Translate this page into:
A comparative study on the role of Omani honey with various food supplements on diabetes and wound healing
⁎Corresponding author. salla.reddy@hct.edu.om (Hemadri Reddy Salla) hemadrisvu2020@gmail.com (Hemadri Reddy Salla)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Honey has been used traditionally for the treatment of various diseases as well as a regular diet in many preparations without knowing its impact on health in condition like diabetes. Antioxidant activity was studied by DPPH assay and phosphomolybdenum method for mountain honey (MH) and cultivar honey (CH). This assay revealed that antioxidant activity of MH was higher at lower concentration (75%) than CH, whereas in phosphomolybdenum method, MH at 100%, had a greater antioxidant activity than CH. Effect of honey and food supplements (Turmeric, Lemon, Ginger and Garlic) either alone or in respective combinations were tested on alloxan induced diabetic mice. After 21 days of treatment it was found that the synergistic effect of MH with turmeric was greater in reducing the blood glucose level (143.8 ± 24.74–122.4 ± 11.82). The influence of the honey alone as well as in combination with different food supplements were studied to determine the effect on body weight in diabetic mice. This study revealed that MH is not affecting the weight in diabetic mice while the CH treated mice showed reduction (7.7 ± 0.41 g) in body weight. The two types of honey were also studied to evaluate the effect on wound healing and this study revealed that MH has significant influence on wound healing relative to CH.
Keywords
Antioxidant
Antidiabetic
Weight loss
Wound healing
Lemon
Ginger
Turmeric
Garlic and honey
1 Introduction
Diabetes mellitus (DM) is one of the serious public health problem worldwide. According to recent analysis, this condition affects approximately 415 million people and is expected to raise to 642 million by the year 2040 (IDF, 2017). The Middle East and North African (MENA) region, which includes all Arab states, is currently burdened with the second highest diabetes prevalence rate (10.7%, age-adjusted) after North America and Caribbean region (11.5%). Oman is a Middle Eastern country of about 3 million people and presently has one of the world’s highest diabetes popularity (Al-Lawati, 2017). Omani lifestyles now feature less physical activity and the diet involves more unhealthy foods high in the intake of fast food, refined sugar, and saturated fat. These lifestyle changes are causative factors explaining why diabetes is now the most predominant non-communicable disease in Oman (Boutayeb, 2005). Several alternative therapies including honey as an important constituent in the management of diabetes, elevation of plasma insulin levels and dropping of blood glucose levels have been observed in patients with diabetes after usage of honey (Ali-Wali, 2003, 2004).
The medical world is looking for the health benefits of natural products, medicinal herbs, and also honey, in the management of diabetes. Classic medical treatment using recipes of traditional medicine including the use of apicultural products, the diabetic patients can maintain the normal level of insulin in the blood and also their overall health condition (Bobiş et al., 2018). Several studies in the recent decades explained to a large extent many medicinal effects of honey such as antioxidant, hepatoprotective, cardio protective, antibacterial, anti-inflammatory and antitumor agent.
Honey is a wonderful gift of nature, produced by Apis mellifera from nectar of plant. It is considered as an excellent food and source of traditional medicine (Andualem, 2013). Sumerian tablet used as drug and ointments, contained honey was mentioned during 2100-2000BC (Nazarian et al., 2011). Many studies show that honey is effective for cardiovascular diseases, diabetes, skin infections, wounds, intestinal disorder, respiratory infections etc (Samarghandian et al., 2017). Natural honey is sticky, more concentrated, darker in color and viscous in nature due to low water content. The color of liquid honey varies from clear and colorless to dark amber or black. It is purely based on and varies with botanical origin, age, and storage conditions. Transparency or clarity depends upon the amount of suspended particles such as pollens. According to Al-Farsi et al. (2018), the Sidr Honey (CH) possess Glucose-22.7%, Fructose-31.6%, Sucrose-6.76%, insoluble matter-0.15% and pH 6.83, whereas the multifloral honey (MH) contains Glucose-28.0%, Fructose-35.6%, Sucrose-0.44%, insoluble matter-0.10% and pH 4.17.
Antioxidant capacity of honey is vital in many disease circumstances and is due to an extensive range of compounds including phenolics, peptides, organic acids, flavonoids enzymes and so on (Samarghandian et al., 2017). Also honey can be used as an alternative therapy for burns, infections, and skin ulcers as a natural remedy due to the existence of antimicrobials which enhances immune system thereby reduces inflammation, infection and has been introduced into modern medical practices (Al-Waili et al., 2011). Due to honey's acidity, hydrogen peroxide content, osmotic effect, nutritional and antioxidant contents, stimulation of immunity, and to unidentified compounds play role in wound healing through stimulation of tissue growth, enhanced epithelialization, and minimized scar formation.
Honey is one of the oldest known medicines since from ancient time. Its medical and therapeutic importance has been recently rediscovered. Especially, Omanis are keen to have honey present on their dining tables, especially during Eid, when they eat meat and honey. As well as specifically for patients in which the honey helps to speed up healing. The Arabs consider honey as therapeutic and the Arabic name for bee is ‘inhalant’, which means a gift of Allah. No doubt honey is an integral part of Arabic cuisine and remedies (Al Saadi et al., 2016). However, Omani people use honey with different combinations of food like ginger, lemon, milk etc, as a health supplement without understanding the benefits and side effects. Though Omani honey is much used, its importance from a medical point of view was not much explored. Therefore it is very essential to consider and study the role of various food supplements in combination with honey from human health point of view. Therefore the current research was aimed to investigate the effects of honey with different edibles namely lemon, garlic, ginger and turmeric on diabetics to establish the impact of honey on blood sugar level, antioxidant capacity, body weight and their wound healing efficacy. The results of this study may provide the basis for further exploration of honey as a potential natural pharmaceutical product to treat or manage various acute or chronic diseases.
2 Materials and methods
2.1 Chemicals and reagents
DPPH (1,1-Diphenyl-2-picrylhydrazyl)/ALDRICH, Methanol, Ascorbic acid/ALDRICH, Alloxan monohydrate/ACROS (Organics), Tri-sodium citrate, Distilled water, Phosphomolybdenum reagent, Glucometer device/ONETOUCH Select plus.
All animal experiments in the present study was conducted according to the TRC (Sultanate of Oman) guidelines.
2.1.1 Collection of samples
Mountain honey-MH (multifloral honey), cultivar honey-CH (Sidr honey), turmeric, lemon, garlic and ginger were collected from different places in the Sultanate of Oman, Muscat, Rustaq and Bahla. The respective honey samples were harvested in the month of early September 2017 and collected from local beekeepers and stored at room temperature in a dark place throughout the experimental period. Whereas food supplements used in the experiment were collected freshly time to time during the experiment time.
2.1.2 Preparation of honey with supplements
Twenty percent (20%) honey is prepared by mixing 20 g of honey in 80 ml of distilled water. The various food supplements (lemon, turmeric, garlic & ginger) extracts mixtures were prepared by mixing 1 ml of pure crude extract of the supplements in 100 ml of distilled water 1% (v/v).
2.1.3 Preparation of honey-ginger/turmeric extracts solution
Ginger and turmeric were washed with tap water and sliced into uniform pieces using sterile knife and dried in microwave at 40 °C for 48 h. The dried ginger and turmeric pieces were crushed using electric grinder to fine powder. Ginger and turmeric extracts were obtained by mixing 1 g of ginger/turmeric powder in 100 ml (1% W/V) of deionized water.
2.1.4 Preparation of garlic extract
Fresh garlic bulbs were cleaned and peeled, and homogenized using blender. The homogenized bulbs were squeezed using cheese cloth. The extract was prepared by diluting the concentrated garlic juice with distilled water (1 ml in 99 ml distilled water).
2.1.5 Preparation of lemon extract
The extract was prepared by diluting the pure lemon juice with distilled water (1 ml in 99 ml distilled water).
2.2 Preliminary analysis
2.2.1 Total phenolic content (TPC)
The total phenolic content was assayed using the Folin-Ciocalteu method according to Socha et al. (2009) and Ferreira et al. (2009) with slight modifications. The honey solutions were prepared at a concentration of 8%. 0.5 ml of the stock solution was mixed with 0.3 ml of Folin-Ciocalteu reagent and followed by 2 ml of 15% sodium carbonate, the final volume was adjusted to 5 ml with distilled water and the mixture was incubated for 90 min. The absorbance of the mixture was measured at 765 nm against the blank. A standard curve of Gallic acid was prepared for quantification, using a concentration range between 8 and 40 mg/l and the results were expressed as mg Gallic acid/kg honey.
2.2.2 Total flavonoid content (TFC)
Total flavonoids content in honey was determined by a colorimetric method according to Zhishen et al. (1999). One ml of 0.2% honey was mixed with 4 ml of distilled water followed by 0.3 ml of 5% sodium nitrite. After 5 min, 0.3 ml of 10% aluminum chloride was added, six min later 2 ml of 1 M of sodium hydroxide was added and the final volume was increased to 10 ml. The mixture was shaken and absorbance was measured at 510 nm using a spectrophotometer. A calibration curve was made using a standard solution of catechin 20–100 mg/l. The results were presented as mg of catechin equivalents per kg of honey.
2.3 Antioxidant study
2.3.1 DPPH free radical-scavenging
The antioxidant characteristics of mountain and cultivar honey samples were examined by determining the free radical-scavenging activity of the DPPH radical based on the method Ferreira et al. (2009). In brief, honey samples were dissolved in distilled water at various concentrations (50%, 75%, and 100%) from 50 to 100 mg/ml. 0.5 ml of honey was mixed with 2.7 ml of methanolic solution containing DPPH radicals (0.0039 g/ml). The mixture was vigorously shaken and left to stand for 15 min in dark. The decrease of the DPPH radical was determined by measuring the absorbance of mixture at 517 nm against blank. The blank consisted of a honey solution (1.5 ml) at the same concentration values previously described and 3.5 ml of methanol. The negative control consisted of 3.5 ml of methanol with 1.5 ml of the DPPḢ solution.
Ascorbic acid was used as a standard. The radical scavenging activity (RSA) was calculated as the percentage of DPPH discoloration using the following equation:where As is the absorbance of the solution when the sample extract is added at a particular level and ADPPH is the absorbance of the DPPH solution.
2.3.2 Total antioxidant capacity
Three hundred ul (0.3 ml) of honey (honey + 20 ml Methanol) was mixed with 2.7 ml of Phosphomolybdenum reagent (0.335 g of sodium phosphate and 0.078 g of ammonium molybdate in 3.3 ml sulphuric acid) in test tube. The samples in a test tube were kept in a water bath at 95 °C for 90 min. The blank was prepared by mixing 0.3 ml of methanol without honey. The absorbance of the samples was measured at 695 nm using UV–Vis spectrophotometer. Ascorbic acid was used as control. Different concentrations were used for honey as well as ascorbic acid: −100%, 75% and 50%. The total antioxidant capacity was estimated (Shumaila et al., 2013) using the following formula:
Total antioxidant capacity (%) = [(Abs of control) - (Abs of sample)] / (Abs of control) X 100
2.4 Hypoglycemic activity of honey with supplements against alloxan induced diabetic mice
2.4.1 Induction of diabetes
The Healthy male experimental mice is segregated as different groups were fasted overnight (12–14 h) and their weight and blood glucose level was noted. Mice were then made diabetic by a single intraperitoneal injection (150 mg/kg body weight) of alloxan monohydrate. Two days after alloxan injection, plasma blood glucose level of each animal was determined by taking the blood from the tail of mice and recorded by glucometer select plus model.
2.4.2 Experimental design
The healthy male mice were divided into 8 groups for the estimation of blood glucose level with 5 animals in each group as follows. Group 1: negative control (Diabetic mice, no treatment), group 2: positive control (treated with glibenclamide-5 mg/ml), group 3: normal mice, group 4: treated with Mountain honey (MH), group 5: treated with MH + G, group 6: treated with MH + L, group 7: treated with MH + G* (mountain honey with ginger) and group 8: treated with MH + T. The same experimental regimen was maintained for cultivar honey (CH). All experimental mice were received treatment after 2 days of alloxan injection except normal mice (nondiabetic) and diabetic control (positive & negative) groups. The dosages were administered to the mice orally thrice a week along with regular mice feed and water. The blood sugar levels and body weights were measured prior to the treatment and after three weeks of treatment using glucometer device and weighing balance respectively.
2.5 Effect of Omani honey on wound healing in mice
Twelve healthy female mice from animal house facility, Higher College of Technology, were selected. They were segregated into 4 groups and each group consists of three mice as follows. Group1: treated with MH, group 2: treated with CH, group 3: positive control (treated with silverin – a Silver Sulphadiazine cream) and group 4: negative control (natural healing without treatment). The creation of wound was made based on the method of Jimi et al. (2017). Briefly, the longitudinal wound measuring 1 cm on the dorsal side of the mouse was made by using sterile scissor and razor. The experimental groups received the respective treatment using swaps around the wound continuously for 2 weeks. The wounds of all groups were treated every day for two weeks and the wounds were measured (±cm) at regular interval (7th and 14th day).
2.6 Statistical analysis
Results analyzed in the present study were analyzed using Microsoft excel 2013, Descriptive statistics, mean ± SE. All Data presented are mean values of triplicates (n = 3), obtained from three replicates; unless stated otherwise.
3 Results and discussion
3.1 Total phenol content
Polyphenols are the widely distributed groups of phytoconstituents with enormous biological role in plant kingdom. The reaction between Folin-Ciocalteu and phenolic compounds results in the formation of blue color complex which allow quantification using gallic acid as standard. The total content of phenolic present in the MH is 1274 mg/kg while in CH is 1394 mg/kg. According to several reports it was observed that the studied honey samples possess higher contents of phenols compare to honey samples of other countries; Malaysian honey 305.5–419.9 mg/kg (Khalil et al., 2012); Czech honey 39.2–167.1 mg/kg (Lachman et al., 2010). High percentage of phenol content in Omani honey indicates the superior quality of the honey. The variation in phenolic content of honey samples from one country to other may be due to different geographical and botanical sources of honey.
3.2 Total flavonoid content
Flavonoids are plant pigments that are synthesized from the amino acid phenyl alanine having several subclasses viz Anthocyanin’s, flavons, flavonone, flavonone glycosides, flavonol glycosides etc. The presence of flavonoids in honey depends on nectar, pollen or propolis (Hamdy et al., 2009). The flavonoid content was measured using a colorimetric method, which is based on the formation of a complex between the aluminum ion and the carbonyl and hydroxyl groups of flavonoids that produce a yellow color. The total flavonoids found were highest in MH which was 1315 mg/kg followed by CH having slightly less concentration of flavonoids was 1013 mg/kg. The content of flavonoid is directly proportional to the antioxidant capacity of the samples. These values of flavonoids in Omani honey samples are higher in compare to various honey samples reported by Al-Farsi et al. The quantity of flavonoids present in the honey samples are based on botanical and geographical differences as well to climate and environmental factors such as humidity, temperature and soil composition.
3.3 Antioxidant study
It was observed from the findings shown in the Fig. 1, that MH at 75% (0.400 ± 0.155) and CH at 100% (0.366 ± 0.011) has shown the highest antioxidant activity. This shows that the MH even at low concentration is better and more effective than CH comparatively. MH on the other hand comes from honey bees which naturally feed on plant nectar with natural ingredients like non reducing sugars and other substances with low glycemic index. Hence, MH even at low concentrations is more effective and beneficial than CH at high concentration though both have hydrogen donating properties. The antioxidant capacity of the honey samples have been directly related to the biochemical constituents like total phenols, total flavonoid contents, and total water-soluble vitamins. The mechanism of antioxidant activity is due to the unpaired electron of DPPH which forms a pair with the electron of hydrogen donated by free radical scavenging antioxidant from honey, thus converting the purple colored odd electron DPPH to its reduced form which is yellow. The degree of decolourization is measured by UV–Visible spectrophotometer in order to determine the scavenging activity of honey based on the concentration of phenols, flavonoids.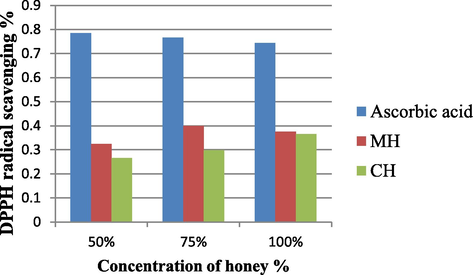
Free radical scavenging activity of different honey mixture and standard determined by the reduction of DPPH. All results expressed in the figure are mean of triplicates.
The MH at 100% showed (0.293 ± 0.0253), highest antioxidant capacity followed by the CH at 100% (0.233 ± 0.0072) as shown in Fig. 2. It was noticed that the antioxidant capacity is directly proportional to the concentration of natural ingredients which is responsible for the antioxidant potential in the samples used in the study. The antioxidant capacity of the honey samples varied from one to another based on chemical composition of honey which in turn depends on the composition of nectar. The nectar composition depends on where it originates from like different plants as well as environmental factors, beekeeping practices, and storage conditions.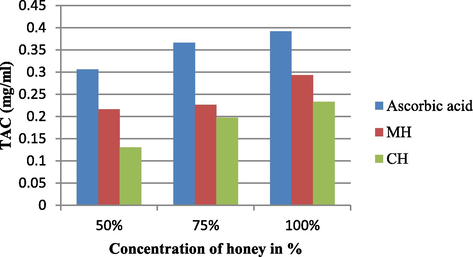
The Total Antioxidant Capacity (TAC) of MH and CH at different concentration. All results expressed in the figure are mean of triplicates.
3.4 Hypoglycemic study
3.4.1 Effect of honey in combination with food supplements on blood sugar levels in diabetic mice
From the Table 1(a)–(d) it is noticed that the consumption of honey alone and with supplements has shown diverse results against blood sugar level in diabetic mice. The Alloxan administered mice with sugar level 110 mg/dl and above was considered as diabetic mice. It was reported that honey composed of fructose at high concentration (Fasanmade and Alabi, 2008) as well as glucose. But in this study it was noticed that the cultivar honey reduced the blood glucose level from 154.6 ± 33.84 to 146 ± 13.00 (↓8.6 ± 20.84) than the mountain honey 134 ± 12.94 to 133.6 ± 18.09 (↓0.4 ± 5.15). Note: Values expressed in the tables (a–d) are mean ± SE (n = 3), G*: Ginger, +ve control: standard drug, −ve control: diabetic but no treatment, Normal control: normal mice without treatment, ↓: decrease, ↑: increase.
Change (mg/dl)
After treatment
Initial blood sugar
Animal group
S. no
a: Effect of MH alone and in the combination with supplements on blood sugar (mg/dl)
↓0.4 ± 5.15
133.6 ± 18.09
134 ± 12.94
MH
1
↓7 ± 6.48
120 ± 20.45
127.6 ± 13.97
MH + G
2
↓21 ± 12.92
122.4 ± 11.82
143.8 ± 24.74
MH + T
3
↓18 ± 12.08
124 ± 9.3
146 ± 21.38
MH + G*
4
↓20 ± 2.07
115.8 ± 18.18
135.8 ± 16.11
MH + L
5
b: Effect of CH alone and in the combination with supplements on blood sugar (mg/dl)
↓8.6 ± 20.84
146 ± 13
154.6 ± 33.84
CH
1
↑18.6 ± 1.32
133.6 ± 16.25
115 ± 14.93
CH + G
2
↓5 ± 9.39
116.6 ± 10.11
121.6 ± 19.50
CH + T
3
↑17 ± 6.24
138 ± 11.53
121 ± 5.29
CH + G*
4
↑20 ± 13.87
153.6 ± 20.42
133 ± 6.55
CH + L
5
c: Effect of supplements alone on blood sugar (mg/dl)
↓16.8 ± 11.43
118.5 ± 24.74
135.3 ± 13.31
T
1
↑4 ± 20.27
137 ± 25.23
133 ± 45.50
G*
2
↓9 ± 22.96
147.6 ± 28.09
153.6 ± 5.13
L
3
↑6 ± 8.35
132.3 ± 18.50
126.3 ± 26.85
G
4
d: control groups blood sugar level (mg/dl)
↓3.7 ± 13.26
118.3 ± 19.50
122 ± 6.24
Control (+)
1
↑17.7 ± 8.00
136.3 ± 23.69
118.6 ± 15.69
Control (−)
2
↑9.4 ± 14.59
126 ± 36.59
116.6 ± 22.0
Normal Control
3
Though Honey contains fructose and glucose, the glycemic index is lower than refined sugar thereby the impact of honey on diabetics is lesser than refined sugar. Several reports suggests that fructose in honey may moderate the hypoglycemic effect of honey (Erejuwa et al., 2012). Also the honey with relatively high concentration of fructose has a lower glycemic index (GI) than other types of honey (Ischayek and Kern, 2006). In addition, in a comparison between honey in combination with supplements both MH from 143.8 ± 24.74 to 122.4 ± 11.82 (↓21 ± 12.92) and CH from 121.6 ± 19.50 to 116.6 ± 10.11 (↓5 ± 9.39) with turmeric showed the greatest reduction in the blood glucose level than other supplement combinations.
Turmeric is known to have many health benefits, and turmeric is one of the five medicinal plants used effectively as a single drug (Vuthipongse, 1986; Sithicharoenchai, 1986; Suppasil-Nanakorn, 1993). Turmeric alone is found to reduce the blood sugar from 135.3 ± 13.31 to 118.5 ± 24.74 (↓16.8 ± 11.43), lemon from 153.6 ± 5.13 to 147.6 ± 28.09 (↓9 ± 22.96) was also shown reduction in blood sugar level in diabetic mice. But lemon in combination with CH has shown increasing the blood sugar (↑20 ± 13.87), while lemon with MH the reduction (↓20 ± 2.07) of blood sugar level in diabetic mice was observed. From the overall observations MH in combination with different supplements studied was found to be more effective than CH treatment groups.
These findings clearly suggest that natural mountain honey is safer than cultivar honey from a diabetic point of view. Since, the glycemic index (GI) of the honey which contains more fructose (MH) have less GI than the honey (CH) which is having less fructose were GI will be high. Several reports revealed that lower GI foods are not affecting and rising blood sugar levels in type II diabetics (Bobiş et al., 2018). The positive control glibenclamide drug was used and showed a decline (↓3.7 ± 13.26) in blood glucose level in diabetic mice. While in normal (↑9.4 ± 14.59) control (not diabetic; normal mice) and negative (↑17.7 ± 8.00) control (diabetic; no treatment), the blood sugar level was elevated.
3.4.2 Effect of honey in combination with food supplements on body weight in diabetic mice
Results showed in Table 2(a)–(c) shows the effect of honey in combination with additives on the body weight in diabetic mice. Overall MH (mean difference ↑2.66 ± 1.374 g) alone and in combination with supplements has resulted in progressive increase in body weight of all the treated groups of diabetic mice similar to the standard drug treated mice (↑3.22 ± 0.360 g). All values placed in the tables (a-c) are mean ± SE (n = 3). + ve control: standard control drug, −ve: diabetic but no treatment, normal control: not diabetic no treatment, ↓: decrease, ↑: increase.
S no
Treatment group
Initial body weight
After treatment
Change (g)
a: Effect of MH alone and in combination with supplements on body weight in diabetic mice
1
Normal Control
24.98 ± 1.907
23.76 ± 2.512
↓1.22 ± 0.605
2
Control (−)
28.14 ± 2.126
28.9 ± 0.842
↑0.76 ± 1.280
3
Control (+)
25.86 ± 1.469
29.08 ± 1.109
↑3.22 ± 0.360
4
MH
25.36 ± 1.870
28.02 ± 0.496
↑2.66 ± 1.374
5
MH + G
23.32 ± 0.965
26.14 ± 2.538
↑2.82 ± 1.573
6
MH + T
23.28 ± 3.471
24.7 ± 3.232
↑1.42 ± 0.239
7
MH + G*
25.34 ± 3.092
27.26 ± 3.205
↑1.92 ± 0.113
8
MH + L
27.8 ± 1.437
30.84 ± 1.350
↑3.04 ± 0.087
b: Effect of CH alone and in combination with supplements on body weight in diabetic mice
1
CH
18 ± 2.107
10.3 ± 2.517
↓7.7 ± 0.410
2
CH + G
29 ± 3.350
14.7 ± 1.528
↓14.3 ± 1.822
3
CH + T
19.7 ± 6.006
8.7 ± 2.517
↓11 ± 3.489
4
CH + G*
21.8 ± 2.994
12.4 ± 0.551
↓9.4 ± 2.443
5
CH + L
24.6 ± 4.574
18 ± 2.136
↓6.6 ± 2.438
c: Effect of supplements alone on body weight in diabetic mice
1
T
28.1 ± 3.381
10 ± 0.902
↓18.1 ± 2.479
2
G
26.1 ± 3.764
10.8 ± 2.254
↓15.3 ± 1.510
3
G*
29.2 ± 2.762
17.2 ± 2.100
↓12 ± 0.662
4
L
19.6 ± 3.262
15.1 ± 3.408
↓4.5 ± 0.146
However CH alone shown the (18 ± 2.107 to 10.3 ± 2.517) mean difference (↓7.7 ± 0.410 g) and with supplement formulations has reduced the body weight in all treated diabetic mice. This was the major difference found and noticed among the two types of honey and this might be based on glycemic index. As mentioned earlier under antidiabetic study CH contains low concentration of fructose resulted in high glycemic index (Nemoseck et al., 2011). It was suggested that the honey which has a high glycemic index is assumed to increase the body weight, in controversy the results of Chepulis and Starkey (2007) reported that honey fed rats had significantly lowered the body weight, adiposity etc.
From the current results we noticed that the uptake of MH is better than the CH in gaining weight during diabetic condition. The MH seems to possess more of natural sugars than the CH leads to the diabetic mice gaining weight than losing with MH treatment. In addition, supplements alone also reduced the body weight of the mice. A great reduction of weight in diabetics was noticed with turmeric treatment from 28.1 ± 3.381 to 10 ± 0.902 mean difference (↓18.1 ± 2.479 gms) followed by garlic (↓15.3 ± 1.510), ginger (↓12 ± 0.662) and lemon (↓4.5 ± 0.146 gms). As seen in Table 2(a)–(c) the synergistic influence of honey with different food supplements does not influence the body weight during diabetes, but the individual supplements showed reduction of body weight in diabetics.
The supplement Turmeric showed great impact on blood sugar and in relation with body weight. This might be due to the presence of active ingredients in the turmeric called curcumin and curcuminoids. This evidences that even MH alone (↑2.66 ± 1.374 gms) and in combination with lemon (lemon ↑3.04 ± 0.087 gms) increased the body weight significantly. In comparison the synergistic effect with turmeric is very low (↑1.42 ± 0.239 gms), this suggests that turmeric in general causes weight reduction in individuals.
3.5 Wound healing study
Honey has been known to heal wounds and cutaneous ulcers and lately its healing properties have been rediscovered (Ndayisaba et al., 1992; Zumla and Lulat, 1989). It was noticed from the results shown in Table 3 and Fig. 3 that honey from either type can be used for wound healing. Note: All the results expressed in the table are the mean of triplicates. Here MH: mountain honey; CH: cultivar honey; control (+): standard drug (Silver Sulphadiazine); control: no treatment.
Groups
Initial wound size (cm)
After 14 days of treatment wound left (cm)
Control
1
0.6
Control (+)
1
0.53
MH
1
0.2
CH
1
0.27
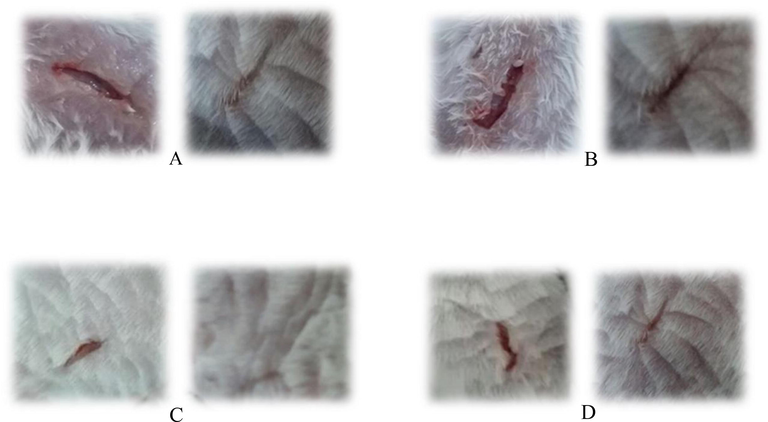
Wound healing by honey: where, A: normal control (without treatment), B: positive control (treated with Silver Sulphadiazine), C: mountain honey treated wound and D: cultivar honey treated wound; where: Left side figure denotes after 7 days of treatment; right side figure denotes after 14 days of treatment (A–D).
Between MH and CH, the MH treated mice recovered almost completely (initial wound inducted was 1 cm; wound leftover after 14 days of treatment was 0.2 cm) while the CH treated was not completely healed (initial wound inducted was 1 cm; wound leftover after 14 days of treatment was 0.27 cm). The progress of wound healing was monitored regularly to determine the effectiveness of honey samples in wound dressing and duration of wound healing. In comparison, Fig. 3(c) and (d) clearly showed that the MH treated wound was recovered quickly and completely. The results of honey treated wounds were found to be more effective than the positive control treatment (initial wound inducted was 1 cm; wound leftover after 14 days of treatment was 0.53 cm). This is due to the bioactive constituents and biophysical property of honey that makes it effective in wound care as well as healing. The viscosity, inhibition factor, acidic pH and content of sugar has an osmotic action which draws out fluid from the wound. All these factors contribute to promote wound healing (Subrahmanyam et al., 2001; Jalali et al., 2007). The above discussed properties might help to explain some biological and therapeutic properties of honey, particularly as an antibacterial agent or wound healer (Al-Waili et al., 2011).
4 Conclusion
The present study concludes that honey alone and in combination with various food supplements used in diabetic mice affect blood sugar levels and body weight due to its effectiveness properties of its nature and composition. In comparison MH showed maximum antioxidant activity than CH studied. Honey being mixture of sugars, gives reason to believe that the consumption of honey would raise blood sugar, but from this study it was noticed that honey does not raise the blood glucose level either alone or in combination with different supplements. The diabetic mice when studied for body weight the cultivar honey and supplements treated diabetic mice lost their weight whereas the MH treated mice gained the weight even in combination with supplements. Although the effectiveness of MH and CH in wound healing were found to be practically equal, the MH was slightly more effective than the CH with respect to duration. The results of animal experimentation may not be actually extrapolated to human situation, but this study provides evidence that consumption of honey influences and affects the diabetics in blood sugar variation and body weight variation.
Acknowledgement
The authors are highly grateful to TRC-FURAP, Higher College of technology, and Ministry of Manpower for providing required fund and support. The authors are also thankful to biology lab technicians.
References
- Diabetes mellitus: a local and global public health emergency. Oman Med. J.. 2017;32:177-179.
- [Google Scholar]
- Intrapulmonary administration of natural honey solution, hyperosmolar dextrose or hypos molar distill water to normal individuals and to patients with type-2 diabetes mellitus or hypertension: their effects on blood glucose level, plasma insulin and C-peptide, blood pressure and peaked expiratory flow rate. Eur. J. Medi. Res.. 2003;31:295-303.
- [Google Scholar]
- Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: comparison with dextrose and sucrose. J. Med. Food. 2004;7:100-107.
- [Google Scholar]
- Honey for wound healing, ulcers, and burns; data supporting its use in clinical practice. Sci. World J.. 2011;11:766-787.
- [Google Scholar]
- Combined Antibacterial activity of sting less bee (Apis mellipodae) honey and garlic (Allium Sativum) extract agent’s standard and clinical pathogenic bacteria. Asian Pac. J. Trop. Biomed.. 2013;3:725-731.
- [Google Scholar]
- Honey and diabetes: the importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid. Med. Cell. Longev.. 2018;2018:4757893.
- [Google Scholar]
- The double burden of communicable and non-communicable diseases in developing countries. Trans. Royal Soc. Trop. Med. Hygiene. 2005;100:191-199.
- [Google Scholar]
- The long-term effects of feeding honey compared with sucrose and a sugar-free diet on weight gain, lipid profiles, and DEXA measurement in rats. J. Food Sci.. 2007;73:1-7.
- [Google Scholar]
- Fructose might contribute to the hypoglycemic effect of honey. Molecules. 2012;17:1900-1915.
- [Google Scholar]
- Differential effect of honey on selected variables in alloxan-induced and fructose-induced diabetic rate. Afr. J. Biomed. Res.. 2008;11:191-196.
- [Google Scholar]
- Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem.. 2009;114:1438-1443.
- [Google Scholar]
- Determination of flavonoid and phenolic acid content of clover, cotton and citrus floral honeys. J. Egypt Pub. Health Assoc.. 2009;84:245e259.
- [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 7th edition [cited 2017 April]. Available from: http://www.diabetesatlas.org/.
- US honeys varying in glucose and fructose content elicit similar glycemic indexes. J. Am. Diet. Assoc.. 2006;106:1260-1262.
- [Google Scholar]
- Experimental evaluation of repair process of burn-wounds treated with natural honey. J. Anim. Vet. Adv.. 2007;6:179-184.
- [Google Scholar]
- Novel skin splint for accurately mapping dermal remodeling and epithelialization during wound healing. J. Cell Physiol.. 2017;232:1225-1232.
- [Google Scholar]
- Content and antioxidant properties of processed Tualang honey (agromas) collected from different regions in Malaysia. Int. J. Pharm. Pharm. Sci.. 2012;4:214-219.
- [Google Scholar]
- Synergistic effect of honey with food additives as antimicrobials and on weight loss and various physiological studies in mice. IJPSR. 2016;7:4461-4466.
- [Google Scholar]
- Evaluation of antioxidant activity and total phenolics of selected Czech honeys. Food Sci. Technol.. 2010;43:52-58.
- [Google Scholar]
- Origin of honey proteins and method for its quality control. Pak. J. Bot.. 2011;42:3221-3228.
- [Google Scholar]
- Honey promotes lower weight gain, adiposity, and triglycerides than sucrose in rats. Nutr. Res.. 2011;31:55-60.
- [Google Scholar]
- Honey and health: a review of recent clinical research. Pharmacogn. Res.. 2017;9:121-127.
- [Google Scholar]
- Assessment of antioxidant potential, total phenolics and flavonoids of different solvent fractions of Monotheca buxifolia fruit. Osong Public Health Res. Perspect.. 2013;4:246-254.
- [Google Scholar]
- Medicinal plant project and primary health care. In: Conference and Training Course on Medicinal Plants Project and Primary Health Care. Prachin Buri: Wang-Nam-Yen Hospital; 1986. p. :13-16. (in Thai)
- [Google Scholar]
- Effects of topical application of honey on burn wound healing. Ann. Burns Fire Disasters. 2001;16:135-137.
- [Google Scholar]
- Suppasil-Nanakorn, S., (Ed.), 1993. Medicinal Plants and Development: Development Job-plan of Medicinal Plants for Herbal Medicine, NESDB plan number VI, 1987–1991, Central Research Division, Faculty of Medicine, Khon Kaen University, Khon Kaen, first ed., 109–120, 121–129 (in Thai).
- Antioxidant activity and phenolic composition of herb honeys. Food Chem.. 2009;113:568-574.
- [Google Scholar]
- Development of herbal medicines in primary health care. In: Conference and Training Course on Medicinal Plants Project and Primary Health Care. Prachin Buri: Wang Nam Yen Hospital; 1986. p. :13-16. (in Thai)
- [Google Scholar]
- The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem.. 1999;64:555-559.
- [Google Scholar]







