Translate this page into:
Rational drug design, synthesis, and in vivo biological activity of new indolyl-imidazolone hybrids as potential and safer non-steroidal anti-inflammatory agents
⁎Corresponding authors. drasifhusain@yahoo.com (Asif Husain), shahalam@nu.edu.om (Shah Alam Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
The study aimed to synthesize and evaluate the potential anti-inflammatory and analgesic activities of rationally designed hybrid molecules of imidazolone and indole nuclei linked through a methylene bridge.
Methods
Indolyl-imidazolone hybrids were synthesized in three simple steps starting from 2-phenyl-1H-indole (1). In the first step, compound 1 was converted to 2-phenyl-1H-indole-3-carbaldehyde (2) using standard conditions of the Vilsmeier Haack reaction. Benzoyl glycine was reacted with 2 (step 2) followed by treatment with aromatic/aliphatic amines (step 3) to furnish the indolyl-imidazolone hybrids. In vivo anti-inflammatory and analgesic activity along with ulcerogenicity of the prepared hybrids were evaluated in experimental animals. Molecular properties and pharmacokinetic profile were also predicted using online computational software. Cyclooxygenase-2 (COX-2) enzyme (PDB: 3pgh) was used for molecular docking studies. Indomethacin and aspirin were used as reference compounds for the comparison purpose.
Results
The percentage inhibition in edema in rats and reduction in frequency of acetic acid induced writhes in mice indicated that two compounds namely 3-(3-Hydroxpropyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4g) and 3-(2,4-Dinitrophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]- 4H-imidazol-4-one (4b) could be useful in treating pain and inflammatory conditions. Both the hybrid molecules exhibited better biological spectrum than the standard drug indomethacin. Additionally, both the potent compounds were noted to be less ulcerogenic than indomethacin. Pharmacokinetic profile predicted using ADMETsar and SwissADME cheminformatic software indicated compound 4g to be orally bioavailable with high blood brain barrier permeability. However, molecular docking studies revealed that compound 4b binds to COX-2 enzyme more strongly than 4g as indicated by a lower binding energy and formation of hydrogen bond interactions with amino acid residues in the binding pocket.
Conclusions
It could be concluded that hybrid compounds 4b and 4g are promising lead candidates and should be further studied to develop compounds for the treatment of inflammatory conditions.
Keywords
Analgesic
Anti-inflammatory
Hybrid
Imidizalolone
Indole
Ulcerogenic
1 Introduction
Synthetic low molecular weight compounds such as paracetamol, ibuprofen, indomethacin, diclofenac, celecoxib etc., belonging to Non-steroidal anti-inflammatory drugs (NSAIDs) constitute a frequently and most widely used classes of medicines across the globe to treat pain, fever, inflammatory diseases and in particular arthritis (Moore et al., 2019, Abdu et al., 2020). But the chronic use of NSAIDs leads to gastroenteropathy including gastric damage, gastric upset, heartburn, nausea, irritation, bleeding besides hepatorenal toxicity (Allison et al., 1992, Cryer and Kimmey 1998, Khan et al., 2018). These side effects of conventional NSAIDs are produced because of inhibition of a constitutive cytoprotective cyclooxygenase-1 (COX-1) enzyme along with inducible form of COX-2. Expression of COX-2 enzyme mainly occurs in inflammatory cells and therefore development of drugs like celecoxib, rofecoxib that selectively inhibit COX-2 enzyme offered the hope in a battle against the inflammatory diseases because these were demonstrated to be safer and less ulcerogenic than non-selective NSAIDs (Khan et al., 2019). But long-term usage of two promising COX-2 inhibitors namely rofecoxib and valdecoxib were found to cause cardiovascular complications in patients and were eventually withdrawn from the market. Failure of these two selective COX-2 inhibitors which were thought by many as ‘ideal’ anti-inflammatory agents’ did had its setbacks but it further pressed the scientific community to search for new & safer NSAIDs (Verrico et al., 2003, Dogné et al., 2005, Bindu et al., 2020).
Indole nucleus is a versatile scaffold which is also a part of the structure of indomethacin; a clinically used NSAID. It is also a pharmacophore of analgesic drug, pravodoline. Several research studies and review articles have reported that compounds endowed with indole moiety exhibits varied and important pharmacological activities in addition to anti-inflammatory and analgesic actions (Rani et al., 2004, Singh et al., 2008, Bi et al., 2010, Chavan et al., 2011, Thanikachalam et al., 2019).
Imidazole, a five membered heterocyclic moiety that contains two nitrogen atoms on the first and third positions, is a bioactive moiety present in several synthetic and natural products. Imidazole with a ketonic functionality is known as imidazolone, which is also bioactive. It has been studied extensively owing to its wide spectrum of medicinal properties (Jung et al., 2004, Hassanein et al., 2008). Imidazole ring is present in several clinically used drugs such as metronidazole (antimicrobial), ketoconazole, clotrimazole (antifungal), midazolam (sedative), cimetidine (H2-blocker; antiulcer), pantoprazole, omeprazole (proton pump inhibitor, antiulcer), clonidine (antihypertensive), eprosartan (vasodilator), etomidate (general anesthetic), fexinidazole (anti-trypanosomiasis) and dacarbazine (anticancer), etc. They have also been utilized as important structural fragments and building blocks to synthesize an array of heterocyclic compounds of potential pharmaceutical interest (Kumar 2010, Chopra et al., 2020). It has been shown that combining two or more classes of biologically active heterocyclic nuclei thorough hybridization result in compounds that are capable of exhibiting enhanced bioactivity (Husain et al., 2016, Kaur and Silakari 2017, Lucarini et al., 2020, Husain et al., 2021). Imidazole is a common structural feature of both H2 blockers and proton pump inhibitors. Moreover, it is also a structural component of anti-inflammatory molecule flumizole. Thus, we contemplated that imidazole ring containing compounds could impart gastroprotective activity to the synthesized compounds. Furthermore, it was hypothesized that clubbing imidazole with indole moiety, a pharmacophore of NSAID indomethacin and many other clinically useful drugs, through a suitable linker in one molecule would yield potent and safer NSAIDs.
A rational drug design approach was used in the current study to synthesize some new low molecular weight indole derivatives linked to imidazolone moiety in a hope that such a combination of bioactive moieties may enhance overall biological spectrum of the synthesized hybrid compounds along with their safety profile. Hence, we outline here synthesis and potential anti-inflammatory and analgesic properties of hybrid molecules of imidazolone and indole nuclei linked through a methylene bridge. The rational drug design strategy adopted to synthesize the indolyl-imidazolone hybrid molecules is presented in Fig. 1.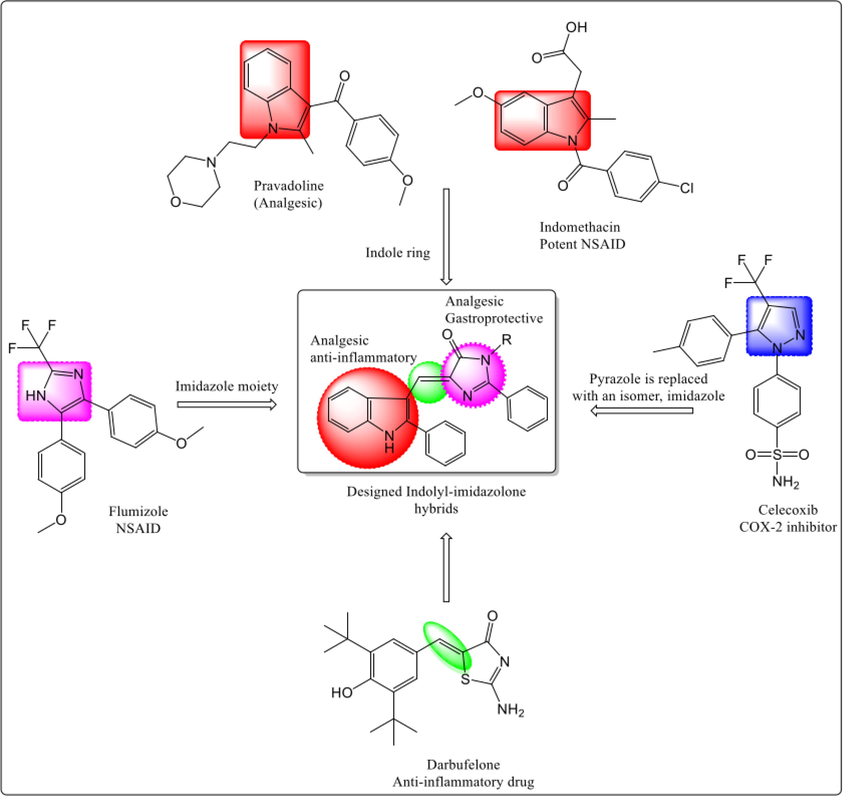
Rational drug design approach used to prepare indolyl-imidazolone hybrids.
2 Experimental
All the chemicals and solvents used in the experiment were of high purity and used without purification. Melting points were determined on a paraffin bath using open capillary tubes. Microanalysis was carried out on Perkin-Elmer model 240 analyzer. Chemical structures were characterized by acquiring analytical data using IR, NMR and mass spectrometry techniques. Proton (1H) NMR data was acquired either on a Varian E-360 MHz or Bruker spectrospin DPX-300 MHz instrument. Tetramethylsilane (TMS) was employed as an internal reference compound for chemical shift values. Molecular weight and molecular fragmentation pattern were studied with the help of mass spectra acquired on a Jeol JMS-D 300 instrument at 70 eV. Completion of a chemical reactions was regularly monitored on TLC plates. A mixture of benzene and petroleum ether (8:2), benzene, acetone (8:2) or toluene, ethyl acetate and formic acid (5:4:1) was used for developing chromatogram. Iodine vapors were used to locate the spots on TLC plates.
2.1 Synthesis of 2-phenyl-1H-indole (1)
It was synthesized following a reported method (Khan et al., 2012). Solid product obtained after usual work up was further purified by crystallization using benzene solvent to get colorless shining flakes of 2-phenyl-1H-indole (1). Yield- 75.7%, Rf 0.72 (Benzene: Petroleum ether; 8:2), m.p. 188–189 °C [Reported m.p. 188–189 °C]. The spectral data is in agreement with the earlier published data (Khan et al., 2012).
2.2 Synthesis of 2-phenyl-1H-indole-3-carbaldehyde (2)
Compound (2) was synthesized following a reported method. (Khan et al., 2012). The crude product obtained after usual work up was further purified by crystallization from acetone to obtain colorless flakes. Yield- 88%, Rf 0.32 (Benzene: Petroleum ether; 8:2), m.p. 246–247 °C [Reported m.p. 247 °C]. The spectral data is in agreement with the earlier published data (Khan et al., 2012).
2.3 Synthesis of 2-phenyl-4-[(2-phenyl-indolin-3-yl) methylene)-oxazol-5(4H)-one (3)
It was synthesized following a reported method. It was crystallized using acetone to yield orange needles. Yield 54.5% (Khan et al., 2012), Rf 0.82 (Toluene: Ethyl acetate: Formic acid = 5:4:1), m.p. 209–210 °C [Reported m.p. 210 °C]. The spectral data is in agreement with the earlier published data (Khan et al., 2012).
2.4 General method for preparation of 2,3,5-trisubstituted-4H-imidazol-4-one derivatives (4a-g)
Compound (3) (0.01 mol, 3.6 g) and various aliphatic/aromatic amine (0.01 mol) in equimolar amounts were thoroughly mixed and then fused on an oil bath for 60–75 min. The resulting mixture was allowed to cool down to ambient temperature and then crushed ice was added to it which resulted in the formation of precipitates. The solid mass was filtered and was purified by crystallization using a suitable solvent(s) to get pure crystals.
2.4.1 3-(4-Nitrophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]-4H-imidazol-4-one (4a)
Dark orange crystals, crystallized using ethanol/methanol mixture, yield 64%, Rf 0.52 (Benzene: Acetone, 8:2), m.p. 191–193 °C; IR (cm−1, KBr): 3310 (NH), 3049, 1735 (C = O), 1645, 1585. 1H NMR (CDCl3): δ 7.11–7.73 (m, 17H, H-4,5,6 of indole + 14H of three phenyl rings), 8.05 (br, 1H, H-7-indole), 8.21 (s, 1H, olefinic proton), 11.23 (s, 1H, –NH); MS: (m/z) 484 (isotopic M+); C30H20N4O3.
2.4.2 3-(2,4-Dinitrophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4b)
Orange crystals, crystallized using ethanol/methanol mixture, yield 46%, Rf 0.50 (Benzene: Acetone, 8:2), m.p. 203–205 °C; IR (cm−1, KBr): 3275 (NH), 2997, 1731 (C = O), 1619, 1531. 1H NMR (CDCl3): δ 7.03–7.68 (m, 16H, H-4,5,6 of indole + 13H of three phenyl rings), 7.89 (br, 1H, H-7 of indole), 8.20 (s, 1H, olefinic proton), 11.11 (s, 1H, –NH); MS: (m/z) 529 (isotopic M+); C30H19N5O5.
2.4.3 3-(4-Fluorophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4c)
Orange-red shining crystals, crystallized using ethanol/methanol mixture, yield 77.5%, Rf 0.46 (Benzene: Petroleum ether, 8:2), m.p. 215–217 °C; IR (cm−1, KBr): 3282 (NH), 3005, 1726 (C = O), 1627, 1581. 1H NMR (CDCl3): δ 6.96–7.53 (m, 17H, H-4,5,6 of indole + 14H of three phenyl rings), 7.92 (br, 1H, H-7 of indole), 8.06 (s, 1H, olefinic proton), 11.19 (s, 1H, –NH); MS: (m/z) 457 (M+); C30H20N3OF.
2.4.4 3-(2-Fluorophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4d)
Orange shining crystals, crystallized using ethanol/methanol mixture, yield 57%, Rf 0.41 (Benzene: Petroleum ether, 8:2), m.p. 222–223 °C; IR (cm−1, KBr): 3290 (NH), 2998, 1734 (C = O), 1637, 1583; 1H NMR (CDCl3): δ 6.91–7.60 (m, 17H, H-4,5,6 of indole + 14H of three phenyl rings), 7.77 (br, 1H, H-7 of indole), 7.93 (s, 1H, olefinic proton), 11.16 (s, 1H, –NH); MS: (m/z) 457(M+); C30H19N3OF.
2.4.5 3-Benzyl-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]-4H-imidazol-4-one (4e)
Brown crystals, crystallized using ethanol/methanol mixture, yield 52%, Rf 0.64 (Benzene: Petroleum ether, 8:2), m.p. 235–236 °C; IR (cm−1, KBr): 3463, 3239 (NH), 3023, 1731 (C = O), 1626, 1481; 1H NMR (DMSO‑d6): δ 3.45 (d, 2H, -N-CH2-), 7.10–7.63 (m, 18H, H-4,5,6 of indole + 15H of three phenyl rings), 7.81 (br, 1H, H-7 of indole), 7.93 (s, 1H, olefinic proton), 11.23 (s, 1H, –NH); MS: (m/z) 453 (M+). C31H23N3O.
2.4.6 3-(2-Hydroxpropyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4f)
Reddish brown crystals, crystallized using ethanol/methanol mixture, yield 44%, Rf 0.40 (Benzene: Petroleum ether, 8:2), m.p. 218–219 °C; IR (cm−1, KBr): 3471, 3293 (NH), 3011, 1723 (C = O), 1622, 1487; 1H NMR (DMSO‑d6): δ 1.19 (d, 3H, –CH3), 3.46 (m, 1H, –CH-OH), 3.91 (d, 2H, -N-CH2-), 5.31 (s, 1H, –OH), 7.09–7.61 (m, 13H, H-4,5,6 of indole + 10H of two phenyl rings), 7.87 (br, 1H, H-7 of indole), 8.17 (s, 1H, olefinic proton), 11.12 (s, 1H, –NH); MS: (m/z) 421 (M+); C27H23N3O2.
2.4.7 3-(3-Hydroxpropyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl)methylene]-4H-imidazol-4-one (4g)
Red crystals, crystallized using ethanol/methanol mixture, yield 53.4%, Rf 0.46 (Benzene: Petroleum ether, 8:2), m.p. 265–266 °C; IR (cm−1, KBr): 3482, 3286 (NH), 3010, 1732 (C = O), 1630, 1489; 1H NMR (DMSO‑d6): δ 1.85 (m, 2H, –CH2-), 3.54 (m, 2H, –CH2-OH), 3.68 (t, 2H, -N-CH2-), 5.1 (s, 1H, –OH), 7.09–7.61(m, 13H, H-4,5,6 of indole + 10H of two phenyl rings), 7.79 (br, 1H, H-7 of indole), 8.18 (s, 1H, olefinic proton), 11.24 (s, 1H, –NH); MS: (m/z) 421 (M+); C27H23N3O2.
2.5 Pharmacology
The prepared molecules were tested for their anti-inflammatory and analgesic activities in Wistar Albino rats (175–200 g) and Albino mice (20–25 g), respectively with rodents of either sex used. It was after obtaining an approval from the Institutional Animal Ethics Committee (IAEC) that the studies were conducted. Animals were handled humanely and in the most acceptable manner throughout the course of the study. Experimental rats/mice were kept in cages in groups of six at the ambient temperature of 25 ± 2 °C with a 12 h light/12 h dark cycle, unhindered access to food and water ad libitum was provided. Compounds displaying good anti-inflammatory activity (greater than 60% inhibition) had then been tested further for their analgesic action through the acetic acid-induced writhing test. The molecules that that had been tested for their analgesic action were also assessed for ulcerogenicity.
2.5.1 Anti-inflammatory activity
The prepared hybrid compounds were investigated for the anti-inflammatory activity using the carrageenan-induced rat paw edema (CIRPE) method (Winter et al., 1962). The rats were arbitrarily segregated into nine groups with six in each having a control group, standard group and seven test compounds groups. The rats in control group were given only 0.5% carboxymethyl cellulose (CMC) suspension while those in other groups were treated with prepared molecules (4a-g) and standard drug (Indomethacin) at a dose level of 20 mg/kg p.o. Percentage inhibition of edema (anti-inflammatory activity) was calculated following a reported method of Winter et al., using the standard formula. The results of the anti-inflammatory activity are presented in Table 1. nt: not tested; Doses: anti-inflammatory (20 mg/kg), analgesic (25 mg/kg) and ulcerogenic activity (60 mg/kg). *p < 0.05; **p < 0.01; aMean ± SEM, n = 6, Relative to the standard, indomethacin and aspirin respectively for anti-inflammatory and analgesic activity, one-way ANOVA followed by Dunnett’s t test was used for analysis of results; bRelative to their respective control and data were analyzed by one-way ANOVA followed by Dunnett’s t test.
S. No.
Treatment
Anti-inflammatory activity (% inhibition)a
Analgesic activity (% protection)
Ulcerogenic activity (severity index)b
1.
Control
–
–
–
2.
Indomethacin
66.85 ± 0.81
–
2.25 ± 0.21
3.
Aspirin
Nt
62.22 ± 0.80
Nt
4.
4a
57.10 ± 0.81*
Nt
Nt
5.
4b
80.20 ± 0.78**
80.74 ± 0.71**
0.91 ± 0.35*
6.
4c
66.85 ± 0.83
83.33 ± 0.74**
1.25 ± 0.21*
7.
4d
52.50 ± 0.78**
Nt
Nt
8.
4e
62.00 ± 0.83*
76.85 ± 0.78*
0.91 ± 0.25**
9.
4f
62.00 ± 0.79*
82.22 ± 0.74**
0.83 ± 0.25**
10.
4g
81.10 ± 0.74**
85.50 ± 0.79**
1.16 ± 0.25*
2.5.2 Analgesic activity
Analgesic activity was assessed using a commonly used acetic acid induced writhing method (Siegmund et al., 1957) in Albino mice. A total of five compounds (4b, 4c, 4e, 4f and 4g) that exhibited more than 60% inhibition of edema i.e., showed good anti-inflammatory activity were chosen to undergo further screening for their analgesic activity. The writhing inducing agent used was a 1% aqueous acetic acid solution (i.p. injection; 0.1 mL). Seven groups (1 group for control; 1 group for standard drug and five groups for test compounds) having six mice in each group were made. Before receiving the acetic acid injections, the mice were kept individually in the cages and habituated for 30 min. Analgesic activity was tested after i.p. administration of test compounds and the positive control (aspirin) at the dose of 25 mg/kg. All the molecules were injected as CMC suspension (1%). Control group received only 1% CMC. 0.1 mL of 1% acetic acid solution was administered to mice intraperitoneally (i.p.) after 20 min of drug administration and after a further 20 min, severity of writhing response was recorded. The analgesic activity is calculated as % protection. The results of the analgesic activity are presented in Table 1.
2.5.3 Acute ulcerogenicity
Five compounds (4b, 4c, 4e, 4f and 4g) were tested for their ulcerogenic potential after administration of these or positive control indomethacin to the rats (Cioli et al., 1979) with a dose of 60 mg/kg. Control group rats received only vehicle (1% CMC suspension) through oral route. Almost a day (24 h) prior to the administration of these compounds’ food was stopped while water was allowed. Past the test/standard treatment, normal diet of the rats was resumed for 17 h and then were sacrificed. Removal of the stomach and it’s opening along the greater curvature was done next after which it was washed with distilled water and with the help of normal saline, was gently cleaned. A magnifying glass was used for examining the mucosal damage. The assessment of the mucosal damage was done as per the standard scoring system of Cioli et al. Table 1 displays the result of the ulcerogenicity experiment.
2.6 Analysis of the computational data for molecular properties alongside pharmacokinetic profile
ChemDraw 16 was used to draw the 2D chemical structures of hybrid molecules. The generated SMILES notations were then used to obtain the molecular properties after feeding it into the Molinspiration online cheminformatic software (https://www.molinspiration.com). The oral bioavailability was assessed based on the violations of Lipinski’s rule of five for molecular weight, logP, hydrogen bond donor and hydrogen bond acceptor values. The compiled data are presented in Table 2. Pharmacokinetic profile (ADMET) of hybrid molecules was also generated using two online bioinformatic prediction tools viz., admetSAR and Swiss ADME. The predicted data for ADME parameters are shown in Table 3. TPSA: total polar surface area; HBA: hydrogen bond acceptor; HBD: hydrogen bond donor; MW: molecular weight; nRot bonds: no of rotatable bonds: INMT: Indomethacin. GIA: Gastrointestinal absorption; BBBp: blood brain barrier permeation; RAT: LD50, rat acute toxicity in mol/kg; FT: pLC50 of fish toxicity in mg/L.
Comp no
miLogP
TPSA
MW
HBA
HBD
nRot bonds
Violation of Lipinski rule
4a
6.33
96.51
484.51
7
1
5
1
4b
6.43
142.34
529.51
10
1
6
2
4c
6.54
50.69
457.51
4
1
4
1
4d
6.54
50.69
457.51
4
1
4
1
4e
6.70
50.69
453.55
4
1
5
1
4f
4.83
70.92
421.50
5
2
5
0
4g
4.74
70.92
421.50
5
2
6
0
INMT
3.99
68.54
357.79
5
1
4
0
Comp no
GIA
BBBp
RAT
FT
p-gp substrate
CYP1A2 inhibitor
CYP2C19 inhibitor
CYP2C9 inhibitor
CYP2D6 inhibitor
CYP3A4 inhibitor
4a
Low
No
2.380
1.570
No
No
Yes
No
No
No
4b
Low
No
2.253
1.134
No
No
Yes
No
No
No
4c
High
No
2.718
0.841
No
No
No
No
No
No
4d
High
No
2.718
0.841
No
No
No
No
No
No
4e
High
Yes
2.725
0.963
No
No
No
No
No
No
4f
High
Yes
2.707
1.086
Yes
No
Yes
Yes
No
Yes
4g
High
Yes
2.509
1.330
Yes
Yes
Yes
Yes
No
Yes
INMT
High
Yes
4.072
0.261
No
Yes
Yes
Yes
No
No
2.7 Molecular docking studies
Two most promising compounds (4b and 4g) which exhibited potent anti-inflammatory and analgesic spectrum were then further selected for molecular docking analysis. Energy of 2D structures (.mol files) of both the ligands and reference drug indomethacin were minimized using CHARMM force field in the discovery studio and were saved as.pdb files. 3D structure of COX-2 protein (PDB: 3pgh) having a resolution of 2.50 Å and with a non-selective ligand was downloaded from the RCSB site Water and hetero atoms were deleted within the protein structures while hydrogen atoms attached and then energy minimized structures were saved as.pdb files using Accelrys drug discovery studio (DS). Molecular docking studies of indolyl-imidazolone hybrids on to the 3pgh were carried out using recent version of Auto Dock Vina 1.1.2 (Trott and Olson 2010) on windows 10 operating system.
3 Results and discussion
3.1 Chemistry
A set of seven new indolyl-imidazolone hybrid conjugates was prepared as illustrated in Scheme 1. A methylene linker was used to connect 2-phenyl-3- indolyl to the carbon atom present at the 5th position of 4-imidazolone moiety. A phenyl ring at 2nd position and different aliphatic/aromatic substituents were placed at the nitrogen atom at 3rd position of 4- imidazolone ring to correlate the structure activity relationship (SAR) of prepared 2,3,5 trisubstituted imidazolone derivatives. Compound 2-phenyl-1H-indole (1), was prepared following the reaction conditions of ‘Fischer indole reaction’ i.e. in the presence of fused zinc, acetophenone was condensed with phenyl hydrazine HCl to obtain (1). ‘Vilsmeier Haack reaction’ conditions were used to prepare compound (2). Briefly, compound (1) was reacted with dimethylformamide (DMF) and phosphorus oxychloride (POCl3) to synthesize 2-phenyl-1H-indole-3-carbaldehyde (2). 2-phenyl-4-[(2-phenyl-1H-indol-3-yl)methylene)oxazol-5(4H)-one (3) was prepared by reacting 2 with a solution of benzoyl glycine in acetic anhydride. The reaction was carried out in the presence of fused sodium acetate. Finally, in the last step, compound (3) was fused with aliphatic/aromatic amines to furnish seven novel 2,3,5-trisubstituted-4H-imidazol-4-ones or indolyl-imidazolone derivatives (4a-g).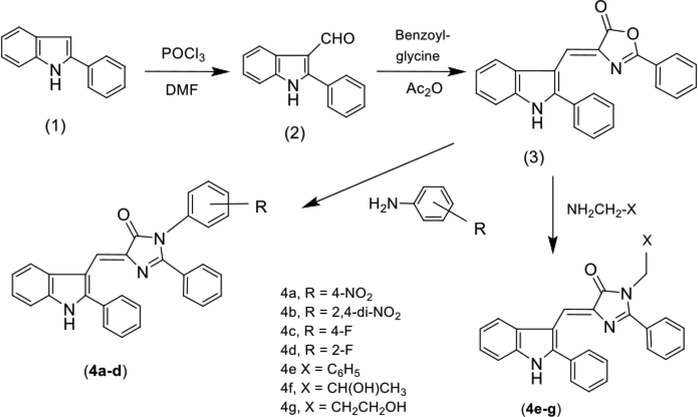
Protocol for synthesis of indolyl-imidazolones (4a-g).
The chemical structures of the prepared conjugates were characterized with the help of their elemental analysis & spectral data. IR spectrum of each compound displayed characteristic peaks for carbonyl group (C⚌O) in the range of 1723–1734 cm−1 and NH stretching bands (3275–3382 cm−1). In proton (1H NMR; ppm) spectrum, the signals for the aromatic protons, NH protons of the indole ring and methylene bridge protons showed characteristic chemical shift peaks at appropriate δ values. A sharp singlet (δ 8.06–8.21) was observed for the methylene bridge ⚌CH–) linking indole moiety at the 3rd position to the 5th position on to the imidazolone ring. The proton of secondary nitrogen (NH) of the indole ring was observed downfield as a singlet (δ 11.12–11.24). Compounds 4e showed additional signal for benzylic protons while 4f and 4g showed distinct signals for hydroxy and alkyl protons. Furthermore, a characteristic molecular ion peak was observed in the mass spectrum confirming the chemical structure of the prepared hybrid compounds. Elemental analysis for C, H and N revealed the values to be within ±0.4 % of the theoretical values.
3.2 Pharmacology
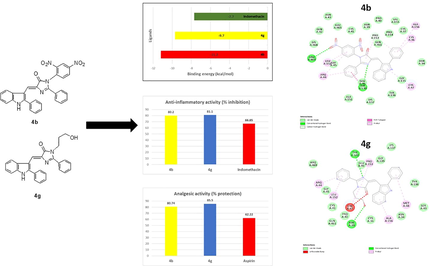
Newly synthesized indolyl-imidazolone conjugates were tested for their potential anti-inflammatory, analgesic and possible gastrotoxicity in the rodents. The in-vivo anti-inflammatory activity of the indolyl-imidazolone derivatives and positive control indomethacin was studied at the same dose (20 mg/kg, po) for comparison purpose. Anti-inflammatory activity of the hybrid derivatives was observed in the range of 52.50% to 81.10% (Table 1). The results of percentage inhibition in edema indicated that two compounds, 3-(2,4-Dinitrophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]- 4H-imidazol-4-one (4b) and 3-(3-Hydroxpropyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]-4H-imidazol-4-one (4g) showed excellent anti-inflammatory activity, 80.20% and 81.10% respectively. In fact, their anti-inflammatory activity was much more pronounced than the reference NSAID indomethacin (66.85%). One more compound, 3-(4-Fluorophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]-4H-imidazol-4-one (4c) was found to be equipotent (66.85%) to the standard drug. Other four compounds, 4a, 4d, 4e and 4f were noted to be weaker anti-inflammatory agent than the standard drug. SAR studies suggest that addition of a nitro group drastically improves the anti-inflammatory activity. 2,4 dinitro derivative (4b) showed an inhibition of 80.20% in comparison to 57.1% of mono nitro derivative (4a). Replacing 4-nitro group, a strongly withdrawing/deactivating group as in 4a with a weak electron withdrawing group (F) in 4c increases the activity. However, it could be noted that altering the position of 4-F to 2-F in 4d makes the compound less active. Furthermore, replacement of a substituted aromatic ring at the 3rd position of imidazolone ring with benzyl group (4e) or with 2-hydroxy propyl group (4f) results in moderately active compounds.
Compounds (4b, 4c, 4e, 4f & 4g) that displayed powerful anti-inflammatory activity i.e., more than 60% inhibition of edema were also selected for investigation of their analgesic potential in a mice model using acetic acid induced writhing assay. Hybrid conjugates exhibited excellent analgesic activity in the range of 76.85 to 85.50 %. All the five compounds are found to be more powerful analgesic agents than the standard drug acetyl salicylic acid (aspirin) which only showed 62.22% protection at a dose of 25 mg/kg i.p. (Table 1).
Five most potent anti-inflammatory compounds were also assessed for gastrotoxicity (ulcerogenicity) in rats. To evaluate the ulcerogenicity of 4b, 4c, 4e, 4f & 4g along with the reference drug indomethacin, a single dose of 60 mg/kg (po) was administered to Albino Wistar rats. All the five compounds under investigation displayed lower ulcerogenic activity (severity index ranging from 0.83 to 1.16) than the positive control NSAID, indomethacin, which produced greater gastrotoxicity as evidenced by a high severity index score of 2.25 (Table 1). Compounds 4b and 4g, the two most potent analgesic and anti-inflammatory agents showed a severity index of 0.91 and 1.16 and are found to be almost twice as safer as indomethacin.
3.3 In silico studies
Molecular properties obtained using online molinspiration webtool highlights that amongst the prepared conjugates, 4f and 4g do not violate Lipinski’s rule of five. Indomethacin, that is orally active also obeys the Pfizer rule and thus it could be suggested that the most potent compound 4g would also be orally bioavailable. Compound 4b showed two violations of Lipinski’s rule of five for two molecular properties; one for the molecular weight and another for the LogP value. Because low molecular weight compounds are associated with a good absorption rate, compound 4b due to its high lipophilicity and high molecular weight, will suffer from poor absorption rate through gastrointestinal tract (GIT). Amongst all the synthesized compounds, only compounds 4a and 4b are predicted to have low gastrointestinal absorption by SwissADME. Compounds 4e-g are more non-polar than 4a-c as they are expected to cross the blood brain barrier (BBB) (Table 3). In contrast to 4b, compound 4g and indomethacin are capable of permeating through BBB and are also noted to have high and favorable absorption in GIT. It was observed that 4g is a substrate of p-glycoprotein and thus it is expected to be effluxed easily from cell. Further, it can be observed that compounds 4c-e do not inhibit CYP1A2, CYP2C19, CYP2C9, CYP2D6 and CYP3A4 metabolizing enzymes. Compound 4b is predicted to be CYP2C19 inhibitor while compound 4g may inhibit all CYP forms except CYP2D6 and therefore might show more drug interactions. Obtained computational data further indicated that the prepared hybrid derivatives would exhibit safer spectrum in both rat acute toxicity and fish toxicity as their predicted LD50 and LC50 values are quite high.
Compounds 4b and 4g were selected for molecular docking studies on to the COX-2 enzyme. The analysis was performed to study the various ligand-target interactions and their binding energy. Fig. 2 illustrates the binding energies of the best molecular docking poses between ligand (4b, 4g & indomethacin) and COX-2 enzyme. It can be noted that compound 4b has the higher strength and affinity (lowest binding energy, −11.2 kcal/mol) than 4g (-9.7 kcal/mol) and indomethacin (-7.7 kcal/mol) to interact with 3pgh (COX-2 protein).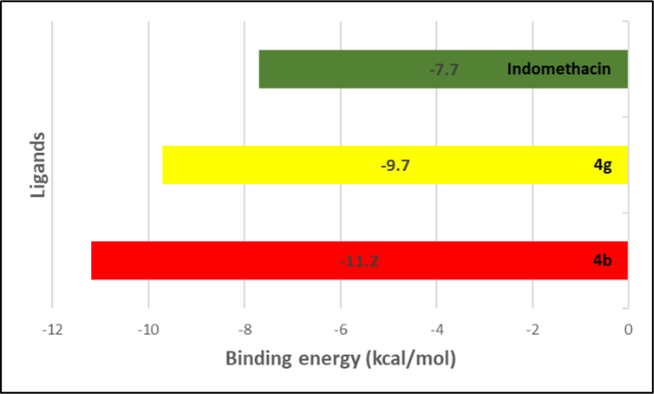
Binding energies of best poses of compounds 4b, 4g and indomethacin.
2D, 3D interactions of 4b, 4g and indomethacin with the 3pgh and hydrogen bond surface of receptor with ligand in the binding pocket are shown in Figs. 3-5. Compound 4b, 4g and indomethacin are noted to form three, two and one hydrogen bonds, respectively. 4b showed formation of H-bond with three amino acid residues of chain A viz., (i) Gly135 through NH of indole ring, (ii) Tyr130 through N1 of imidazolone ring and (iii) Arg469 through oxygen atom of nitro group. Similarly, 4g forms one H-bond with Tyr130 through N1 of imidazolone ring and another H-bond with Asn39 residue through hydroxyl group. Indomethacin interacts with Arg120 by forming a H-bond. These compounds also interact through van der walls and π- π interactions (Figs. 3-5).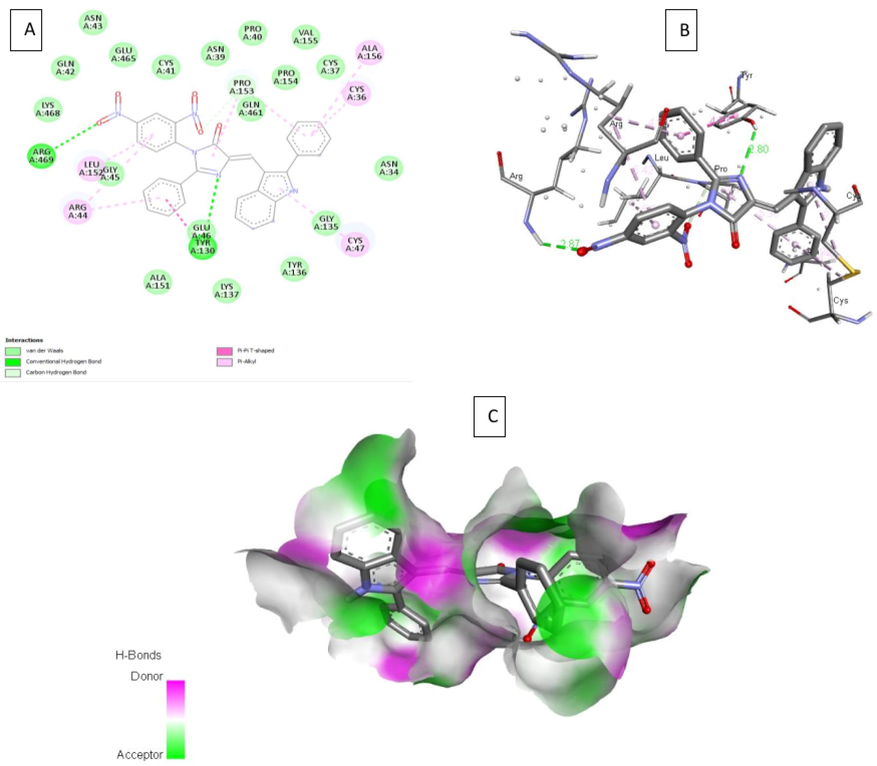
A) 2D interaction of 4b with the 3pgh; B) 3D interaction of 4b with COX-2; C) H- bond surface of receptor with ligand 4b.
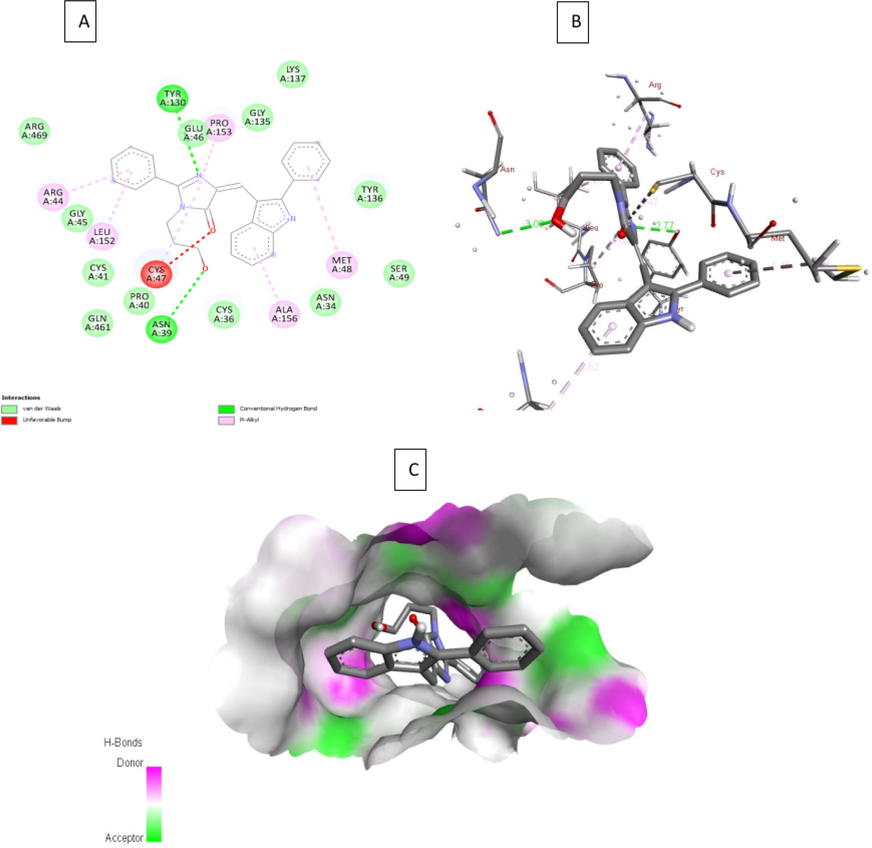
A) 2D interaction of 4g with the 3pgh; B) 3D interaction of 4g with COX-2; C) H- bond surface of receptor with ligand 4g.
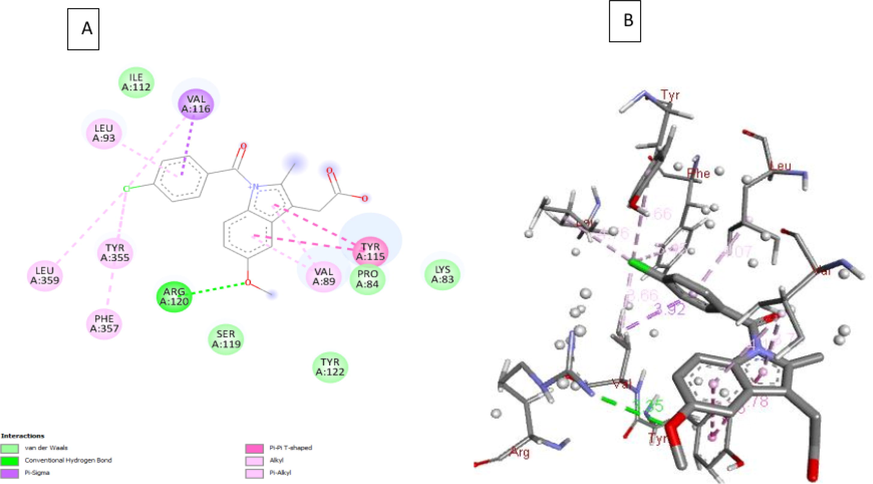
A) 2D interaction of indomethacin with the 3pgh; B) 3D interaction between indomethacin and COX-2.
4 Conclusion
A set of seven new indole linked imidazolone hybrid derivatives were successfully designed, prepared and their chemical structures characterized based on analytical data. Among the synthesized hybrid molecules, we have identified two most active compounds namely; 3-(2,4-Dinitrophenyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]- 4H-imidazol-4-one (4b) and 3-(3-Hydroxpropyl)-2-phenyl-5-[(2-phenyl-1H-indol-3-yl) methylene]-4H-imidazol-4-one (4g) as lead compounds for further studies, modification and development. Both the identified hybrids possess powerful anti-inflammatory & analgesic activities with respect to indomethacin and aspirin, respectively. They were observed to be less ulcerogenic in comparison to the respective standard NSAID, indomethacin. Computational studies also support the results of in vivo studies. Biological test and molecular docking results showed that the compound 4b and 4g possess excellent anti-inflammatory and analgesic activities. These compounds could be further studied and modified accordingly to develop compounds for the treatment of inflammatory conditions.
Acknowledgement
Authors are thankful to the Researchers Supporting Project number (RSP-2021/335), King Saud University, Riyadh, Saudi Arabia. One of the authors (AH) would also like to thank Jamia Hamdard for providing necessary research facilities to carry out the research work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): Usage and co-prescription with other potentially interacting drugs in elderly: A cross-sectional study. PloS One. 2020;15(10) e0238868
- [Google Scholar]
- Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N. Engl. J. Med.. 1992;327(11):749-754.
- [Google Scholar]
- Synthesis and characterization of novel indole derivatives reveal improved therapeutic agents for treatment of ischemia/reperfusion (I/R) injury. J. Med. Chem.. 2010;53(18):6763-6767.
- [Google Scholar]
- Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol.. 2020;180
- [Google Scholar]
- Synthesis, characterization and evaluation of analgesic and anti-inflammatory activities of some novel indoles. Trop. J. Pharm. Res.. 2011;10(4):463-473.
- [Google Scholar]
- Imidazole: An emerging scaffold showing its therapeutic Voyage to develop valuable molecular entities. Current Drug Research Reviews Formerly: Current Drug Abuse Reviews.. 2020;12(2):103-117.
- [Google Scholar]
- The role of direct tissue contact in the production of gastrointestinal ulcers by anti-inflammatory drugs in rats. Toxicol. Appl. Pharmacol.. 1979;50(2):283-289.
- [Google Scholar]
- Gastrointestinal side effects of nonsteroidal anti-inflammatory drugs. Am. J. Med.. 1998;105(1):20S-30S.
- [Google Scholar]
- Synthesis and biological evaluation of novel imidazolone derivatives as potential COX-2 inhibitors. Arch. Pharmacal. Res.. 2008;31(5):562-568.
- [Google Scholar]
- Synthesis, molecular properties, toxicity and biological evaluation of some new substituted imidazolidine derivatives in search of potent anti-inflammatory agents. Saudi Pharm. J.. 2016;24(1):104-114.
- [Google Scholar]
- Coumarin linked heterocyclic hybrids: A promising approach to develop multi target drugs for Alzheimer's disease. J. Mol. Struct.. 2021;1241:130618.
- [Google Scholar]
- Synthesis and cytotoxic activity of 1-(1-benzoylindoline-5-sulfonyl)-4-phenylimidazolidinones. Arch. Pharmacal. Res.. 2004;27(5):478-484.
- [Google Scholar]
- Multiple target-centric strategy to tame inflammation. Future Med. Chem.. 2017;9(12):1361-1376.
- [Google Scholar]
- Indole derivatives with anticonvulsant activity against two seizure models. Pharmacophore.. 2012;3(1):55-61.
- [Google Scholar]
- Cyclo-oxygenase (COX) inhibitors and cardiovascular risk: are non-steroidal anti-inflammatory drugs really anti-inflammatory? Int. J. Mol. Sci.. 2019;20(17):4262.
- [Google Scholar]
- Synthesis, molecular docking with COX 1& II enzyme, ADMET screening and in vivo anti-inflammatory activity of oxadiazole, thiadiazole and triazole analogs of felbinac. J. Saudi Chem. Soc.. 2018;22(4):469-484.
- [Google Scholar]
- Review of imidazole heterocyclic ring containing compounds with their biological activity. Pharmacophore. 2010;1(3):167-177.
- [Google Scholar]
- Effects of new NSAID-CAI hybrid compounds in inflammation and lung fibrosis. Biomolecules.. 2020;10(9):1307.
- [Google Scholar]
- Pharmacoepidemiology of non-steroidal anti-inflammatory drugs. Therapies. 2019;74(2):271-277.
- [Google Scholar]
- Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem.. 2004;39(5):449-452.
- [Google Scholar]
- A method for evaluating both non-narcotic and narcotic analgesics. Proc. Soc. Exp. Biol. Med.. 1957;95(4):729-731.
- [Google Scholar]
- Thiazolyl/oxazolyl formazanyl indoles as potent anti-inflammatory agents. Eur. J. Med. Chem.. 2008;43(11):2597-2609.
- [Google Scholar]
- An insight into the medicinal perspective of synthetic analogs of indole: A review. Eur. J. Med. Chem.. 2019;180:562-612.
- [Google Scholar]
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2010;31(2):455-461.
- [Google Scholar]
- Adverse drug events involving COX-2 inhibitors. Ann. Pharmacother.. 2003;37(9):1203-1213.
- [Google Scholar]
- Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med.. 1962;111(3):544-547.
- [Google Scholar]







