Translate this page into:
A 8-week, randomized, double-blind, placebo-controlled human trial to evaluate the efficacy and safety of Saccharum officinarum wax alcohols (Policosanol) on improvement of blood cholesterol
⁎Corresponding author at: Ilwonbio Co., Ltd, & Department of Korean Physiology, College of Korean Medicine, Wonkwang University, 460 Iksandaero, Iksan, Jeonbuk 54538, South Korea. desson@wku.ac.kr (Kang-Beom Kwon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The prevalence of atherosclerotic cardiovascular disease appears to be reduced, according to a large body of research, by lowering blood levels of the ratio of low-density lipoprotein cholesterol (LDL-C)/ high-density lipoprotein cholesterol (HDL-C), triglyceride (TG)/HDL-C, and LDL-C.
Objective
The objective of the investigation was to determine the safety and endurance of policosanol (20 mg/d), as well as its efficacy in healthy individuals. Two parallel groups in this randomized, double-blind, placebo-controlled human experiment received either a policosanol (20 mg/d) or a placebo for eight weeks. 80 people were randomly assigned, with a mean (SD) age (years) of 42.61 (13.51), a mean (SD) BMI (kg/m2) of 24.53 (3.57), a mean (SD) weight (kg) of 66.71 (14.30), and a mean (SD) height (cm) of 164.21. (7.99).
Results
At 8 weeks, when compared to the baseline group, the policosanol (20 mg/d) batch displayed considerably greater LDL-C (-4.87 ± 11.30 mg/dL; p = 0.014), total cholesterol (-6.82 ± 14.32 mg/dL; p = 0.007), triglyceride (-9.37 ± 19.27 mg/dL; p = 0.008), non HDL-C reductions (-10.32 ± 13.75 mg/dL; p = 0.0001), and significantly greater augmentation of HDL-C (3.50 ± 4.55 mg/dL; p = 0.010). Policosanol (20 mg/d) treatment also significantly reducing the serum levels of TC/HDL-C (p = 0.0001) and LDL-C/HDL-C (p = 0.0002), triglyceride/HDL-C (p = 0.001), and T-cholesterol-HDL-C/HDL-C (p = 0.0001) ratios.
Conclusion
In humans, policosanol delivery resulted in a lowering of LDL-C levels and an improvement in other lipid markers, demonstrating the product potential to regulate hypercholesterolemia.
Keywords
Policosanol
Low-density lipoprotein cholesterol
Triglyceride
Hypercholesterolemia
Cardiovascular disease
1 Introduction
Major prevention refers to the controlling of cardiovascular risk factors, such as increased LDL-C, in patients who have never had a coronary incident. Depending on population - based studies data showing a consistent, positive, and graded association between heart disease events and LDL-C density and death rates, it is believed that focusing on a major prevention of LDL lessening will minimize risk in both patient populations with and without cardiovascular illness at a wide array of concentrations (Reiter-Brennan et al., 2020; Ference et al., 2017; Virani et al., 2020).
Individuals lacking known cardiovascular illness often have considerably lower initial probabilities for cardiovascular risk than do those who have. The overall risk decrease from treating hypercholesterolemia would often be lower for individuals who already have cardiovascular disease (CVD), therefore the choice to prescribe LDL medication will rely on the overall CVD risk (Reiter-Brennan et al., 2020; Ference et al., 2017). Research findings with clinical end-points have shown a connection between cardiovascular events and high intensities of LDL-C and total cholesterol in serum (Ference et al., 2017), aside from the benefits of take down LDL-C (Arnett et al., 2019; Lloyd et al., 2013; Heart, 2002; Ford et al., 2016; Kim and Le, 2020; Kazi et al., 2017; Packard, 2015).
A group of long-chain aliphatic primary alcohols that were initially extracted from wax made from sugar cane (Saccharum officinarum L.) widely known as policosanol. (PC) (Arruzazabala et al., 2000). Three substances make up the majority of the combination: octacosanol (60–70% by weight), triacontanol (10–20% by weight), and hexacosanol (4–10% by weight). Rice bran, wheat germ and bee wax are only a few examples of alternative natural materials from which the combination can be produced (Wang et al., 2003; Aleman et al., 2001), however the commercially available products predominantly comprise sugar cane policosanol (SCP). In fact, PC supplementation has been approved as a cholesterol-lowering product in several nations (Yanai et al., 2015), and SCP has been utilized in Cuba in a significant number of individuals for its cholesterol-lowering characteristics.
Policosanol has been shown to reduce lipid level in individuals suffering from type II hyperlipidemia (Canetti et al., 1997; Mas et al., 1999; Aneiros et al., 1995). Policosanol has other significant pleiotropic properties, which include the reduction of LDL oxidation sensitivity and the prevention of platelet adhesion (Castaño et al., 2002a; Menéndez et al., 2000; Arruzazabala et al., 1998). Policosanol has been shown in clinical tests to be both tolerable and safe (Fernández et al., 2004; Castaño et al., 2002b; Menéndez et al., 2000; Arruzazabala et al., 1998; Canetti et al., 1997; Mas et al., 1999; Aneiros et al., 1995).
There is still more to learn about the mechanism underlying PC-induced cholesterol reduction. According to several studies, HMG-CoA, also known as 3-hydroxy-3-methylglutaryl-coenzyme A, is the rate-limiting step in the production of cholesterol, appears to be less likely to be synthesized and more likely to be degraded as a result of PC (Kabir and Kimura, 1993; Menéndez et al., 2001). Further research has shown that PC can reduce blood cholesterol by promoting AMP-kinase phosphorylation in mouse liver and hepatoma cell cultures (Banerjee et al., 2011). This clinical research was done to look into the safety and tolerability besides the efficacy of policosanol medication on the lipid panel in Korean persons.
2 Subjects and methods
2.1 The ingredients of the test and placebo products
Table 1 lists the ingredients of the placebo and test products.
Composition of materials
Test product (%)
(Policosanol)Placebo product (%)
(Placebo)
Policosanol (policosanol sugar cane wax alcohol)
6.1
–
Cellulose, Crystalline
45.7
45.7
Maltodextrin
37.8
43.9
Cross linked sodium carboxymethyl cellulose
5.0
5.0
Magnesium stearate
1.0
1.0
Silicon dioxide
1.0
1.0
HPMC
2.5
2.5
Glycerin ester of fatty acid
0.3
0.3
Titanium dioxide
0.3
0.3
Food coloring
0.3
0.3
Total
100
100
2.2 Study population and design
The investigation was executed according to compliance with the ethical guidelines of the Helsinki Declaration (World Medical Association, 2013). The Wonkwang University Korean Medical Hospital's institutional review board (IRB) (Iksan, South Korea) examined and confirmed study procedures. To prepare medical staff to offer consistent intervention, all the investigators employed the same standardized protocols, written instructions, precise directions, and training materials. Throughout a training session for all the investigators, the protocol was taught, and the coordinating center addressed any issues that came up during the study follow-up. Prior to taking part in the trial, each participant signed an informed consent form.
The study's clinical trial registry number is IRB 2020–18. The study involved 80 healthy male and female participants, aged 19 to 75 were divided into two groups for our 8-week, randomized, double-blind, placebo-controlled human trial of individuals with baseline LDL-C (low-density lipoprotein cholesterol) concentrations of at least 100–159 mg/dL in the fasting blood test at the time of screening. According to protocol, 80 healthy volunteers were divided into two groups at random and given either policosanol (20 mg/day) or a placebo (both n = 40) for eight weeks. At day 0 and at the completion of the drug trial, lipid levels in serum were assessed.
2.3 Treatment and exclusion criteria
Test products were identical and labelled clearly before distribution. Treatment was randomly assigned using a 1:1 randomization ratio and a computer-generated random allocation scheme with balanced blocks. Test tablets were given once daily after a meal. Participants having triglyceride levels of 200 mg/dL or higher and total cholesterol levels of 240 mg/dL or higher in the fasting blood test at the time of screening were excluded from the experiment. Individuals were disqualified if they had severe kidney damage, a record of liver problems, a diagnosis of a tumor, were alcoholics, or suffered from chronic diseases. Additionally, individuals who had taken another lipid-lowering drug within six months prior to entering the study, those with a record of allergy to the wax alcohols of Saccharum officinarum (Policosanol), and those who had any additional particular circumstances that, in the doctor's opinion, would threaten their health and well-being during the clinical trial were also excluded. Additionally, the individuals were required to give specifics about their food intake and regular exercise at each visit (Kim et al., 2020; Kang et al., 2016; Nyambe-Silavwe and Williamson, 2016).
2.4 Assessments of safety and tolerability
In the safety analysis, everyone who was randomly assigned and subjected to minimum one study intervention dose was taken into account. Standard laboratory techniques employed at Precise Lab Solution (Jeonju, South Korea) to investigate hematocrit, hemoglobin, RBC, and, WBC were examined using standard laboratory protocols. Blood biochemistry includes leptin, adiponectin, creatinine, blood urea nitrogen (BUN), glucose, gamma-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), creatine kinase (CK), total protein, albumin, total bilirubin, and alkaline phosphatase (ALP). Specific gravity and pH are included in the urine test. Vital signs including blood pressure, heart rate, and body temperature were all measured as part of safety evaluations, and safety occurrences were tracked as well.
2.5 Statistical evaluation
Fisher's exact tests, independent t-tests, chi-square tests, and were utilized to contrast the initial traits of the placebo and test groups. An independent t-test was used to compare the two groups' anthropometric features including urinalysis, and blood biochemistry. The outcomes were displayed as mean ± SD. P < 0.05 was used as the significant level. The SAS computer program version 9.3 was used to carry out all statistical experiments (Cary, North Carolina, USA-based SAS Institute).
3 Results
The consort flow chart (Fig. 1) presents information about the subject's disposition. 25 males (15 randomly assigned to policosanol, 10 to a placebo) and 55 women (25 randomly assigned to policosanol, 30 to a placebo) were included in the study. Two trial subjects from the policosanol group and two from the placebo group withdrew due to pregnancy; this was not related to any adverse effects. The fact that the majority of the features were the same in both groups indicates that randomization was successful because the beginning characteristics of the two groups did not significantly differ from one another (Table 2). Values are presented as mean ± SD or number (%). 1) Analyzed by independent t-test between the groups. 2) Analyzed by chi-square test between the groups. 3) Analyzed by Fisher’s exact test between the groups.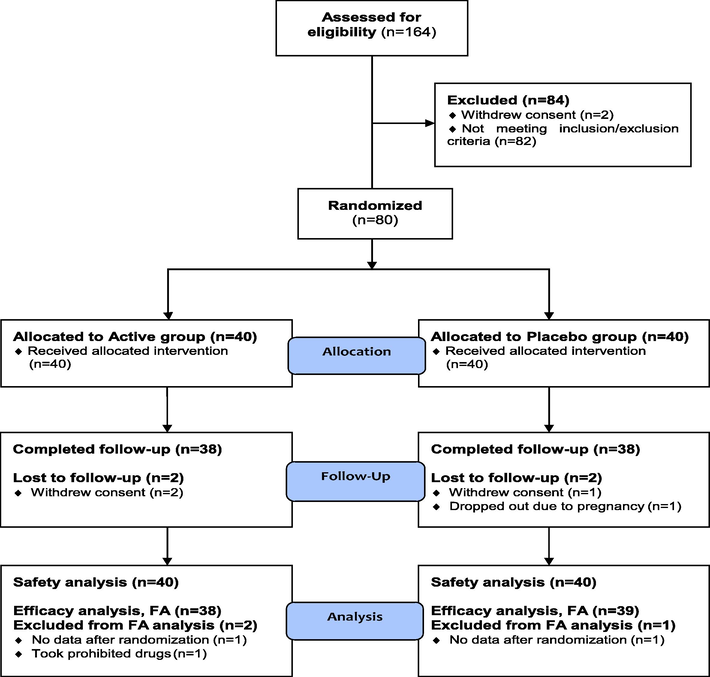
Flowchart for the randomized, double-blind, placebo-controlled human trial.
Policosanol (n = 40)
Placebo (n = 40)
Total (n = 80)
p-value1)
Sex (M/F)
15/25
10/30
25/55
0.228 2)
Age (years)
43.55 ± 14.78
41.68 ± 12.23
42.61 ± 13.51
0.538
Height (cm)
164.68 ± 8.33
163.75 ± 7.72
164.21 ± 7.99
0.608
Weight (kg)
68.18 ± 14.95
65.24 ± 13.64
66.71 ± 14.30
0.361
BMI (kg/m2)
24.95 ± 3.93
24.11 ± 3.18
24.53 ± 3.57
0.299
SBP (mmHg)
122.25 ± 14.95
119.38 ± 13.22
120.81 ± 14.10
0.365
DBP (mmHg)
72.98 ± 11.16
72.78 ± 10.01
72.88 ± 10.54
0.933
Pulse (beats/min)
74.83 ± 10.10
77.93 ± 10.27
76.38 ± 10.24
0.177
Alcohol (n, %)
17 (42.50)
18 (45.00)
35 (43.75)
0.822 2)
Alcohol (units/week)
5.90 ± 3.05
7.78 ± 6.30
6.87 ± 5.02
0.269
Smoking (n, %)
4 (10.00)
5 (12.50)
9 (11.25)
1.000 3)
Smoking (n/day)
8.25 ± 7.89
10.20 ± 3.19
9.33 ± 5.43
0.626
3.1 Primary outcome
After 8 weeks, the LDL-C concentration was considerably lesser in the policosanol group in comparison to the placebo group. According to the statistical examination, level of LDL-C was −4.87 ± 11.30 mg/dL lower in policosanol batch at 8 weeks than they were in placebo batch (p = 0.014). The concentration of total cholesterol in the placebo batch and the policosanol batch were noticeably different after 8 weeks. The statistical study indicates that the total cholesterol concentrations were −6.82 ± 14.32 mg/dL after 8 weeks, they were lowered in the policosanol group versus the placebo group (p = 0.007). Compared to the group receiving a placebo, where triglyceride levels rose, the policosanol group had a drop in triglyceride levels. At week 8, there was a mean change of −9.37 ± 19.27 mg/dL in the policosanol group, compared to 5.33 ± 27.25 mg/dL in placebo batch (p = 0.008). Compared to the placebo group, the HDL-C levels increased in the policosanol group. At week 8, the mean change in the policosanol group was 3.50 ± 4.55 mg/dL, compared to 0.26 ± 6.09 mg/dL in the placebo group (p = 0.010). While non-HDL-C levels rose in the placebo group, they decreased in the policosanol group. At week 8, the mean decrease in the policosanol group was −10.32 ± 13.75 mg/dL, as opposed to 0.56 ± 7.88 mg/dL as respect to the placebo group (p < 0.0001) (Table 3). Values are presented as mean ± SD. 1) Analyzed by independent t-test for change value between the groups. *p < 0.05, **p < 0.01, ***p < 0.001.
Policosanol (n = 38)
Placebo (n = 39)
p-value1)
Baseline
8 week
Change value
Baseline
8 week
Change value
LDL-C
[0 ∼ 140 mg/dL]132.92 ± 16.22
128.05 ± 16.04
−4.87 ± 11.30
125.33 ± 17.17
126.08 ± 19.31
0.74 ± 7.89
0.014*
Total cholesterol
[130 ∼ 220 mg/dL]207.87 ± 23.36
201.05 ± 20.96
−6.82 ± 14.32
209.97 ± 23.52
210.79 ± 25.40
0.82 ± 8.67
0.007**
Triglyceride
[34 ∼ 150 mg/dL]114.50 ± 43.19
105.13 ± 41.01
−9.37 ± 19.27
110.31 ± 44.47
115.64 ± 53.91
5.33 ± 27.25
0.008**
HDL-C
[42 ∼ 74 mg/dL]57.55 ± 11.99
61.05 ± 12.59
3.50 ± 4.55
59.49 ± 12.77
59.74 ± 12.97
0.26 ± 6.09
0.010*
Non HDL-C[-]
(mg/dL)150.32 ± 24.25
140.00 ± 21.46
−10.32 ± 13.75
150.49 ± 21.53
151.05 ± 23.70
0.56 ± 7.88
<0.0001***
3.2 Secondary outcome
Table 4 shows the impact of policosanol on atherogenic parameters in serum; policosanol administration resulted in a decrease in serum T-cholesterol-HDL-C/HDL-C ratio (-0.33 (SD 0.33) v. 0.00 (SD 0.31), p < 0.0001), triglyceride/HDL-C ratio (-0.28 (SD 0.40) v. 0.09 (SD 0.57), p = 0.001) LDL-C/HDL-C ratio (-0.21 (SD 0.27) v. 0.00 (SD 0.21), p = 0.0002), and total cholesterol/HDL-C ratio (-0.33 (SD 0.33) v. 0.00 (SD 0.31), p < 0.0001) when compared to placebo values. Values are presented as mean ± SD. 1) Analyzed by independent t-test for change value between the groups. **p < 0.01, ***p < 0.001.
Policosanol (n = 38)
Placebo (n = 39)
p-value1)
Baseline
8 week
Change value
Baseline
8 week
Change value
Total cholesterol/HDL-C
3.75 ± 0.77
3.42 ± 0.70
−0.33 ± 0.33
3.65 ± 0.75
3.66 ± 0.76
0.00 ± 0.31
<0.0001***
LDL-C/HDL-C
2.41 ± 0.59
2.20 ± 0.58
−0.21 ± 0.27
2.19 ± 0.48
2.19 ± 0.50
0.00 ± 0.21
0.0002***
Triglyceride/HDL-C
2.12 ± 0.97
1.84 ± 0.86
−0.28 ± 0.40
2.02 ± 1.13
2.11 ± 1.27
0.09 ± 0.57
0.001**
(T-cholesterol-HDL-C)/HDL-C
2.75 ± 0.77
2.42 ± 0.70
−0.33 ± 0.33
2.65 ± 0.75
2.66 ± 0.76
0.00 ± 0.31
<0.0001***
3.3 Safety parameters
Without experiencing any negative or adverse reactions, all subjects finished the protocol. Neither of the groups experienced any subject complaints. The biochemistry of the blood (creatinine, BUN, glucose, GGT, LD, ALT, AST, CK, ALP, total bilirubin, albumin, total proteins), CBC (WBC, RBC, hemoglobin, hematocrit, platelets count), and urinalysis (specific gravity, pH) did not alter significantly after the 8-week experiment (Table 5). Values are presented as mean ± SD. 1) Analyzed by independent t-test for change value between the groups.
Policosanol (n = 40)
Placebo (n = 40)
p-value1)
Baseline
12 week
Change value
Baseline
12 week
Change value
CBC
WBC (✕103/μl)
5.75 ± 1.39
5.75 ± 1.56
0.00 ± 1.08
5.50 ± 1.65
5.54 ± 1.68
0.03 ± 1.24
0.908
RBC (✕103/μl)
4.68 ± 0.46
4.70 ± 0.42
0.02 ± 0.25
4.46 ± 0.44
4.44 ± 0.41
−0.01 ± 0.20
0.553
Hemoglobin (g/dL)
14.18 ± 1.31
14.12 ± 1.15
−0.06 ± 0.73
13.48 ± 1.60
13.46 ± 1.54
−0.02 ± 0.73
0.795
Hematocrit (%)
42.09 ± 3.65
41.83 ± 3.17
−0.26 ± 2.37
40.36 ± 4.50
40.10 ± 4.14
−0.27 ± 2.17
0.984
Platelets count (✕103/μl)
254.45 ± 66.69
247.23 ± 47.68
−7.23 ± 52.77
276.63 ± 59.66
280.08 ± 59.44
3.45 ± 29.97
0.270
Blood biochemistry
Total protein (g/dL)
6.89 ± 0.43
6.97 ± 0.48
0.08 ± 0.36
6.86 ± 0.34
6.88 ± 0.38
0.03 ± 0.35
0.530
Albumin (g/dL)
4.29 ± 0.26
4.28 ± 0.23
−0.01 ± 0.23
4.18 ± 0.22
4.20 ± 0.22
0.01 ± 0.20
0.639
Total bilirubin (mg/dL)
0.78 ± 0.29
0.76 ± 0.32
−0.02 ± 0.33
0.73 ± 0.43
0.76 ± 0.33
0.03 ± 0.28
0.446
ALP (IU/L)
178.15 ± 57.48
185.35 ± 63.93
7.20 ± 23.76
162.18 ± 43.59
167.93 ± 46.79
5.75 ± 22.21
0.779
CK (U/L)
117.43 ± 71.04
124.03 ± 69.64
6.60 ± 59.81
124.95 ± 113.20
109.98 ± 91.20
−14.98 ± 125.00
0.329
AST (IU/L)
24.68 ± 10.25
26.18 ± 10.30
1.50 ± 11.87
22.38 ± 6.49
21.70 ± 5.57
−0.68 ± 6.35
0.311
ALT (IU/L)
23.35 ± 16.96
23.55 ± 16.33
0.20 ± 15.87
19.45 ± 7.59
18.88 ± 7.04
−0.58 ± 7.44
0.781
LDH (U/L)
185.68 ± 39.67
196.35 ± 64.35
10.68 ± 60.91
178.03 ± 36.95
178.33 ± 34.96
0.30 ± 22.21
0.316
gamma-GT GGT (IU/L)
23.78 ± 15.55
24.55 ± 19.46
0.78 ± 7.13
22.18 ± 10.92
21.85 ± 12.36
−0.33 ± 8.21
0.524
Glucose (mg/dL)
86.10 ± 11.47
87.28 ± 12.18
1.18 ± 7.13
84.15 ± 9.70
84.65 ± 8.07
0.50 ± 9.56
0.721
BUN (mg/dL)
12.95 ± 2.95
12.25 ± 3.12
−0.69 ± 3.10
12.97 ± 4.07
12.63 ± 2.64
−0.34 ± 3.32
0.628
Creatinine (mg/dL)
0.90 ± 0.17
0.89 ± 0.17
−0.02 ± 0.07
0.90 ± 0.15
0.88 ± 0.14
−0.02 ± 0.08
0.883
Urinalysis
Specific gravity
1.02 ± 0.01
1.02 ± 0.01
0.00 ± 0.01
1.02 ± 0.01
1.02 ± 0.01
0.00 ± 0.01
0.913
pH
5.81 ± 0.72
6.19 ± 0.83
0.38 ± 0.86
6.06 ± 0.74
6.11 ± 0.94
0.05 ± 0.99
0.120
4 Discussion
Controlling the blood's level of cholesterol is the therapeutic objective for treating hyperlipidemia and the CVD it causes. The blood LDL, and TG levels are reduced while HDL levels are raised in order to control cholesterol. For managing cholesterol, there are already drugs accessible, however, the patient may suffer from the adverse effects and expenses of taking them. The statin-induced myopathies are the most common adverse effects (AE) with statins, which were initially obtained from fungus (Azemawah et al., 2019). Nausea, discomfort, cholelithiasis, cholecystitis, hepatic problems, and coagulation abnormalities are among the adverse events (AE) associated with fibrates, a different family of medication used to treat hyperlipidemia (Okopień et al., 2018). Despite these findings, a natural remedy could be a less expensive option with fewer and milder side effects than current drugs.
This randomized clinical trial examined the impact of policosanol on individuals' serum lipid levels. According to the latest findings, 8 weeks of policosanol use significantly decreased TG, TC, and LDL-C levels while concurrently raising HDL-C levels and significantly lowering the ratios of total cholesterol to HDL-C, LDL-C to HDL-C, triglycerides to HDL-C, and T-cholesterol to HDL-C. Prior research provided the findings of a randomized, double-blind, placebo-controlled investigation on the effects of policosanol on elderly adults with type II hyperlipidemia. Considering the total cholesterol findings (6.1 mmol/L), the participants took 5–10 mg of policosanol each day for a year. In the long run, the policosanol therapy group considerably reduced the LDL-C/HDL-C ratio (22.2%), TG (11.9%), TC (15.4%), and LDL-C (20.5%), while increasing HDL-C by as much as 12.7% (Castaño et al., 2002b).
Furthermore, policosanol showed both efficiency and tolerance in individuals with high global cardiovascular risk, resulting in a marked decline in serum LDL-C/HDL-C, TC/HDL-C, TG, TC, and LDL-C as well as an increase in HDL-C. No drug-related adverse clinical or biochemical consequences were seen after the effective course of treatment (Castaño, G., et al., 1999). Uncertainty exists regarding the precise molecular pathways by which policosanol lowers lipid levels. However, other investigations have determined that the activity of policosanol involves a variety of mechanisms, involving the activation of AMP-kinase, the inhibition of cholesteryl ester transfer protein, and the suppression of HMG-CoA reductase (Kim et al., 2017; Singh et al., 2006; Mccarty, 2002).
There is presently no supplement that has been shown to be both secure and successful in controlling hyperlipidemia. In the current trial, we demonstrate the safety and efficacy of policosanol in the regulation of hyperlipidemia. Policosanol intake boosted HDL-C and decreased blood levels of LDL-C, TG, and TC. This study also demonstrated the safety of taking policosanol supplements, as there were no significant negative side effects. Furthermore, policosanol can be regarded as secure and useful in assisting individuals in controlling their blood cholesterol. The study showed policosanol, a natural wax, helped control cholesterol levels by regulating lipid markers. For individuals with mild to severe hypercholesterolemia, policosanol is a safe and effective therapeutic choice because it had no harmful effects on the individuals' physiological parameters and did not change their health status.
5 Conclusion
There were some limitations to the current investigation. To begin with, 80 participants participated in the study. To come to a firmer conclusion about policosanol efficacy, it would be beneficial to carry out a follow-up study with a bigger sample size and in more than one location. In addition, our study lasted for 8 weeks only. To get to a firmer conclusion regarding policosanol long-term efficiency, it could be beneficial to conduct research over a longer period of time.
Acknowledgement
This present study was supported by Wonkwang University, South Korea in 2021.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- One-year dog toxicity study of D-002, a mixture of aliphatic alcohols. J. Appl. Toxicol.. 2001;21(3):179-184.
- [CrossRef] [Google Scholar]
- Effect of policosanol in lowering-cholesterol levels in patients with type II hypercholesterolemia. Curr. Ther. Res. Clin. Exptl.. 1995;56:176-182.
- [Google Scholar]
- 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol.. 2019;74(10):e177-e232.
- [CrossRef] [Google Scholar]
- Effect of policosanol on platelet aggregation in type II hypercholesterolemic patients. Int. J. Tissue React.. 1998;20(4):119-124.
- [Google Scholar]
- Protective effect of policosanol on atherosclerotic lesions in rabbits with exogenous hypercholesterolemia. Braz. J. Med. Biol. Res.. 2000;33(7):835-840.
- [CrossRef] [Google Scholar]
- State of the art comprehensive review of individual statins, their differences, pharmacology, and clinical implications. Cardiovasc. Drugs Ther.. 2019;33(5):625-639.
- [CrossRef] [Google Scholar]
- Activation of AMP-kinase by policosanol requires peroxisomal metabolism. Lipids. 2011;46(4):311-321.
- [CrossRef] [Google Scholar]
- Effects of policosanol on primary hypercholesterolemia: A 3-year open follow-up. Curr. Ther. Res. Clin. Exptl.. 1997;58:868-875.
- [Google Scholar]
- A long-term, open-label study of the efficacy and tolerability of policosanol in patients with high global coronary risk. Cur. Ther. Res.. 1999;60(7):379-391.
- [CrossRef] [Google Scholar]
- Effects of policosanol on older patients with hypertension and type II hypercholesterolaemia. Drugs R. D.. 2002;3(3):159-172.
- [CrossRef] [Google Scholar]
- Effects of policosanol and lovastatin on lipid profile and lipid peroxidation in patients with dyslipidemia associated with type 2 diabetes mellitus. Int. J. Clin. Pharmacol. Res.. 2002;22(3–4):89-99.
- [Google Scholar]
- Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J.. 2017;38(32):2459-2472.
- [CrossRef] [Google Scholar]
- A pharmacological surveillance study of the tolerability of policosanol in the elderly population. Am. J. Geriatr. Pharmacother.. 2004;2(4):219-229.
- [CrossRef] [Google Scholar]
- Long-term safety and efficacy of lowering low-density lipoprotein cholesterol with statin therapy: 20-year follow-up of west of Scotland coronary prevention study. Circulation. 2016;133(11):1073-1080.
- [CrossRef] [Google Scholar]
- MRC/BHF Heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet (London, England). 2002;360(9326):7-22.
- [CrossRef] [Google Scholar]
- Biodistribution and metabolism of orally administered octacosanol in rats. Ann. Nutr. Metab.. 1993;37(1):33-38.
- [CrossRef] [Google Scholar]
- Effect of supplementation of low-molecular-weight chitosan oligosaccharide, GO2KA1, on postprandial blood glucose levels in healthy individuals following bread consumption. Food Sci. Biotechnol.. 2016;25(3):911-914.
- [CrossRef] [Google Scholar]
- Statins for primary prevention of cardiovascular disease: review of evidence and recommendations for clinical practice. Med. Clin. North Am.. 2017;101(4):689-699.
- [CrossRef] [Google Scholar]
- Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med.. 2017;39(4):889-899.
- [CrossRef] [Google Scholar]
- Effects of statins for primary prevention in the elderly: recent evidence. J. Lipid Atheroscler.. 2020;9(1):1-7.
- [Google Scholar]
- Acute effects of Amomum villosum Lour. fruit extract on postprandial glycemia and insulin secretion: A single-blind, placebo-controlled, crossover study in healthy subjects. Saudi. J. Biol. Sci.. 2020;27(11):2968-2971.
- [CrossRef] [Google Scholar]
- Long-term effects of statin treatment in elderly people: extended follow-up of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER) PloS One. 2013;8(9):e72642.
- [Google Scholar]
- Effects of policosanol in patients with type II hypercholesterolemia and additional coronary risk factors. Clin. Pharmacol. Ther.. 1999;65(4):439-447.
- [CrossRef] [Google Scholar]
- Policosanol safely down-regulates HMG-CoA reductase - potential as a component of the Esselstyn regimen. Med. Hypotheses. 2002;59(3):268-279.
- [CrossRef] [Google Scholar]
- Effects of policosanol treatment on the susceptibility of low density lipoprotein (LDL) isolated from healthy volunteers to oxidative modification in vitro. Br. J. Clin. Pharmacol.. 2000;50(3):255-262.
- [CrossRef] [Google Scholar]
- Policosanol modulates HMG-CoA reductase activity in cultured fibroblasts. Arch. Med. Res.. 2001;32(1):8-12.
- [CrossRef] [Google Scholar]
- Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: a randomised, controlled, single-blind, cross-over intervention. Br. J. Nutr.. 2016;116(3):443-450.
- [CrossRef] [Google Scholar]
- Benefits and risks of the treatment with fibrates–a comprehensive summary. Expert Rev. Clin. Pharmacol.. 2018;11(11):1099-1112.
- [CrossRef] [Google Scholar]
- Long-term follow-up of lipid-lowering trials. Curr. Opin. Lipidol.. 2015;26(6):572-579.
- [CrossRef] [Google Scholar]
- ACC/AHA lipid guidelines: Personalized care to prevent cardiovascular disease. Cleve. Clin. J. Med.. 2020;87(4):231-239.
- [CrossRef] [Google Scholar]
- Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther.. 2006;318(3):1020-1026.
- [CrossRef] [Google Scholar]
- American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139-e596.
- [CrossRef] [Google Scholar]
- Effects of policosanols and phytosterols on lipid levels and cholesterol biosynthesis in hamsters. Lipids. 2003;38(2):165-170.
- [CrossRef] [Google Scholar]
- Effects of dietary fat intake on HDL metabolism. J. Clin. Med. Res.. 2015;7(3):145-149.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102769.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







