the current study, covering both the extended conformations of the reference ligand and the interacting macromolecular residues. Docking parameters for each target were assessed by comparing conformation and chemical similarity between the reference ligand and active site of macromolecule, then used for computational screening against anti-cancer targets (Mujwar, 2021).
2.4 Screening of drug-likeness and ADME/T prediction
Evaluating the drug-likeness properties of drug candidates accelerates drug discovery and development progress. All 19 molecules were subjected to a virtual screening process to assess their drug-likeness, pharmacokinetic, and associated toxicity. The physicochemical and ADME/T properties of compounds were calculated using SwissADME (https://www.swissadme.ch/), DataWarrior 6.0 software, and an online platform PkCSM (https://biosig.lab.uq.edu.au/pkcsm/prediction). Molsoft, an online tool (https://molsoft.com/mprop/), was also employed to calculate drug-likeness in the form of a metric called 'Molsoft Score' by evaluating the drug-like properties of chemical compounds, including the number of hydrogen bond acceptors (HBA), the number of hydrogen bond donors (HBD), the molecular weight, the water partition coefficient (MolLogP), the water solubility (MolLogS), molecular polar surface area (MolPSA), molecular polar surface volume (MolVol), acid dissociation constant (pKa) and blood–brain barrier (BBB) assessment. The analysis of BOILED-Egg conducted by SwissADME (Shabbir et al., 2023) was subsequently utilized to validate the drug-likeness through passive gastrointestinal absorption and brain permeability evaluation based on physicochemical descriptors such as WLOGP and TPSA.
3 Results
3.1 Macromolecular target selection and preparation
Macromolecular targets involved in the pathophysiology of diverse cancer types such as cervical, liver, colorectal, breast, and lung cancer were reviewed with reference to recent literature, and essential targets were shortlisted (Table 1) for further analysis due to their direct involvement in cancer progression (Sarnik et al., 2021). A molecular docking study of phytochemical ligands against described anti-cancer targets and a three-dimensional form of the macromolecular targets were retrieved from the Protein Data Bank (Berman et al., 2002) and prepared for docking analysis by removing the complex ligands, addition of polar hydrogen, and ADT (AutoDockTool) atom type. The final shortlisted ligands based on low binding energy, high binding affinity, and other parameters discussed in this study, shown in Fig. 2, might lead the way to discovering a potent anti-cancer agent.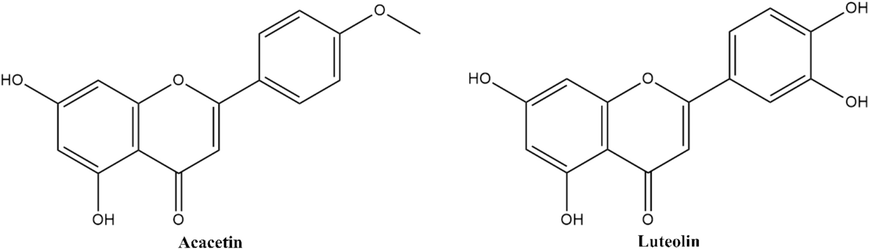
Structural representation of shortlisted phytochemicals acacetin and luteolin.
3.2 Screening of drug-likeness and ADME/T prediction
A virtual screening was performed of 19 phytoconstituents from Oxalis corniculata, taking into account drug similarity, Lipinski's “rule of five” and toxicity parameters. Among the 19 phytochemicals screened, two compounds, acacetin and luteolin, performed well in all the screening parameters. The physicochemical properties of these 2 compounds are computed and shown in Table 2 using tools SwissADME and Data Warrior 6.0. These identified phytochemicals had satisfactory spectrum with a molecular weight of less than 500 Dalton, less than 10 hydrogen bond acceptors, less than 5 hydrogen bond donors, and logP values of less than 5. Their pharmacokinetic parameters, such as absorption, partition coefficient, and solubility, were also predicted and found to be important factors, as shown in Table 3. The drug-likeness scores of the two compounds were computed using Molsoft, an online tool. The compounds exhibiting positive scores are considered to be in the drug-like range. As illustrated in Fig. 3A, acacetin and luteolin, along with standard drugs (curcumin, doxorubicin, and paclitaxel), were predicted to have positive scores of 0.20–0.97, suggesting that they seem drug-like. Further BOILED-Egg analysis from SwissADME predicts passive gastrointestinal absorption and brain access using WLOGP and TPSA physicochemical descriptors. The white area represents the physicochemical domain where the gastrointestinal tract most likely absorbs molecules, and the yellow area signifies the physicochemical space where molecules are most likely to penetrate the brain. It is important to note that the yolk and white regions are not completely separate. BOILED-Egg analysis of standard drugs doxorubicin and paclitaxel showed neither to be absorbed nor permeant to the blood–brain barrier (BBB); their prediction did not fall in the range of the plot with a TPSA of 206.07 Å2, 221.29 Å2 and a WLOGP of −0.32, 3.41 respectively while proposed phytochemicals acacetin, luteolin, and curcumin (control) fall within the range (Fig. 3B). These results suggest that acacetin and luteolin are promising leads for the development of an anti-cancer drug for different forms of cancer. MW − Molecular weight, cLogP − Partition coefficient between water and n-octanol, cLogS − Water solubility at 25˚ and pH=7.5, HBA − Hydrogen bond acceptor, HBD − Hydrogen bond donor, TPSA − Topological polar surface area (Å2), RB − Rotatable bond.
Name
MW
cLogP
cLogS
HBA
HBD
(TPSA)
Mutagenic
Tumorigenic
Reproductive Effective
Irritant
RB
Acacetin
284.266
2.6114
−3.17
5
2
79.90
none
none
None
none
2
Luteolin
286.238
1.99
−2.56
6
4
111.13
none
none
None
none
1
Properties
Acacetin
Luteolin
Molecular weight
284.267
286.239
TPSA
79.90
111.13
LogP
2.8798
2.2824
H-bond acceptor
5
6
H-bond donor
2
4
Absorption
Water solubility (log mol/L)
−3.284
−3.094
P-Glycoprotein substrate
Yes
Yes
P-Glycoprotein I inhibitor
No
No
P-Glycoprotein II inhibitor
No
No
Caco2 permeability (log Papp in10-6 cm/s)
1.137
0.096
Intestinal absorption (human) (% Absorbed)
94.318
81.13
Skin permeability (log Kp)
2.737
−2.735
Distribution
VDss (human, log L/kg)
0.346
1.153
BBB permeability (logBB)
0.196
−0.907
CNS permeability (log PS)
−2.159
−2.251
Fraction unbound (human) (Fu)
0.08
0.168
Metabolism
CYP3A4-substrate
Yes
No
CYP2D6-substrate
No
No
CYP2C19-inhibitor
Yes
No
CYP1A2-inhibitor
Yes
Yes
CYP2D6-inhibitor
No
No
CYP2C9-inhibitor
Yes
Yes
CYP3A4-inhibitor
Yes
No
Excretion
Renal OCT2 substrate
No
No
Total clearance (log ml/min/kg)
0.663
0.495
Toxicity
Hepatotoxicity
No
No
hERG I inhibitor
No
No
hERG II inhibitor
No
No
AMES toxicity
No
No
Skin sensitization
No
No
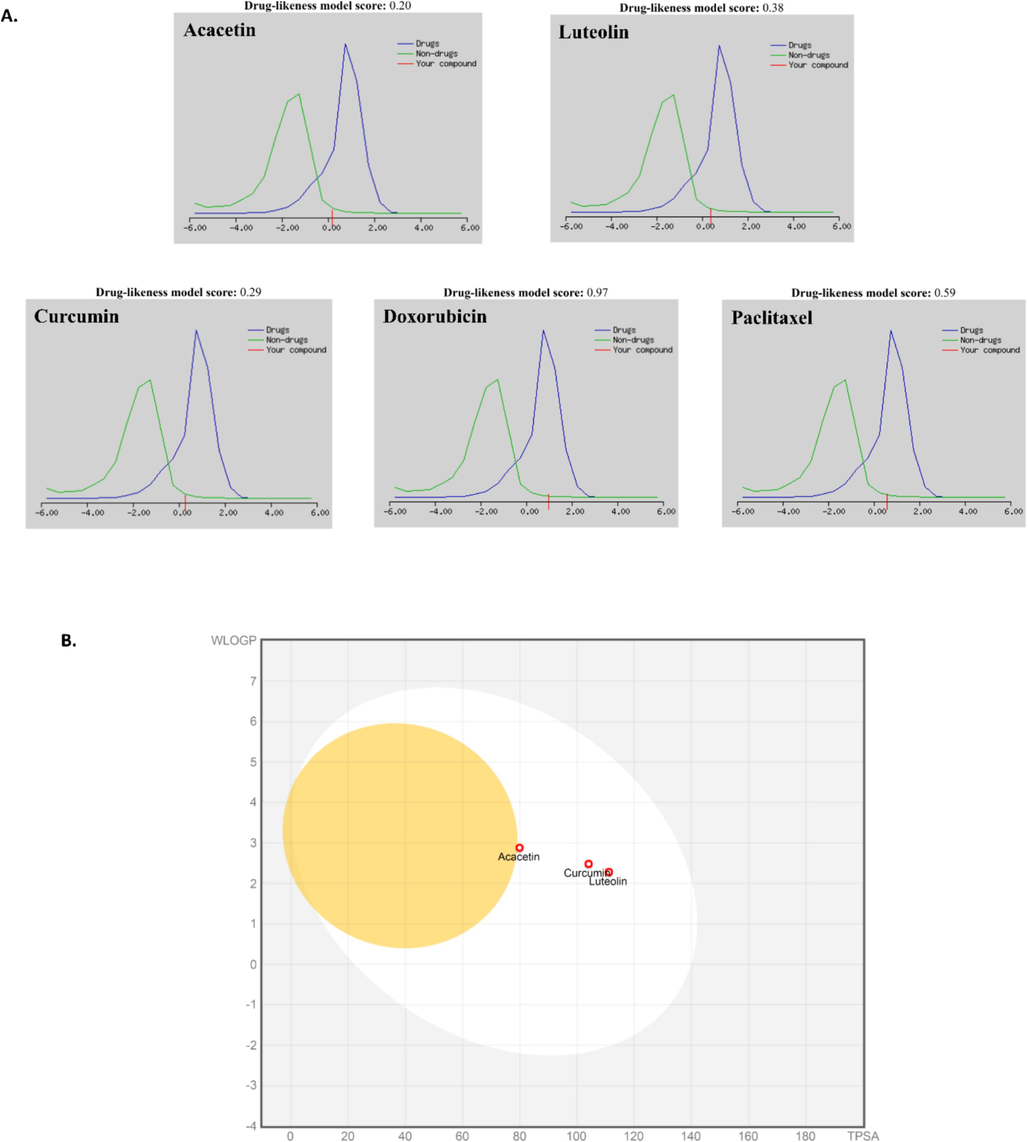
Drug-likeness representation of acacetin and luteolin. (A.) Using MolSoft (B.) BOILED-Egg analysis of acacetin and luteolin compared with FDA-approved anti-cancer drugs.
3.3 Molecular docking
The docking protocol used for each macromolecular target investigated in this study was verified by redocking each reference ligand. The validated docking parameters, including the screening criteria, were further evaluated for screening a previously generated herbal-based ligand library against all anti-cancer targets implicated in different cancer types. The finalized grid parameters considered in the current study for molecular docking of the respective targets are tabulated in Table S1. After virtual evaluation, the lead receptors PD-L1, PARP, and BRAF were shortlisted depending on their minimum binding score (Table 4 and Fig. 4) in different types of cancer. The binding scores for various macromolecular targets analyzed in the present study are documented in Table S2 − S6. This comprehensive analysis highlights the potential of specific phytochemicals in targeting key receptors involved in cancer progression.
Sr. No.
Cancer Types
Molecules
Binding Energy (kcal/mol)
PD-L1
(PDB: 7bear)
PARP
(PDB: 7kk4)
BRAF
(PDB: 8c7y)
1
Cervical Cancer
Acacetin
−8.68
−
−
Luteolin
−8.70
−8.52
−
2
Liver Cancer
Acacetin
−8.68
−
−
Luteolin
−8.70
−
−
3
Colorectal Cancer
Acacetin
−8.68
−
−8.22
Luteolin
−8.70
−
−9.03
4
Breast Cancer
Acacetin
−8.68
−
−
Luteolin
−8.70
−8.52
−
5
Lung Cancer
Acacetin
−8.68
−
−8.22
Luteolin
−8.70
−
−9.03
6
Standard Drugs
Curcumin
−8.62
−8.33
−8.78
Doxorubicin
−8.09
−9.56
−6.7
Paclitaxel
−5.10
−7.38
−4.88
7
−
Reference ligand
−8.36
−7.89
−7.86
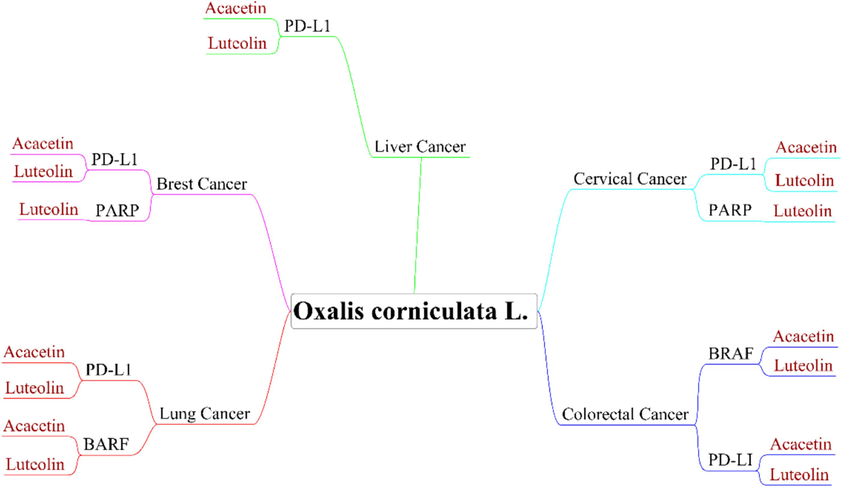
Schematic representation of the shortlisted phytochemicals acacetin and luteolin from Oxalis corniculata and their possible targets from different cancer types.
3.4 The binding affinities of the phytocompounds
Docking analysis revealed that acacetin and luteolin are active and non-toxic phytoconstituents from O. corniculata and exhibit anti-cancer potential against various types of cancer. These phytochemicals exert their therapeutic effects via targeting multiple therapeutic targets, including PD-L1, BRAF, and PARP. The highest binding affinities of acacetin and luteolin were observed against PD-L1 and BRAF receptors. The binding affinities of the phytocompounds and their interactions in two dimensions and the binding mode in three dimensions are shown in Fig. 5 (A − E). Various binding interactions observed in docking studies indicate that acacetin and luteolin can form complexes with amino acid residues inside the binding pockets of the targets (Table 5). These findings underscore the potential of these compounds as multi-target anti-cancer agents.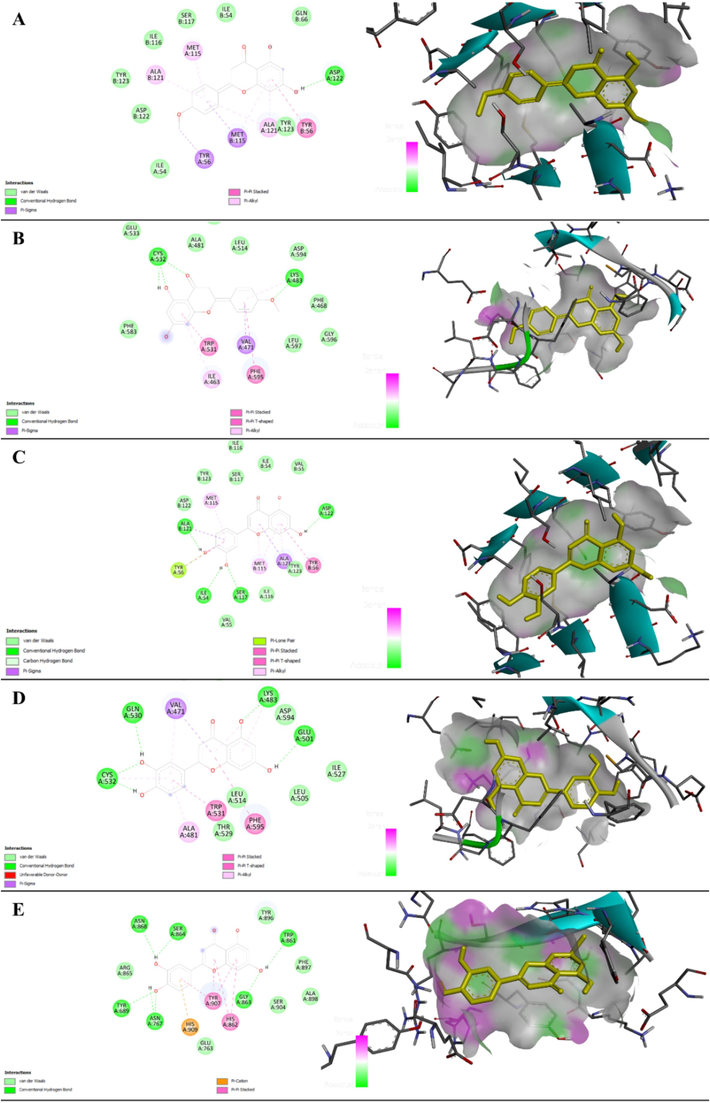
Binding affinity and amino acid interactions of acacetin complexed with PD-L1 (A) and BRAF (B), luteolin complexed with PD-L1 (C), BRAF (D), and PARP (E) shown in the form of two-dimensional interactions and three-dimensional binding mode.
Sr. No.
Interacting Molecules
Types of Interactions
Van der Walls
Conventional Hydrogen Bond
Pi-Sigma
Pi-Pi Stacked
Pi-Alkyl
A
Acacetin and PD-L1 (PDB: 7bear)ASP122, TYR123, ILE116, SER117, ILE54, GLN66, ASP122, TYR123
ASP122
MET115, TYR56
TYR56ALA121, MET115, ALA121
BAcacetin and BRAF
(PDB: 8c7y)GLU533, ALA481, LEU514, ASP594, PHE468, GLY596, LEU597
CYS532, LYS483
VAL471
TRP531, PHE595
ILE463
CLuteolin and PD-L1
(PDB: 7bear)ASP122, TYR123, SER117, ILE166, ILE54, VAL55, TYR123, ILE116, VAL55
SER117, ILE54, ALA121, ASP122
ALA121
TYR56
MET115
DLuteolin and BRAF
(PDB: 8c7y)THR529, LEU514, LEU505, ILE527, ASP594
CYS532, GLN530, LYS483, GLU501
VAL471
TRP531, PHE595
ALA481
ELuteolin and PARP
(PDB: 7kk4)ARG865, TYR896, PHE897, ALA898, SER904, GLU763
ASN767, TYR689, ASN868, SER864, TRP861, GLY863
−
TYR907, HUS862
−
4 Discussion
This work aimed to use a combination strategy of molecular docking and virtual screening to examine the structural interactions of bioactive chemicals from O. corniculata with important molecular targets implicated in cancer etiology. Herbal medicines, such as curcumin, are popular due to their low side effects and proven efficacy in cancer treatment (Gaikwad & Srivastava, 2021). These natural sources have undergone extensive preclinical and clinical testing, leading to the belief that medicinal plants can provide innovative anti-cancer therapies that could transform the oncology sector. Oxalis corniculata is reported for different pharmacological activities, including anti-cancer activities, but precise mechanisms underlying the anti-cancer properties remain incompletely elucidated. However, as far as we know, this is the first report where in silico screening has been performed to identify potential anti-cancer compounds from this plant. Secondary metabolites, including flavonoids and quassinoids, are linked to its anti-cancer potential (Gupta et al., 2022), and due to its antioxidative properties and ability, O. corniculata crude extract induces apoptosis in cancer cell lines (Gholipour et al., 2022).
Chemical compounds exhibit activity and selectivity but may not possess all the properties essential for the selectivity of a viable drug candidate. In silico analysis based on various scaffold structures of phytochemicals from this plant, a ligand library of 19 ligands from different chemical classes was generated (Table S2 − S6). Suitable therapeutic targets involved in cancer progression for various cancer types (Table 1) were identified, and the corresponding molecular binding sites for docking experiments were prepared. The three-dimensional structures of selected protein targets were extracted from the Protein Data Bank and converted into individual receptors for docking analysis.
Molecular docking serves as a crucial computational instrument within the realm of pharmaceutical research to identify the potential compounds and evaluate the interaction between protein and ligand entities at the specific binding site (Zahra et al., 2023). The molecular docking procedure includes verifying the docking protocol for each macromolecular target studied and screening the previously prepared herbal-based ligand library against PDL-1 (Reck et al., 2016), BRAF (Arora et al., 2021), PARP (Knelson, 2021), and all five different targets implicated in various cancer types. The lead molecules were shortlisted based on minimum binding energy and maximum analyzed binding interactions with the macromolecular target. Among the 19 phytoconstituents identified in O. corniculata, acacetin, and luteolin demonstrate binding energies of −8.68, −8.22, and −8.70, −8.22, −8.52 kcal/mol, respectively, when interacting with cancer-related targets such as PDL-1, BRAF and PARP (Table 4). Five stabilizing interactions were observed (Table 5): van der Waal, conventional hydrogen bond, pi-sigma, pi-pi stacked, and pi-alkyl helped stabilize these complexes (Fig. 5A-E). These findings highlight the potential of these compounds in developing novel therapeutic agents for cancer treatment. The result suggests these compounds could formulate multi-target therapeutics derived from plants, capable of exerting inhibitory effects across different types of cancer.
Furthermore, acacetin and luteolin exhibited favorable properties such as appropriate molecular weight, hydrogen bond acceptors and donors, and logP values. Their pharmacokinetic parameters aligned with Lipinski's rule of five. Drug-likeness and absorption analyses of these two compounds, alongside positive controls (doxorubicin, paclitaxel, and curcumin), confirmed their suitability using the MolSoft online platform and Boiled-Egg analysis, falling within an acceptable range. This indicates their potential as promising starting points for anti-cancer drug development. In view of these findings, the two selected compounds emerge as promising candidates for cancer therapeutics. Their demonstrated interactions with therapeutic targets, favorable drug-like properties, and ADME/T profiles underline their potential efficacy. Nevertheless, comprehensive exploration and validation of their in vitro and in vivo activities across various cancer types are imperative for advancing these compounds toward clinical applications.
5 Conclusion
We utilized virtual screening and molecular docking techniques to investigate the interactions between bioactive compounds from Oxalis corniculata and cancer-associated targets. Our findings revealed that compounds acacetin and luteolin exhibited significant affinity towards PD-L1, BRAF, and PARP, indicating their potential as versatile multi-target pharmaceutical agents. Notably, these compounds lacked mutagenic or carcinogenic properties and displayed favorable bioactivity, pharmacokinetics, and ADME/T characteristics. Despite these promising attributes, further research is imperative to validate their therapeutic efficacy in clinical settings.
Funding
RB and Priyanka are supported by CSIR-SRF fellowship. NK lab is supported by CSIR-IMTECH grants.
CRediT authorship contribution statement
Ram Bharti: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation. Somdutt Mujwar: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Priyanka: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation, Formal analysis, Data curation. Thakur Gurjeet Singh: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation. Neeraj Khatri: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Acknowledgments
Authors are grateful to CSIR-IMTECH for consistent support and motivation. This is CSIR-IMTECH communication number 01/2024.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In-vitro Cytotoxicity and In-silico Insights of the Multi-target Anticancer Candidates from Haplophyllum tuberculatum. Borneo J. Pharm.. 2021;4:192-201.
- [CrossRef] [Google Scholar]
- FDA Approval Summary: Olaparib Monotherapy or in Combination with Bevacizumab for the Maintenance Treatment of Patients with Advanced Ovarian Cancer. Oncologist. 2021;26:e164-e172.
- [CrossRef] [Google Scholar]
- The Protein Data Bank.. 2002;urn:issn:0907–4449 58:899-907.
- [CrossRef]
- Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer. Cochrane Database Syst. Rev.. 2017;2017
- [CrossRef] [Google Scholar]
- Dean, J.L., Thangavel, C., McClendon, A.K., Reed, C.A., Knudsen, E.S., 2010. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 2010 29:28 29, 4018–4032. DOI: 10.1038/onc.2010.154.
- EGFR-targeted multifunctional polymersomal doxorubicin induces selective and potent suppression of orthotopic human liver cancer in vivo. Acta Biomater. 2017;64:323-333.
- [CrossRef] [Google Scholar]
- Gaikwad, S., Srivastava, S.K., 2021. Role of Phytochemicals in Perturbation of Redox Homeostasis in Cancer. Antioxidants 2021, Vol. 10, Page 83 10, 83. DOI: 10.3390/ANTIOX10010083.
- Apoptosis Effects of Oxalis Corniculata L. Extract on Human MCF-7 Breast Cancer Cell Line. Galen Medical J.. 2022;11:e2484.
- [CrossRef] [Google Scholar]
- Current Therapeutic Strategies in BRAF-Mutant Metastatic Colorectal Cancer. Front. Oncol.. 2021;11:601722
- [CrossRef] [Google Scholar]
- Isolation, Anticancer Evaluation, Molecular Docking, Drug likeness and ADMET Studies of Secondary Metabolites from Psoralea corylifolia seeds. ChemistrySelect. 2022;7:e202202115.
- [Google Scholar]
- PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: From bench to bed. Front. Oncol.. 2022;12:849352
- [CrossRef] [Google Scholar]
- Medicinal Plants in Light of History: Recognized Therapeutic Modality. J. Evid Based Complementary Altern. Med.. 2014;19:216-219.
- [CrossRef] [Google Scholar]
- The promise of mTOR inhibitors in the treatment of colorectal cancer. Expert Opin. Investig. Drugs. 2012;21:1775-1788.
- [CrossRef] [Google Scholar]
- Knelson, E.H., Patel, S.A., Sands, J.M., 2021. PARP Inhibitors in Small-Cell Lung Cancer: Rational Combinations to Improve Responses. Cancers 2021, Vol. 13, Page 727 13, 727. DOI: 10.3390/CANCERS13040727.
- Efficacy and safety of EGFR inhibitor gefitinib in recurrent or metastatic cervical cancer: a preliminary report. Med. Oncol.. 2023;40:1-6.
- [CrossRef] [Google Scholar]
- Can EGFR be a therapeutic target in breast cancer? Biochimica et Biophysica Acta (BBA) - Reviews on. Cancer. 2022;1877:188789
- [CrossRef] [Google Scholar]
- AKT Inhibitors: New Weapons in the Fight Against Breast Cancer? Front. Pharmacol.. 2021;12:662232
- [CrossRef] [Google Scholar]
- Role of p53 in breast cancer progression: An insight into p53 targeted therapy. Theranostics. 2023;13:1421-1442.
- [CrossRef] [Google Scholar]
- Computational bioprospecting of andrographolide derivatives as potent cyclooxygenase-2 inhibitors. Biomed. Biotechnol. Res. J.. 2021;5:446.
- [Google Scholar]
- Naveed, M., ul Ain, N., Aziz, T., Saleem, A., Aqib Shabbir, M., Ali Khan, A., Albekairi, T.H., 2024. Integrated track of nano-informatics coupling with the enrichment concept in developing a novel nanoparticle targeting ERK protein in Naegleria fowleri. Open Chem 22. DOI: 10.1515/CHEM-2023-0198/MACHINEREADABLECITATION/RIS.
- Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod.. 2020;83:770-803.
- [CrossRef] [Google Scholar]
- Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol.. 2020;2020
- [CrossRef] [Google Scholar]
- Annexin A3 as a Potential Target for Immunotherapy of Liver Cancer Stem-Like Cells. Stem Cells. 2015;33:354-366.
- [CrossRef] [Google Scholar]
- Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med.. 2016;375:1823-1833.
- [CrossRef] [Google Scholar]
- Cyclooxygenase-2 as a therapeutic target against human breast cancer: A comprehensive review. Wires Mechanisms of Disease. 2023;15:e1596.
- [Google Scholar]
- Overexpression of NF-kB as a predictor of neoadjuvant chemotherapy response in breast cancer. Breast Dis.. 2021;40:S45-S53.
- [CrossRef] [Google Scholar]
- Sarnik, J., Popławski, T., Tokarz, P., 2021. BET Proteins as Attractive Targets for Cancer Therapeutics. International Journal of Molecular Sciences 2021, Vol. 22, Page 11102 22, 11102. DOI: 10.3390/IJMS222011102.
- Shabbir, M.A., Naveed, M., Rehman, S. ur, Ain, N. ul, Aziz, T., Alharbi, M., Alsahammari, A., Alasmari, A.F., 2023. Synthesis of Iron Oxide Nanoparticles from Madhuca indica Plant Extract and Assessment of Their Cytotoxic, Antioxidant, Anti-Inflammatory, and Anti-Diabetic Properties via Different Nanoinformatics Approaches. ACS Omega 8, 33358–33366. DOI: 10.1021/ACSOMEGA.3C02744/ASSET/IMAGES/LARGE/AO3C02744_0012.JPEG.
- Baicalin Induces Apoptosis and Suppresses the Cell Cycle Progression of Lung Cancer Cells Through Downregulating Akt/mTOR Signaling Pathway. Front. Mol. Biosci.. 2021;7:602282
- [CrossRef] [Google Scholar]
- Investigation of HER-2 status, treatment response and survival analysis in cervical cancer patients. Eur. J. Gynaecol. Oncol.. 2020;41:1031-1038.
- [CrossRef] [Google Scholar]
- Challenge and countermeasures for EGFR targeted therapy in non-small cell lung cancer. Biochimica et Biophysica Acta (BBA) - Reviews on. Cancer. 2022;1877:188645
- [CrossRef] [Google Scholar]
- Targeting cervical cancer: Is there a role for poly (ADP-ribose) polymerase inhibition? J. Cell Physiol.. 2020;235:5050-5058.
- [CrossRef] [Google Scholar]
- Tung, N., Garber, J.E., 2022. PARP inhibition in breast cancer: progress made and future hopes. npj Breast Cancer 2022 8:1 8, 1–5. DOI: 10.1038/s41523-022-00411-3.
- PD-L1 status in breast cancer: Current view and perspectives. Semin Cancer Biol.. 2021;72:146-154.
- [CrossRef] [Google Scholar]
- BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol.. 2022;12:863043
- [CrossRef] [Google Scholar]
- Current progress and future perspectives of neoadjuvant anti-PD-1/PD-L1 therapy for colorectal cancer. Front. Immunol.. 2022;13:1001444.
- [CrossRef] [Google Scholar]
- Effectiveness of PD-1/PD-L1 inhibitors in the treatment of lung cancer: Brightness and challenge. Sci. China Life Sci.. 2020;63:1499-1514.
- [CrossRef] [Google Scholar]
- P21-Activated Kinase 1: Emerging biological functions and potential therapeutic targets in Cancer. Theranostics. 2020;10:9741-9766.
- [CrossRef] [Google Scholar]
- Yao, J. yue, Xu, S., Sun, Y. ning, Xu, Y., Guo, Q. long, Wei, L. bin, 2021. Novel CDK9 inhibitor oroxylin A promotes wild-type P53 stability and prevents hepatocellular carcinoma progression by disrupting both MDM2 and SIRT1 signaling. Acta Pharmacologica Sinica 2021 43:4 43, 1033–1045. DOI: 10.1038/s41401-021-00708-2.
- In-vivo and in-silico analysis of anti- inflammatory, analgesic, and anti pyretic activities of in-silico leaf extract. J. Chil. Chem. Soc.. 2023;68:5813-5821.
- [CrossRef] [Google Scholar]
- Expression pattern of PD-1/PD-L1 in primary liver cancer with clinical correlation. Liver Int.. 2023;43:1995-2001.
- [CrossRef] [Google Scholar]







