Translate this page into:
A common food additive (E452), hexametaphosphate, denatures the digestive enzyme trypsin
⁎Corresponding author at: Department of Biochemistry, College of Science, Building no 5, P.O. Box 2455, King Saud University, Riyadh 11451, Saudi Arabia. amalik@ksu.edu.sa (Ajamaluddin Malik)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Phosphate additions in processed foods are a health risk that has been overlooked. This study examined the effects of a permitted food additive (E452; Sodium Hexametaphosphate (SHMP)) on trypsin solubility, structure, and stability at the intestinal physiological pH of 6.0. SHMP-treated trypsin samples were assessed for conformational changes and aggregation propensity using various spectroscopic and microscopic methods. Far-UV CD spectroscopy and intrinsic tryptophan fluorescence showed a rise in fluorescence intensity and alpha-helical structure up to 10 μM SHMP (∼1:1 M ratio). Secondary structure loss, tryptophan quenching, and solubility decrease with an increase in SHMP concentration (>10 μM). SHMP-treated trypsin aggregates showed poor ThT fluorescence. According to kinetics, SHMP leads to trypsin aggregation instantly. All spectroscopic studies showed trypsin denatured at above 10 μM SHMP. Thus, digestive enzyme structure and solubility loss will impair food digestion and health. Therefore, SHMP should be avoided as a food additive.
Keywords
Amyloid fibril
Trypsin
SHMP
Polyphosphate
Aggregation
1 Introduction
Large-scale epidemiological study links hyperphosphatemia to renal diseases, cardiovascular events, and general population mortality (Uribarri 2007, Sullivan et al., 2009). Hence, the dietary intake of phosphates is a major issue for everyone, not just those with renal impairment (Block et al., 2004, Kestenbaum et al., 2005). Hyperphosphatemia is common in low-income groups because cheap inorganic food additives are added to processed and fast foods consumed in huge quantities by financially impoverished people. For many years, physicians treating chronic kidney disease were advocating for dietary phosphate restriction. Recent research reveals that phosphate additions in food may harm young, healthy people. (Tonelli et al., 2005, Dhingra et al., 2007, Foley et al., 2009). Previously, phosphate was thought to cause only blood vessel and organ calcification. Recent research shows that klotho and FGF23 regulate blood phosphate levels, and that high serum phosphate levels damage the cardiovascular system and accelerate aging in animal models. (Dhingra et al., 2010, Ohnishi and Razzaque 2010).
Organic esters of phosphates are naturally present in meat, sausages, cereals, legumes, dairy products, nuts, eggs, and fish and cannot be eliminated. Hydrolysis and intestinal reabsorption of organically bound natural phosphates are slow. Studies show that the gut absorbs 40–60 % of natural phosphates. (Kayne et al., 1993, Uribarri 2007). However, the gut quickly absorbs “free” inorganic phosphates used as dietary additives. A variety of inorganic phosphates are permitted for usage under strict limits set by food and safety authorities. Sodium, potassium, and calcium phosphate salts (E 339, E 340, and E 341, respectively), diphosphate salts (E 450), triphosphate salts (E 451), and polyphosphate salts (E 452) can all be used lawfully in food processing as preservatives, stabilizers, acidifying agents, taste enhancers, acidity buffers, antiglycating, and emulsifying agents (Additives et al., 2019, Huang et al., 2019). Processed cheese, animal products (fish, ham, sausages), bakery items, and drinks (cola and other soft drinks) use large amounts of inorganic phosphates, making the situation more serious due to the lack of phosphate content labeling. Industrial food processing uses inorganic phosphates since they are cheap and improve food functionality, so it has more phosphate than natural food.
Sodium Hexametaphosphate (SHMP) is a permitted food additive, known as E452. Sodium hexametaphosphate (Na6[(PO3)6]) is a synthetic phosphate salt that goes by a number of other names. They include Graham's Salt, Hex, and Sodium Polymetaphosphate. It is a hexamer of metaphosphates, which suggests that it is cyclic of NaPO3. SHMP is utilized in dairy, meat, and seafood production as a metal ion chelator, deflocculator, adhesive, and swelling reagent. Inorganic polyphosphate excessive use can cause blood chemistry abnormalities, heart issues, central nervous system problems, and cancer. (Kendrick et al., 2011, Brown and Razzaque 2018, Lempart and Jakob 2019, Boyineni et al., 2020, Qadeer and Bashir 2022). Phosphates sequester calcium, making them hazardous. Moreover, this chemical may produce systemic acidosis by hydrolyzing into phosphoric acid. Therefore, it should be avoided. (Additives et al., 2019, Huang et al., 2019).
In general, phosphates have a positive effect on the functional qualities of processed foods, however, it is unclear how inorganic phosphates affect the solubility, structure, and stability of digestive enzymes. To our knowledge, no studies on the effects of polyphosphate on the structure–function of the digestive enzyme trypsin have been conducted. To better understand how polyphosphates affect digestive enzymes, the goal of this work was to evaluate the effects of sodium hexametaphosphate (E451a) on the solubility, structure, and stability of bovine trypsin using various spectroscopic and microscopic techniques. Trypsin is a proteolytic enzyme that is secreted in the duodenum and catalyzes the hydrolysis of peptide bonds, breaking down protein molecules into smaller pieces. The novelty of the presented research is providing the aggregation mechanism of sodium hexametaphosphate to trypsin molecules for food safety concerns.
2 Materials and methods
Sigma Chemical Co. (St. Louis, Missouri, U.S.A.) provided bovine trypsin (Cat. no. T8003; ∼10,000 BAEE units/mg protein), sodium hexametaphosphate (Cat. no.305553; 96 % purity), and thioflavin T (Cat. no. T3516; dye content, ≥65 %). All solutions were prepared using Milli-Q water (Millipore Corp., USA), which was afterward filter sterilized. The analytical grade was used for all of the other reagents that were utilized in this study.
2.1 Sample preparation
A stock solution of bovine trypsin (83 μM) was prepared in 1 mM HCl. Bovine trypsin concentration was determined by spectrophotometric analysis of the absorbance at 280 nm using an extinction coefficient (E1%) of 15.4. Aliquots of bovine trypsin were stored at −20 degrees Celsius. A stock solution of SHMP (100 mM) was freshly prepared in deionized water.
2.2 SHMP-induced unfolding and aggregation of trypsin
SHMP was added to bovine trypsin (8.3 μM) in 20 mM phosphate buffers at pH 6.0 at varying concentrations. To prevent autolysis, SHMP-treated trypsin samples were incubated for equilibration at pH 6.0 for two hours on ice.
2.2.1 Turbidity measurements
At 350 nm, the turbidity of bovine trypsin (8.3 μM) was evaluated in response to varied concentrations of SHMP ranging from 0 to 1000 µM. The absorbance of SHMP-treated trypsin samples was collected in 1.0 cm path length cuvette using a Carry 60 UV–visible spectrophotometer (Agilent Technologies).
2.2.2 Rayleigh light scattering kinetics of SHMP-induced trypsin aggregation
Using Rayleigh light scattering (RLS) measurements, the kinetics of SHMP-induced trypsin aggregation at pH 6.0 were analyzed. The concentration of bovine trypsin was maintained at 8.3 μM, and RLS kinetics were evaluated in the presence of 1000 µM SHMP in a cuvette with a pathlength of 1.0 cm using a stirrer and Peltier-connected Carry Eclipse fluorometer (Agilent Technologies). The RLS intensity was measured in a cuvette with a 1.0 cm path length (3 ml) and a magnetic bar at 350 nm as a function of time (s). Excitation and emission wavelengths were kept constant at 350 nm. The widths of the slits used for excitation and emission were 1.5 and 2.5 nm. Initially, RLS kinetics were recorded in the absence of SHMP for 110 s, then 1000 µM SHMP was added and data were collected for a further 340 s.
2.2.3 Intrinsic fluorescence measurements of SHMP-induced trypsin
At room temperature, SHMP-treated trypsin samples were evaluated for changes in the intrinsic tryptophan fluorescence on a Carry Eclipse spectrofluorometer. Bovine trypsin (8.3 μM) was treated with SHMP at pH 6.0. (0 to 1000 µM). Trypsin samples were excited at 295 nm to monitor changes in the milieu surrounding the Trp fluorophore. Between 300 and 400 nm, the emission spectrum was recorded. Both the excitation slit width and the emission slit width were fixed at 5 nm.
2.2.4 Far-UV circular dichroism (CD) of SHMP-induced trypsin
This study measured the far-UV CD of SHMP-treated trypsin samples using a ChirascanPlus spectropolarimeter (Applied Photophysics, UK). At room temperature, in a 0.1 cm path-length cuvette, far-UV CD measurements were made between 200 and 250 nm. The concentration of trypsin remained constant at 8.3 μM in all samples. SHMP blanks of various concentrations were used to subtract from the spectra of the test samples.
2.2.5 Thioflavin T fluorescence measurement of SHMP-induced trypsin
The Thioflavin T (ThT) stock solution was made in deionized water and filtered through a 0.22-µm syringe filter. The concentration of ThT was calculated using a molar extinction value of 36,000 M−1cm−1 at a wavelength of 412 nm (Khan et al., 2016). Samples of trypsin (8.3 μM) that had been treated with different SHMP concentrations were incubated with 10 µM ThT in the dark for 30 min to detect the formation of amyloid fibrils. ThT fluorescence in SHMP-treated trypsin samples was measured at 440 nm. From 450 to 600 nm, the emission spectrum was measured. In this experiment, a slit width of 5.0 nm was set for both excitation and emission. Furthermore, Th-T Kinetics experiments of SHMP-induced trypsin were carried out at a pH of 6.0.
2.2.6 Transmission electron microscope (TEM) of SHMP-induced trypsin aggregation
The JOEL-1400 Transmission Electron Microscope (JEOL USA, Inc.) captured TEM images of SHMP-induced trypsin aggregation while operating at a 120 kV accelerating voltage. To examine the effect of SHMP on trypsin aggregate morphology, transmission electron microscopy was applied to ten microliters of trypsin samples (8.3 μM) treated with 1000 M SHMP at the intestinal physiological pH. To prepare the samples for examination, a 200-mesh copper grid was used. After two minutes, the grids were washed and negatively stained with 2 % uranyl acetate.
3 Results
3.1 SHMP-induced turbidity in trypsin
The trypsin aggregate formation with SHMP experiment was carried out at a pH of 6.0. Changes in turbidity at 350 nm were used to measure the trypsin aggregation behavior in the presence of SHMP. Fig. 1 showed the results of a scatter plot of the correlation between the change in turbidity at 350 nm and SHMP concentrations. When trypsin was either untreated or treated with 5 or 10 µM SHMP, there was no apparent difference in the turbidity of the solution. The turbidity of the trypsin solutions in samples containing 10–200 µM SHMP increased linearly with increasing concentration up to a plateau at 400 uM SHMP. The absorption of samples with 400–1000 µM SHMP was about 1.1 at 350 nm. The results showed that trypsin aggregates were formed in the presence of 25–1000 µM SHMP at a pH of 6.0.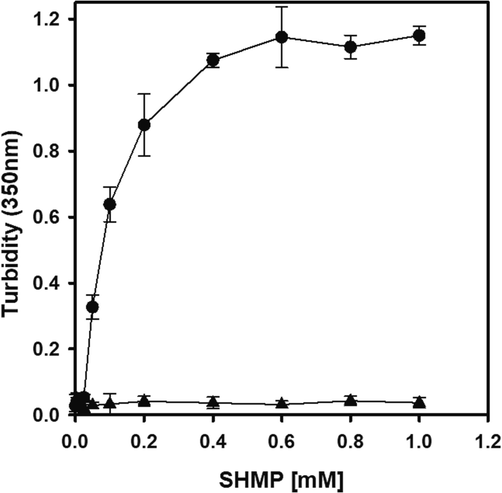
The effect of SHMP on trypsin solution turbidity at pH 6.0. The turbidity of the trypsin solution (8.3 μM) was measured at 350 nm in the presence of 0–1000 µM SHMP (●). The turbidity of the control samples (0–1000 µM SHMP) without trypsin is shown in (▲).
3.2 SHMP-induced changes in the tertiary structure of trypsin
Bovine trypsin has four tryptophan residues which are primarily responsible for intrinsic fluorescence. As ligands interacted with proteins, changes in the microenvironment around tryptophan were detected, as evaluated by changes in the wavelength maximum and intrinsic fluorescence intensity (Khan et al., 2019, Zhang et al., 2019). The fluorescence spectra of trypsin were studied in the presence of 0–1000 µM SHMP by excitation at 295 nm and spectra were recorded between 300 and 450 nm. (Fig. 2). Bovine trypsin has a maximum wavelength of 332 nm at pH 6.0. In the presence of up to 10 µM SHMP, the intensity of trypsin fluorescence initially increased. However, the fluorescence intensity steadily decreased in the presence of 10–200 µM SHMP. In this range of SHMP concentrations, trypsin aggregation was found to be dose-dependent. The maximal fluorescence wavelength was unaffected by the addition of SHMP (0–1000 µM). Fluorescence intensity quenching was associated with trypsin aggregation.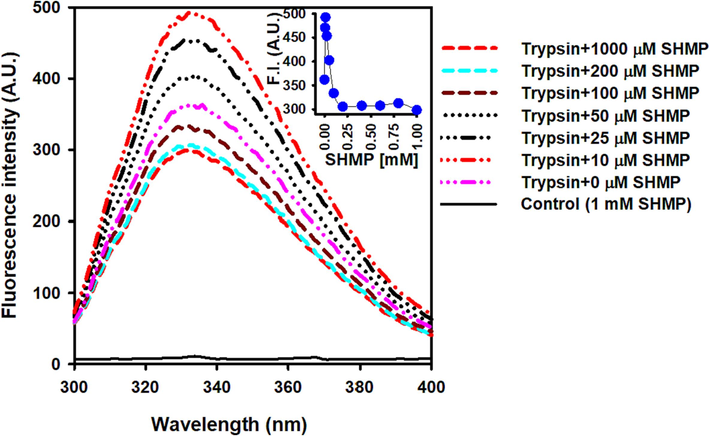
The effect of SHMP on trypsin's tertiary structure. Trypsin intrinsic fluorescence spectra was measured at pH 6.0 in the presence of 0–1000 µM SHMP. In all measurements, the trypsin concentration was 8.3 μM. The fluorescence spectra of the control sample (1000 M SHMP without trypsin) displayed as a solid black line.
3.3 SHMP-induced changes in the secondary structure of trypsin
Trypsin is a protein with two alpha-helices and 13 beta-sheets (Whitlow et al., 1999). As a result, trypsin's far-UV CD spectra showed a single minimum at 208 nm (Liu et al., 2018). Natural trypsin exhibited a far UV CD spectrum at 208 nm at pH 6.0. (Fig. 3). The ellipticity at 208 nm increased in the presence of 5 and 10 µM SHMP, showing secondary structure gain in the trypsin (Fig. 3) The intrinsic tryptophan fluorescence similarly increased in this concentration range (Fig. 2). Increased concentrations of SHMP (10–200 µM) result in a concentration-dependent loss of secondary structure. At this concentration range, negative ellipticities were decreased, but the shape of the far-UV CD spectra remained unchanged. Over 200 µM SHMP, trypsin lost all secondary structure. The aggregation propensity of trypsin is linked to secondary structure loss (Fig. 1). SHMP only causes secondary structure gain or loss, but no structural conversion, according to our findings.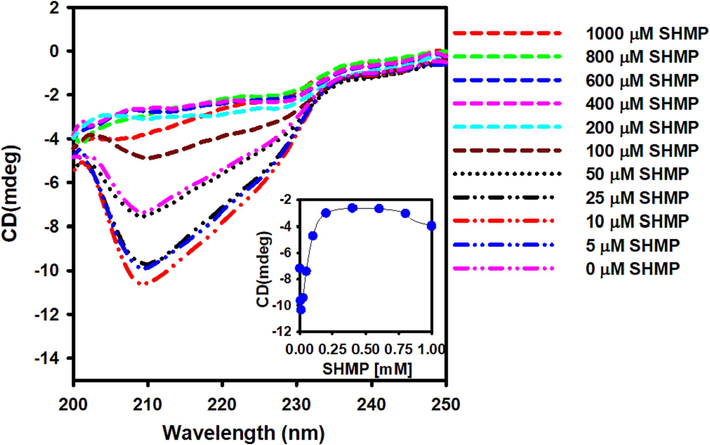
The effect of SHMP on trypsin's far-UV CD spectra. Between 200 and 250 nm, the far UV CD spectra of 0–1000 µM SHMP-treated trypsin were recorded. The trypsin concentration was fixed at 8.3 μM in all the experiments.
3.4 SHMP-induced ThT fluorescence of trypsin
Thioflavin T dye has negligible fluorescence in its unbound condition (aqueous solution). Intense fluorescence is produced by ThT upon binding to the stacked β-sheets of amyloid fibrils (over 10 folds) (Huang et al., 2023). ThT also binds to aggregated protein, but with a low quantum yield (3–5 fold increase) (Malik et al., 2023). As a result, the ThT assay is utilized to identify amyloid fibrils in aggregated proteins. In this study, ThT fluorescence was used to characterize SHMP-induced trypsin aggregation. Increasing the SHMP concentration from 10 to 200 µM leads to a minor increase in ThT fluorescence (Fig. 4), indicating the presence of amorphous structures in the aggregates. Additionally, in the far-UV CD experiment, no cross-beta sheeted structure (a characteristic of amyloid fibrils) was seen (Fig. 3).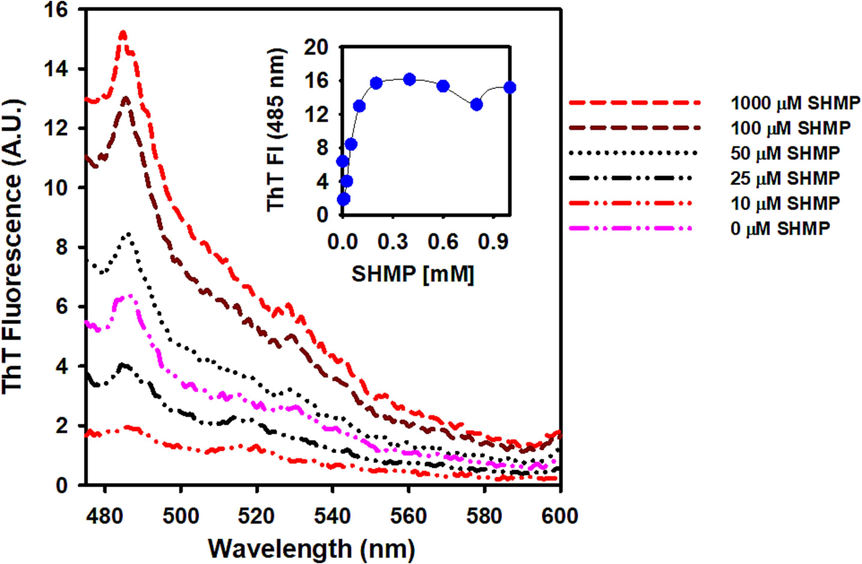
The effect of SHMP on the trypsin ThT fluorescence at pH 6.0. In all experiments, 10 µM ThT was added to the SHMP-treated trypsin samples, the samples were excited at 440 nm and the data was recorded between 450 and 600 nm, with the ex and em slits set to 5 nm.
3.5 SHMP-induced RLS kinetics of trypsin
We investigated the RLS kinetics of SHMP-induced trypsin aggregation at an intestinally physiological pH of 6.0. The RLS kinetics were measured at 350 nm. Fig. 5A showed the kinetic traces of SHMP-induced aggregation at 1000 µM SHMP concentrations. In the absence of SHMP, trypsin did not scatter, indicating that it was soluble. When 1000 µM SHMP was added, light scattering increased rapidly and quickly reached a plateau. The RLS pattern demonstrated that the aggregation process had no lag time. These data suggest that nucleation-independent mechanisms are involved in SHMP-induced trypsin aggregation.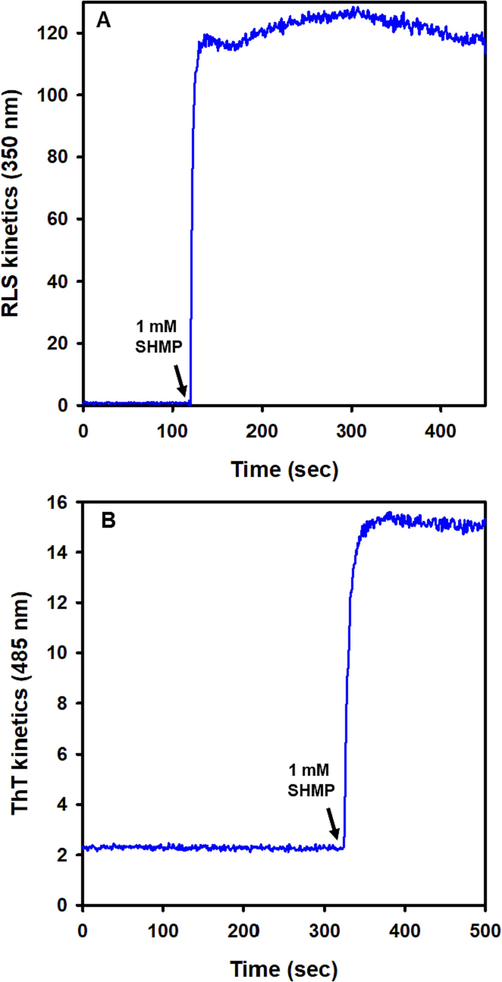
Aggregation Kinetics of trypsin induced by SHMP at pH 6.0. (A) RLS scattering was measured at 350 nm using trypsin samples (8.3 μM) without SHMP. After 110 s, 1000 µM SHMP was added, and RLS was monitored continuously. (B) Effect of SHMP on the ThT fluorescence kinetics. At pH 6.0, 10 µM ThT dye was applied to trypsin samples (8.3 μM) without SHMP, and ThT kinetics were observed at 485 nm. When 1000 µM SHMP was added, ThT fluorescence increased approximately five-fold and reached a plateau within a few seconds.
3.6 SHMP-induced ThT kinetics of trypsin
Using Thioflavin T dye, we analyzed the binding kinetics of ThT in SHMP-induced trypsin (Fig. 5B). The trypsin ThT fluorescence at pH 6.0 (natural condition) without SHMP served as a reference point. Monitoring the increase of RLS kinetics after adding 1000 µM SHMP revealed that the ThT fluorescence rapidly increased. Since there were no amyloid fibrils present, the ThT rise was modest (nearly 5 folds).
3.7 TEM characterization of SHMP-induced trypsin aggregates
High-resolution images of the morphology of SHMP-induced trypsin aggregates were obtained by TEM. (Fig. 6). At pH 6.0, trypsin was soluble; however, trypsin aggregated in the presence of 1000 µM SHMP at the same pH. The TEM image showed that an amorphous-like aggregate was present that corroborates the Far-UV CD data and ThT fluorescence measurements.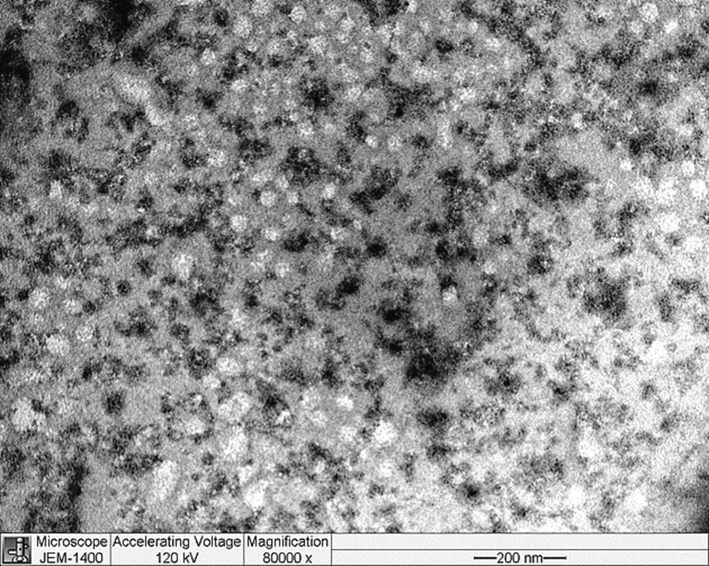
TEM images of SHMP-induced trypsin aggregates. Trypsin (8.3 μM) was treated with 1 mM SHMP at pH 6.0.
4 Discussion
Food processing uses inorganic phosphates to improve texture, water binding, and other properties. (Dobenecker et al., 2021). Inorganic phosphates effectively improve processed dairy and meat products and partially replace sodium chloride. They are cheap and effective (Ursachi et al., 2020, Barcenilla et al., 2022). By increasing protein cross-linking, phosphoates can improve oxidative stability and gel properties (Simsek and Kilic 2020, Li et al., 2021). Inorganic phosphates unfold protein structure, making it more water-binding. Inorganic phosphates are overused in soft drinks to prevent advanced glycation end products. Soft cheese melting salts contain inorganic phosphate salts. (Nagyova et al., 2014). They are also used to prevent agglomeration in high pasteurized sterilized milk, thickened milk, and powdered milk.
The public and policymakers should be aware of the potential harm caused by added inorganic phosphates in processed food because higher serum phosphate levels are linked to mortality in chronic kidney disease patients and cardiovascular events in young, healthy persons. Fast food and other non-cooking processed foods are making people eat more inorganic phosphates than before. Inorganic phosphate intake has increased from 500 mg to 1000 mg per day in the past 30 years. (Calvo and Park 1996, Sherman and Mehta 2009, Kalantar-Zadeh et al., 2010). Dialysis patients have an increased risk of cardiovascular illness due to raised serum phosphate levels (Tonelli et al., 2005, Dhingra et al., 2007, Foley et al., 2009). Everyone, including young, healthy people, should restrict phosphorus intake. Recent research shows that a complicated hormonal (FGF23) feedback system tightly controls phosphate balance and renal excretion. (Liu and Quarles 2007, Prie et al., 2009, Heijboer and Cavalier 2023). In observational studies with high serum phosphate levels, mortality risk increases by 22 % for every 1 mg/dL elevation. (Tonelli et al., 2005).
In this study, we investigated the impact of SHMP on a digestive enzyme (trypsin) in physiological pH conditions using a range of micromolar concentrations of SHMP. The stomach's highly acidic environment is quickly neutralized in the duodenum, where the intestinal pH is around 6. Beginning at pH 6 in the duodenum, the small intestine's pH rises steadily, reaching its highest level at pH 7.4 in the terminal ileum. In the caecum, the pH falls to 5.7, but it rises again gradually to reach pH 6.7 in the rectum (Fallingborg 1999). Trypsin's calculated isoelectric point is 8.69. It has two arginine residues and fourteen lysine residues that are protonated at pH 6.0. According to Protein Calculator v3.4 calculations, the total charge at this pH is 8.5, indicating that trypsin exists in a cationic state at pH 6.0. Because polyphosphates are anionic molecules, a strong electrostatic interaction between cationic trypsin and anionic polyphosphates is expected under intestinal physiological conditions, which can disrupt intramolecular ionic interactions and solvent protein interactions, potentially causing damage to trypsin's native conformation and solubility.
The tertiary and secondary structures of trypsin are altered after being exposed to modest concentrations (5 µM) of SHMP due to electrostatic interactions between the positively protonated residue and the negatively charged phosphates. The intrinsic tryptophan fluorescence initially increased upto 10 µM SHMP. However, increased SHMP concentrations cause tryptophan quenching. Similarly, secondary structure gain was seen up to 10 µM SHMP, but subsequent increase in SHMP concentrations resulted in secondary structure loss. The peak minima did not shift towards 215 nm, indicating that no beta-sheet structure was formed (no amyloid fibril formation). ThT fluorescence increased marginally in aggregated trypsin samples, demonstrating the absence of an amyloid-like structure. Furthermore, TEM images revealed amorphous-like structures in the aggregated trypsin samples (Fig. 6). Trypsin is a protein with a predominant beta-sheet structure. Early SHMP binding results in structure gain, whereas successive SHMP binding results in structure loss and protein aggregation. The many non-native electrostatic interactions are expected to be the cause of aggregation. At pH 6.0, the negatively charged phosphates of SHMP electrostatically interact with the protonated cationic amino acid residues of trypsin, breaking intramolecular ion pairs and the solvent-trypsin interactions.
In the Hofmeister series, the phosphate anion is known as a kosmotropic salt. Several kosmotropes have traditionally been employed to promote protein aggregation at various stages of protein extraction and purification. Because of their synergistic kosmotropic activity, hexa phosphate ions linked by phosphoanhydride bonds bind with cationic trypsin at the intestinal physiological pH and prevent water molecules from establishing a hydration structure at the protein surface. Conformational alterations in trypsin result in an increase in secondary and tertiary structure at approximately a 1:1 ratio of SHMP to trypsin. At high SHMP/trypsin ratios, H-bond interactions between protein and water are disrupted, resulting in structural destabilization of trypsin driven by SHMP, which leads to trypsin unfolding and decreased solubility, eventually leading to trypsin aggregation.
5 Conclusion
In summary, this study sheds light on the multifaceted role of inorganic phosphates in food processing and highlights potential health concerns associated with their excessive consumption. Inorganic phosphates have long been utilized to enhance various food products, offering advantages such as improved texture, water retention, and oxidative stability. However, their widespread use, particularly in processed and fast foods, has led to increased dietary phosphate intake, raising concerns about its health implications. In this study, we have examined the impact of SHMP on trypsin. Under intestinal physiological conditions (pH 6.0), the interaction between cationic trypsin and anionic SHMP disrupts the protein's native conformation and solubility due to strong electrostatic forces. This study found that SHMP induced structural changes in trypsin, with lower concentrations (5 µM) causing structural gains and higher concentrations resulting in structural losses and aggregation. The electrostatic interactions between SHMP's negatively charged phosphates and trypsin's protonated cationic residues are believed to drive these conformational changes and aggregation. These findings shed light on the potential impact of SHMP on protein stability and solubility in the digestive process and highlight the importance of considering such interactions in food processing and dietary choices. While inorganic phosphates offer various benefits to the food industry, their overconsumption poses health risks. Awareness of these risks and the need to limit phosphate intake, particularly in highly processed foods, is essential for maintaining overall health and well-being.
Acknowledgments
The authors are grateful to the Researchers Supporting Project Number (RSPD2023R727), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Additives, E. P. o. F., Flavourings, M. Younes, et al., 2019. Re-evaluation of phosphoric acid-phosphates - di-, tri- and polyphosphates (E 338-341, E 343, E 450-452) as food additives and the safety of proposed extension of use. EFSA J. 17 (6) e05674. https://doi.org/10.2903/j.efsa.2019.5674.
- Microbiological safety and shelf-life of low-salt meat products-A review. Foods. 2022;11(15)
- [CrossRef] [Google Scholar]
- Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol.. 2004;15(8):2208-2218.
- [CrossRef] [Google Scholar]
- Inorganic polyphosphate as an energy source in tumorigenesis. Oncotarget. 2020;11(50):4613-4624.
- [CrossRef] [Google Scholar]
- Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta. Rev. Cancer. 2018;1869(2):303-309.
- [CrossRef] [Google Scholar]
- Changing phosphorus content of the U.S. diet: potential for adverse effects on bone. J. Nutr.. 1996;126(4 Suppl):1168S-1680S.
- [CrossRef] [Google Scholar]
- Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med.. 2007;167(9):879-885.
- [CrossRef] [Google Scholar]
- Relations of serum phosphorus levels to echocardiographic left ventricular mass and incidence of heart failure in the community. Eur. J. Heart Fail.. 2010;12(8):812-818.
- [CrossRef] [Google Scholar]
- Effects of dietary phosphates from organic and inorganic sources on parameters of phosphorus homeostasis in healthy adult dogs. PLoS One. 2021;16(2):e0246950.
- [Google Scholar]
- Intraluminal pH of the human gastrointestinal tract. Dan. Med. Bull.. 1999;46(3):183-196.
- [Google Scholar]
- Serum phosphorus levels associate with coronary atherosclerosis in young adults. J. Am. Soc. Nephrol.. 2009;20(2):397-404.
- [CrossRef] [Google Scholar]
- The Measurement and Interpretation of Fibroblast Growth Factor 23 (FGF23) Concentrations. Calcif. Tissue Int.. 2023;112(2):258-270.
- [CrossRef] [Google Scholar]
- Effect of phosphates on gelling characteristics and water mobility of myofibrillar protein from grass carp (Ctenopharyngodon idellus) Food Chem.. 2019;272:84-92.
- [CrossRef] [Google Scholar]
- Novel flavan-3-ol-dithiothreitol conjugates derived from the degradation of grape seed proanthocyanidins and their neuroprotective potential. Food Chem.. 2023;405(Pt A):134825
- [CrossRef] [Google Scholar]
- Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol.. 2010;5(3):519-530.
- [CrossRef] [Google Scholar]
- Analysis of segmental phosphate absorption in intact rats. A compartmental analysis approach. J. Clin. Invest.. 1993;91(3):915-922.
- [CrossRef] [Google Scholar]
- Phosphate and cardiovascular disease. Adv. Chronic Kidney Dis.. 2011;18(2):113-119.
- [CrossRef] [Google Scholar]
- Serum phosphate levels and mortality risk among people with chronic kidney disease. J. Am. Soc. Nephrol.. 2005;16(2):520-528.
- [CrossRef] [Google Scholar]
- Khan, J. M., A. Ahmed, S. F. Alamery, et al., 2019. Millimolar concentration of sodium dodecyl sulfate inhibit thermal aggregation in hen egg white lysozyme via increased α-helicity. Colloids and Surfaces A. 572 167–173.
- Glycation Induced Generation of Amyloid Fibril Structures by Glucose Metabolites. Protein Pept. Lett.. 2016;23(10):892-897.
- [CrossRef] [Google Scholar]
- Role of polyphosphate in amyloidogenic processes. Cold Spring Harb. Perspect. Biol.. 2019;11(5)
- [CrossRef] [Google Scholar]
- Assessment the influence of salt and polyphosphate on protein oxidation and Nepsilon-(carboxymethyl)lysine and Nepsilon-(carboxyethyl)lysine formation in roasted beef patties. Meat Sci.. 2021;177:108489
- [CrossRef] [Google Scholar]
- How fibroblast growth factor 23 works. J. Am. Soc. Nephrol.. 2007;18(6):1637-1647.
- [CrossRef] [Google Scholar]
- Enhanced trypsin thermostability in Pichia pastoris through truncating the flexible region. Microb. Cell Fact.. 2018;17(1):165.
- [CrossRef] [Google Scholar]
- Agitation does not induce fibrillation in reduced hen egg-white lysozyme at physiological temperature and pH. J. Mol. Recognit. 2023:e3009.
- [CrossRef] [Google Scholar]
- Use of sodium polyphosphates with different linear lengths in the production of spreadable processed cheese. J. Dairy Sci.. 2014;97(1):111-122.
- [CrossRef] [Google Scholar]
- Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J.. 2010;24(9):3562-3571.
- [CrossRef] [Google Scholar]
- Latest findings in phosphate homeostasis. Kidney Int.. 2009;75(9):882-889.
- [CrossRef] [Google Scholar]
- Physiology. Treasure Island (FL): Phosphate. StatPearls; 2022.
- Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol.. 2009;4(8):1370-1373.
- [CrossRef] [Google Scholar]
- Influences of encapsulated polyphosphate incorporation on oxidative stability and quality characteristics of ready to eat beef Doner kebab during storage. Meat Sci.. 2020;169:108217
- [CrossRef] [Google Scholar]
- Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. J. Am. Med. Assoc.. 2009;301(6):629-635.
- [CrossRef] [Google Scholar]
- Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627-2633.
- [CrossRef] [Google Scholar]
- Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin. Dial.. 2007;20(4):295-301.
- [CrossRef] [Google Scholar]
- Crystallographic analysis of potent and selective factor Xa inhibitors complexed to bovine trypsin. Acta Crystallogr. Sect. D, Biol. Crystallogr.. 1999;55(Pt 8):1395-1404.
- [CrossRef] [Google Scholar]
- Interaction between an (-)-epigallocatechin-3-gallate-copper complex and bovine serum albumin: Fluorescence, circular dichroism, HPLC, and docking studies. Food Chem.. 2019;301:125294
- [CrossRef] [Google Scholar]







