Translate this page into:
A comparative study on phytochemical screening, quantification of phenolic contents and antioxidant properties of different solvent extracts from various parts of Pistacia lentiscus L
⁎Corresponding author. med.barbouchi08@gmail.com (Mohammed Barbouchi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Within the framework of discovering new antioxidants from natural sources, this work focus on screening of phytochemical constituents, the variation in the total phenolic content and the antioxidant capacities from different parts (fruits, twigs and leaves) of Pistacia lentiscus L., from two different regions of Morocco; Moulay Idriss Zerhoun (MIZ) and Melloussa (MLS) was studied. Thirty extracts were prepared from fruits, twigs, and leaves using solvents of different polarity (hexane, ethyl acetate, methanol, ethanol and water). The tests of antioxidant activities were assessed by employing two different assays the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and phosphomolybdenum (TAC). The screening tests showed the existence of tannins, flavonoids, saponins, sterols, triterpenes, oses, holosides, reducing sugars and mucilages. The quantitative analysis has proved that the whole plant contains a high amount of total phenolic, this wealth increases as and when the polarity of extraction solvents used increases. The whole plant served as a great source of naturally occurring antioxidants, this study provides that the aqueous extract of Pistacia lentiscus (leaves and twigs) from MIZ is a great natural antioxidant compared to quercetin standard. The results show a very significant positive correlation between the TAC and DPPH assays in relation to the amount of total phenolic.
Keywords
Pistacia lentiscus
Phytochemical screening
Phenolic content
Antioxidant activities
1 Introduction
Currently, the focus of research has been placed on searching new natural antioxidants, in particular of plant origin, has steadily increased. Plants have been an inexhaustible source of medicines and recently, a lot emphasis has been made to find new therapeutic agents based on medicinal plants. Today, a folk favor to use medicinal plants rather than chemical drugs (Dehpour et al., 2009). The antioxidants have high importance in terms of its reducing power of oxidative stress which is one of the causes that could damage biological molecules (Farhat et al., 2013). Pistacia Lentiscus has been known for a long time in traditional medicine for the treatment of various types of diseases. The important has been medically placed grace of its various parts that contain a diversity of chemical constituents.
The purpose of this work was to perform phytochemical screening and to determine the correlation between total phenolic contents and antioxidant activities performed by TAC assay and DPPH assay of different solvent extracts from fruits, twigs and leaves of Pistacia lentiscus L., collected in two different regions of Morocco in order to discover new natural antioxidants.
2 Material and methods
2.1 Plant material
Pistacia lentiscus L. (leaves, twigs and fruits) were collected from two different regions of Morocco: Moulay Idriss Zerhoun (MIZ) Region: Fez-Meknes and Melloussa (MLS) Region: Tangier-Tetouan-AlHoceima, in October 2016. The Geographical coordinates of Moulay Idriss Zerhoun: N 33° 50′ 50,4636″; W 5° 19′ 0,3972″ and Melloussa N 35° 43′ 16,4676″; W 5° 39′ 30,9024″.
The fruits, twigs and leaves of P. lentiscus were air-dried for 15 days at room temperature and then was powdered separately and stored prior to further use.
2.2 Phytochemical screening of plant material
The phytochemical constituents of various parts (leaves, twigs and fruits) of P. lentiscus, were determined by different qualitative tests such as Alkaloid, Tannins, Anthraquinones, Flavonoids, Saponins, Sterols and Triterpenes, Oses and Holosides, Mucilages, Coumarins and Reducing Sugars. The tests were performed by the methods described below: (Diallo, 2005; Abdullahi et al., 2013; Joshi et al., 2013).
2.3 Extraction of crude extracts from different Pistacia lentiscus parts
The different extraction methods were followed to prepare crude extracts from leaves, twigs and fruits of Pistacia lentiscus L., from Melloussa and Moulay Idriss Zerhoun, by various solvents (hexane, ethyl acetate, methanol, ethanol and water).
2.3.1 Infusion
For the aqueous extracts, powdered of the leaves, twigs and fruits of Pistacia lentiscus L., 20 g for each plant parts was extracted with boiling water (200 mL) for 6 h. The aqueous extracts were filtered and evaporated.
2.3.2 Soxhlet extraction
Soxhlet equipment was used in this work. Powdered plant material (25 g) was extracted with solvents of different polarity (hexane, ethyl acetate, methanol and ethanol) for 6 h in about (300 mL). The crude extract of each parts from Pistacia lentiscus was filtered and evaporated.
2.3.3 Extraction yield
with
m1 = mass (g) of different parts (leaves, twigs and fruits) of Pistacia lentiscus starting.
m2 = mass (g) of crude extracts.
2.4 Instrumentation
All spectrophotometric data were acquired using SHIMADZU UVmin-1240 UV-VIS spectrophotometer. Glass cuvettes (1 cm × 1 cm × 4.5 cm).
2.5 Determination of total phenolic content (TPC)
Total phenolic content in the Pistacia lentiscus extracts was determined to use the Folin-Ciocalteu method as described by Singleton et al. (1999). Briefly, 0.3 ml of the crude extract (1 mg/ml) was added to 1.5 ml Folin-Ciocalteu reagent (10/100). The mixing was incubated for six min and mixed with 1.2 ml of Na2SO4 (7.5%). The prepared samples were incubated in darkness for two hours, the absorbance made at 760 nm. Total phenolics were expressed as Gallic acid equivalents (GAE) in mg/g of crude extract. The data were presented on average ± SD for the triplicates. The content of the total phenolic was calculated using the linear equation from the calibration curve: where A is the absorbance and X in mg/g (presented total phenolic content).
2.6 Antioxidant activities
2.6.1 Determination of the free radical scavenging activity (DPPH)
The power of plant extracts to scavenge DPPH free radicals was specified according to the method described by Brand-Williams et al. (1995). Briefly, 0.05 ml of the crude extracts were mixed with 1.95 ml freshly prepared DPPH solution in a concentration of 24 mg in 100 ml ethanol. After 1/2 h of the incubation of samples in darkness, the measured absorbance was made at 515 nm, with a positive control (Ascorbic acid). The percentages of inhibition of the DPPH free radical, as a function of the extracts concentrations, were determined to use the equation:
The antioxidant capacity has been determined from the IC50 value, which is the Concentration of the antioxidant that is needed to trap 50% of DPPH in the test solution. The efficient concentration EC50 was expressed in terms of the concentration of sample extract used for the test (mg/ml) and the quantity of extract in relation to the quantity of initial DPPH (mg/mg DPPH).
The higher the antioxidant capacity, the lower the effective concentration. For rational reasons of clarity, the antiradical power ARP was determined as the reciprocal value of the efficient concentration EC50. (Kroyer, 2004).
2.6.2 Determination of total antioxidant capacity (TAC)
Total antioxidant capacity in the P. lentiscus L. extracts was determined to use the phosphomolybdenum method as described by Prieto et al. (1999). Each sample (0.6 ml) is mixed with 6 ml reagent solution (sodium phosphate (28 mM), sulfuric acid (0.6 M) and ammonium molybdate (4 mM)). The tubes incubated for 90 min at 95 °C. After cooling, the absorbance made at 695 nm against the white which (6 ml reagent solution is mixed with 0.6 ml of methanol) and is incubated within the same basic conditions as the samples. The ascorbic acid (AA) was used as standard and the TAC assay is presented in milligram equivalents of ascorbic acid per gram of crude extract (mg EAA/g of crude extract).
2.7 Statistical analyses
All tests were conducted in triplicates and the data were presented on an average ± SD. The result was statistically analyzed using one-way ANOVA followed by Duncan's. Average values were considered statistically significant when P < 0.05. The correlation between the contents of total phenolic and antioxidant activities was determined as Pearson's correlation coefficient, the difference is considered statistically significant when P < 0.05.
3 Results and discussion
3.1 Phytochemical screening
The phytochemical screening of various parts (leaves, twigs, and fruits) of Pistacia lentiscus L., showed the great presence of tannins, flavonoids, saponins, sterols, triterpenes, oses, holosides, reducing sugars and mucilages. While antraquinones free and anthraquinons combined were absent. Although, the alkaloids present in fruits and absent in the leaves and twigs (See Table 1). High concentration (+++); moderate concentration (++); low concentration (+); absence (----).
Phytoconstituent
Test
Pistacia lentiscus (MIZ)
Pistacia lentiscus (MLS)
Twigs
Leaves
Fruits
Twigs
Leaves
Fruits
Alkaloides
Dragendorff’s Mayer’s
----
--------
----++
++----
--------
----++
++
Tannins Catechics
Stiansy reaction
+++
+++
+++
+++
+++
+++
Tannins Gallics
Lead acetate
+++
+++
+++
+++
+++
+++
Anthraquinons Free
Borntrager’s
----
----
----
----
----
----
Anthraquinons combined
O-heterosides
Reduced GeninsModified Borntrager’s
----
----
----
----
----
---
C-heterosides
----
----
----
----
----
----
Flavonoids
Shinoda’s
+++
+++
+++
+++
+++
+++
Saponins
Foam Index: positive if >100
+++
200+++
125++
100+++
200++
100+++
125
Sterols and Triterpenes
Liberman-burchard
+++
+++
+++
+++
+++
+++
Oses and holosides
Saturated alcohol with thymol
+++
+++
+++
+++
+++
+++
Mucilages
Alcohol 95%
++
++
++
++
++
++
Reducing sugars
Fehling’s
+++
+++
++
+++
+++
++
3.2 Extraction yields of Pistacia lentiscus L
It is apparent through observing the extraction yields presented in Table 2, they differ significantly (p < 0.05) on the one hand, according to the plant parts, on the other hand, the solvent used. Methanol gives the best extraction yield an average of 37.344% on three samples (leaves, fruits, and twigs) of P. lentiscus from two regions, while hexane gave the lowest yield (15.177% on average). As for the plant parts usually, regardless of the extraction solvent, the extract fruits recorded the highest yields.
Yields%
Water
Ethanol
Methanol
Ethyl acetate
Hexane
Pistacia lentiscus from MIZ
Twigs
12,47 ± 0,08d
13,94 ± 0,03f
21,93 ± 0,011c
5,45 ± 0,00d
3,20 ± 0,00d
Leaves
27,80 ± 0,14a
26,70 ± 0,13d
51,33 ± 0,89a
12,00 ± 0,01c
6,32 ± 0,03c
Fruits
21,31 ± 0,14c
43,18 ± 0,09a
51,60 ± 1,07a
42,03 ± 0,69a
48,16 ± 0,61a
Pistacia lentiscus from MLS
Twigs
7,14 ± 0,04e
16,31 ± 0,02a
20,03 ± 0,02c
6,50 ± 0,00d
4,54 ± 0,01d
Leaves
24,69 ± 0,09b
38,33 ± 0,02b
39,67 ± 0,44b
13,62 ± 0,03b
7,71 ± 0,01c
Fruits
25,04 ± 0,12b
36,25 ± 0,13c
39,50 ± 0,33b
13,12 ± 0,02b
21,13 ± 0,03b
Means Yields%
19,74
29,19
37,34
15,62
15,18
3.3 Total phenolic content (TPC)
The results of the TPC in all 30 studied extracts of various parts of Pistacia lentiscus L. by different solvents (Table 3) show a significant difference (p < 0.05) between the concentration of total phenolic. The great distinction between the plant parts appears due to the wealth of some and the poverty of others could be assigned to extraction solvents used, different environmental and climatic conditions (Özcan et al., 2009; Özcan, 2004; Couladis et al., 2003).
Bioactive compounds
TPC (mg GAE/g)
TAC (mg AA/g)
DPPH radical scavenging
IC50 (mg/mL)
EC50 (mg/mgDPPH)
ARP
Aqueous extract
PL from MIZ
Twigs
302,01 ± 1,12b
453,22 ± 0,66b
0,09 ± 0,00
3,89 ± 0,01
25,76 ± 0,05b
Leaves
345,95 ± 1,17a
488,16 ± 0,82a
0,09 ± 0,00
3,83 ± 0,02
26,12 ± 0,12a
Fruits
192,68 ± 3,68h
298,53 ± 1,15g
0,23 ± 0,00
9,75 ± 0,04
10,26 ± 0,04h
PL from MLS
Twigs
167,63 ± 0,76k
409,02 ± 0,41c
0,10 ± 0,00
4,17 ± 0,02
23,98 ± 0,1c
Leaves
299,68 ± 1,12b
269,52 ± 1,23k
0,50 ± 0,00
21,03 ± 0,07
4,76 ± 0,02l
Fruits
160,83 ± 0,67l
258,28 ± 1,15n
0,53 ± 0,00
22,06 ± 0,00
4,53 ± 0,00 m
Ethanolic extract
PL from MIZ
Twigs
232,95 ± 0,94e
319,27 ± 0,91f
0,18 ± 0,00
7,32 ± 0,02
13,66 ± 0,04f
Leaves
255,85 ± 0,90c
352,76 ± 1,15d
0,13 ± 0,00
5,27 ± 0,02
18,99 ± 0,07d
Fruits
125,61 ± 0,45p
217,91 ± 0,41s
1,45 ± 0,01
60,44 ± 0,4
1,66 ± 0,01s
PL from MIZ
Twigs
147,90 ± 0,94mn
246,31 ± 0,66p
0,66 ± 0,00
27,54 ± 0,08
3,63 ± 0,01o
Leaves
243,90 ± 0,85d
349,40 ± 0,58e
0,17 ± 0,00
7,03 ± 0,01
14,22 ± 0,01e
Fruits
127,02 ± 0,72p
211,74 ± 0,25t
1,55 ± 0,00
64,51 ± 0,07
1,55 ± 0,01 t
Methanolic extract
PL from MIZ
Twigs
226,42 ± 0,85f
297,05 ± 0,91h
0,22 ± 0,00
9,28 ± 0,09
10,78 ± 0,1g
Leaves
146,08 ± 0,67n
239,89 ± 0,25q
1,13 ± 0,00
47,28 ± 0,02
2,12 ± 0,00p
Fruits
110,79 ± 0,63r
207,91 ± 0,66u
1,61 ± 0,01
67,08 ± 0,55
1,49 ± 0,01t
PL from MLS
Twigs
202,11 ± 0,85g
292,85 ± 0,49i
0,24 ± 0,00
10,02 ± 0,01
9,94 ± 0,01i
Leaves
150,12 ± 0,81 m
254,58 ± 0,58o
0,57 ± 0,00
23,66 ± 0,08
4,23 ± 0,01n
Fruits
120,69 ± 0,49q
221,74 ± 0,25r
1,35 ± 0,01
56,11 ± 0,36
1,78 ± 0,01r
Ethyl acetate extract
PL from MIZ
Twigs
134,43 ± 0,72o
221,86 ± 0,58r
1,21 ± 0,01
50,59 ± 0,21
1,98 ± 0,01q
Leaves
168,71 ± 0,67k
261,74 ± 0,25m
0,54 ± 0,00
22,31 ± 0,15
4,48 ± 0,03m
Fruits
85,95 ± 0,45t
189,90 ± 0,41w
2,29 ± 0,03
95,25 ± 1,4
1,05 ± 0,02u
PL from MLS
Twigs
175,44 ± 2,92j
266,19 ± 0,25l
0,49 ± 0,00
20,32 ± 0,15
4,92 ± 0,04k
Leaves
186,75 ± 0,72i
277,42 ± 0,41j
0,44 ± 0,00
18,24 ± 0,03
5,48 ± 0,01j
Fruits
105,14 ± 0,76s
201,37 ± 0,49v
1,58 ± 0,02
66,02 ± 0,83
1,52 ± 0,02t
Hexane extract
PL from MIZ
Twigs
48,71 ± 0,27wx
100,26 ± 0,49aa
35,02 ± 0,05
50,59 ± 0,21
1,98 ± 0,01q
Leaves
75,71 ± 0,31u
164,70 ± 0,25x
3,00 ± 0,01
22,31 ± 0,15
4,48 ± 0,03m
Fruits
36,45 ± 0,45y
96,56 ± 0,49ab
93,98 ± 0,34
95,25 ± 1,4
1,05 ± 0,02u
PL from MLS
Twigs
49,58 ± 0,22w
123,10 ± 0,41z
22,82 ± 0,06
20,32 ± 0,15
4,92 ± 0,04k
Leaves
67,83 ± 0,36v
153,10 ± 0,41y
3,00 ± 0,01
18,24 ± 0,03
5,48 ± 0,01j
Fruits
46,82 ± 0,36x
62,48 ± 0,25ac
93,21 ± 0,36
66,02 ± 0,83
1,52 ± 0,02t
Standard Quercetin
0,10 ± 0,00
0,04 ± 0,00
24,03 ± 0,05c
The results revealed that the Pistacia lentiscus leaves are very rich in phenolic compounds (varied from 345,95 ± 1,17 to 67,83 ± 0,36 mg of GAE/g of extract), followed by twigs (varied from 302,01 ± 1,12 to 48,71 ± 0,27 mg of GAE/g of extract) and the fruits (ranged between 192,68 ± 3,68 to 36,45 ± 0,45 mg of GAE/g of extract). The ethanol and water were great solvents than the others in extracting phenolic compounds from the extracts owing to their good solubilities and polarities, followed by methanol and the lower polarity solvents, particularly: ethyl acetate and hexane showed the lower capacity in extracting the phenolic compounds.
It can, therefore, be concluded that higher polar solvents were more effective at extracting phenolic compounds from all parts of the plant than less polar solvents (Galanakis et al., 2013).
There are several studies have confirmed that the Pistacia lentiscus L. plant is rich in phenolic compounds, this wealth increases as and when the polarity of extraction solvents used increases (Bampouli et al., 2014; Botsaris et al., 2015; Zitouni et al. 2016).
3.4 Antioxidant activities of the extracts of Pistacia lentiscus
3.4.1 Total antioxidant capacity (TAC)
The TAC obtained by the phosphomolybdenum method is expressed as (mg EAA/g of crude extract). The results for TAC are presented in Table 3, show a significant difference (p < 0.05) in the TAC of all extracts of Pistacia lentiscus. The Aqueous extract of Pistacia lentiscus leaves from MIZ, showed the highest TAC with 488,16 ± 0,82 mg AA/g of extract. One clearly sees that ethanolic and aqueous extracts of Pistacia lentiscus (leaves and twigs) are recording the greatest TAC, while the hexane extract of Pistacia lentiscus fruits from MLS had the least amount of TAC with 62,48 ± 0,25 mg AA/g.
3.4.2 DPPH free radical scavenging
The antioxidant activity of all extracts of Pistacia lentiscus is assessed by the free radical DPPH reduction method. The results obtaining as given in Table 3. From these results, the greatest antioxidant activity was found in aqueous extracts of Pistacia lentiscus (leaves and twigs) from MIZ, who is stronger than the quercetin standard with a significant difference (p < 0.05). However, there was no statistically significant difference observed between the quercetin standard and the aqueous extracts of Pistacia lentiscus twigs from MLS, while no remarkable antioxidant capacity was found in the hexane extracts of various parts of Pistacia lentiscus from MLS and MIZ.
3.5 Correlation
The dependency of antioxidant activity obtained through each assay, in relationship to the TPC, was also evaluated. The results show a very significant positive correlation in the cases of DPPH scavenging activity (R2 = 0,8742) and TAC (R2 = 0,968), in relationship to the content of phenols.
Although, there is a positive linear correlation among the antioxidant activities assessed by DPPH assay and TAC assay for R2 = 0,839. The results indicated that the phenolic compounds in the different Pistacia lentiscus parts could be the main contributor to the antioxidant activities. This result was in similarity with many previous studies (Botsaris et al., 2015; Zitouni et al., 2016) Figs. 1 and 2.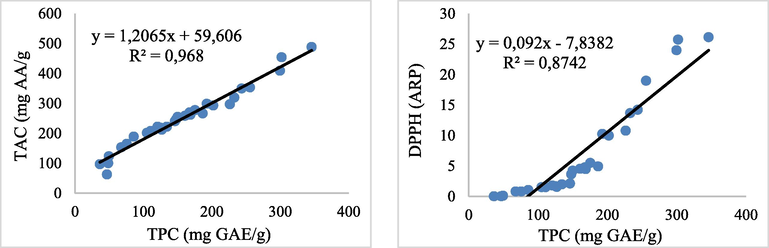
Linear regression between TPC and the antioxidant activities by TAC assay and DPPH assay.
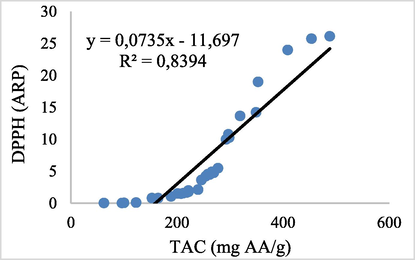
Linear regression between the antioxidant activities by TAC assay and DPPH assay.
4 Conclusions
In this work, we have opted for the study of the phytochemical screening and to determine the TPC and the antioxidant activities by DPPH and TAC assays from the leaves, twigs and fruits of Pistacia Lentiscus L., collected from two different regions of Morocco. The present work proved that the use of different solvents polar in extraction had a big influence significantly (p < 0.05) on the total phenolic contents, total antioxidant capacity and antioxidant activity of obtained extracts, as well as the richness of Pistacia lentiscus L. in secondary metabolites and in phenolic contents. These results showed that the aqueous extracts of P. Lentiscus (leaves) had the highest contents of a phenolic compound and the greatest antioxidant activity in the DPPH and TAC assays. The results indicated that the different part of Pistacia lentiscus L. could be a potential natural source of antioxidants and may have greater importance as a natural antioxidant able to slow down or prevent oxidative stress.
References
- Evaluation of phytochemical screening and analgesic activity of aqueous extract of the leaves of Microtrichia perotitii Dc (Asteraceae) in mice using hotplate method. Med. Plant. Res.. 2013;3:37643.
- [Google Scholar]
- Comparison of different extraction methods of Pistacia lentiscus var. chia leaves: yield, antioxidant activity and essential oil chemical composition. J. Appl. Res. Med. Aromat. Plants.. 2014;1:81-91.
- [Google Scholar]
- Antioxidant and antimicrobial effects of Pistacia lentiscus L. extracts in pork sausages. Food Technol. Biotechnol.. 2015;53:472-478.
- [Google Scholar]
- Use of a free radical method to evaluate antioxidant activity. Food. Sci. Technol.. 1995;28:25-30.
- [Google Scholar]
- Comparative essential oil composition of various parts of the turpentine tree (Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agric.. 2003;83:136-138.
- [Google Scholar]
- Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites.. 2009;60:405-412.
- [Google Scholar]
- Etude de la phytochimie et des activités biologique de Syzygium guineense. willd. (myrtaceae). Mali: PhD. of the University Bamako; 2005. p. :38-47.
- Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crops. Prod.. 2013;49:904-914.
- [Google Scholar]
- A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food. Prop.. 2013;16:382-396.
- [Google Scholar]
- Phytochemical investigation of the roots of Grewia microcos Linn. J. Chem. Pharm. Res.. 2013;5:80-87.
- [Google Scholar]
- Red clover extract as antioxidant active and functional food ingredient. Innov. Food. Sci. Emerg. Technol.. 2004;5:101-105.
- [Google Scholar]
- Characteristics of fruit and oil of terebinth (Pistacia terebinthus L) growing wild in Turkey. J. Sci. Food Agric.. 2004;84:517-520.
- [Google Scholar]
- Essential oil composition of the turpentine tree (Pistacia terebinthus L.) fruits growing wild in Turkey. Food Chem.. 2009;114:282-285.
- [Google Scholar]
- Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol.. 1999;299:152-178.
- [Google Scholar]
- Assessment of phytochemical composition and antioxidant properties of extracts from the leaf, stem, fruit and root of Pistacia lentiscus L. Int. J. Pharmacogn. Phytochem. Res.. 2016;8:627-633.
- [Google Scholar]







