Translate this page into:
The therapeutic potential of skin mucus from Asian swamp eel (Monopterus albus): In vivo evaluation and histological evidence
⁎Corresponding author. syedmahmood@um.edu.my (Syed Mahmood)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
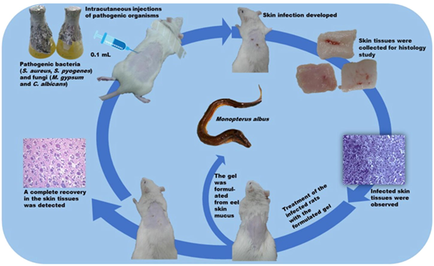
The Asian swamp eel (Monopterus albus), is commonly distributed in Asian countries. However, its therapeutic potential has not been thoroughly investigated yet. The current study aimed to evaluate the in-vivo therapeutic properties of the skin mucus of this fish.
Methods
The eel mucus was collected fleshly and topical gel with carbopol 934 was formulated to study the antibacterial activity on the infected skin of the rats. Sprague Dawley rats were used in the study and divided into 4 groups negative, positive, normal control, and treated groups.
Results
Intracutaneous injections of pathogenic bacteria (Streptococcus pyogenes, Staphylococcus aureus) and fungi (Microsporum gypseum, Candida albicans) were injected into the rats. The development of tinea capitis, impetigo, and cutaneous candidiasis in the animal model was confirmed based on clinical and histopathological observations. To treat the infected rats, a formulated gel of eel skin mucus was applied on the infected rat’s skins topically. The histological analysis confirms a complete recovery in the skin tissues similar to commercial antifungal and antibacterial agents used in the positive control groups.
Conclusion
The present novel eel skin mucus is an efficient therapeutic candidate in treating skin infections associated with pathogenic microbes.
Keywords
Eel skin mucus
Monopterus albus
Intracutaneous injections
Skin infections
1 Introduction
Cutaneous diseases increasingly turned into life-threatening diseases as it was reported that skin infections affect 30–70% of individuals (Hay et al., 2014; Gonzalez et al., 2003). The researches in the field of antibiotics used to treat skin infections topically and systemically has improved significantly. Cutaneous pathogens such as Staphylococcus aureus and Streptococci are prevalent in the topical infection on the skin, while, Gram-negative bacteria, Pseudomonas aeruginosa, are common (Veien, 1998). Fungi are also known to play a significant role in human health and disease. Fungal infection is more prominent in 3rd world countries (Peleg et al., 2010). There is a technical lack in identifying the fungal species compared to the bacteria (Dollive et al., 2012). Phylogenetic markers that include ribosomal RNA gene regions and other highly conserved genes are mainly used for fungal evolution (James et al., 2006).
Monopterus albus (M. albus) i;e Asian swamp eel belongs to synbranchidae family under the order of synbranchiformes (Rossen and Greenwood, 1976). It is native to the tropical and subtropical areas of Malaysia, China, Thailand, Indonesia, northern India and possibly north-eastern Australia (Collins et al., 2002). M. albus is typically freshwater species, but it also can live in brackish water (Schofield and Nico, 2009). There were numerous studies that recorded the ability of M. albus to tolerate a broad spectrum of salinities (Pedersen et al., 2014); for instance, there was a study showed that M. albus can stay many weeks in salinities of 16 g L−1, while it can survive a shorter time at high rates of salinities up to 24 g L−1 (Tok et al., 2009). M. albus can live out of water for an extended period of time (Toh et al., 2011); moreover, it can survive acute and chronic cold temperatures (Saylor et al., 2021).
In terms of the biological activities of eel skin mucus, it recorded antifungal effects against C. albicans, Cryptococcus neoformans and Fusarium species (Nor et al., 2013). Moreover, M. albus extract revealed antibacterial and bacteriostatic effects against V. choleraea and E. coli (Atif et al., 2015). Additionally, it was recorded that eel skin mucus can be considered as an anticancer agent as it led to mitochondria-mediated apoptosis against K562 human leukaemia cell line through inhibiting cell growth and stimulating apoptotic cell death (Kwak et al., 2015).
Eel skin mucus showed significant antibacterial activity against Escherichia coli, Staphylococcus aureus (Hilles et al., 2019), and different oral pathogens, including Streptococcus mutans, Streptococcus pyogenes, Enterococcus faecalis, Pseudomona aeruginosa Klebsiella pneumoniae and Candida albicans (Hilles et al., 2019). It has been demonstrated that eel skin mucus extract from M. albus has antifungal activity against Cryptococcus neoformans; Candida albicans, Candida krusei, Fusarium species (Ikram and Ridzwan, 2013) and Microsporum gypseum (Hilles et al., 2019). Eel skin mucus of M. albus also revealed a potential cytotoxic activity by induction of apoptotic cell death through triggering caspase-3/7, 8 and 9 (Hilles et al., 2020). The screening of the bioactive compound in skin mucus from M. albus using Liquid Chromatography Quadrupole-Time-Of-Flight Mass Spectrometry (LC-QTOF-MS) showed that eel skin mucus contains different bioactive compounds such as Peonidin, Benzyl benzoate, Progenin II, and Salvianolic acid G, these compounds exhibit various biological activities, including antimicrobial, anti-inflammatory, anticancer, and antioxidant properties (Hilles et al., 2019). Since the therapeutic potential of eel skin mucus has not been thoroughly investigated yet, hence, the current study is an attempt to further evaluate the in-vivo therapeutic properties of skin mucus of this fish in the form of eel skin mucus formulated gel to treat skin-related infections associated with certain pathogenic bacterial and fungal strains.
2 Materials and method
2.1 Materials
Eel (M. albus) was collected from a local supplier. The eel species was confirmed by the Marine researcher from the Department of marine science International Islamic University Malaysia. Methanol ethanol (AR grade) was purchased from gardener Sdn Bdn, Malaysia. Bacterial and fungal strains were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Bacterial strains Staphylococcus aureus (ATCC 25923), and Streptococcus pyogenes (ATCC 19615), Fungal strains; Candida albicans (ATCC MYA 4901), and Microsporum gypseum (ATCC 24102). All other chemicals used in the experiments were of AR grades, and Ultra-pure water was used throughout the study.
3 Methodology
3.1 Eel mucus extraction
The extraction procedure was taken from our previously described method of (Sadakane et al., 2007) with a slight modification (Sadakane et al., 2007). The mucus (ESM) was collected from the skin of the eel by smoothly scraping the surface of the eel skin. The collected mucus was homogenised in the presence of ultra-pure water using (T18, Ultra Turax, IKA, Germany) homogeniser for 1 min. Later, the mucus extract was centrifuged at 13,000 rpm for 30 min at 4 °C; the supernatant was freeze-dried for five days at room temperature. The freeze-dried powder was later stored at −20 °C for further use.
3.2 Animal study
Sprague-Dawley rats, eight weeks old, weighing 230–250 g, were used for the study. The rats were purchased from a licensed animal breeding house Sapphire Enterprise, a local animal supplier laboratory in Malaysia. Prior to the experiment, the animals were acclimatised for five days at 23 ± 2 °C with a 12 h light and dark cycle; the humidity was between 40 and 60%. Water and food were in ad libitum.
The animal ethical approval was granted from International Islamic University, Malaysia animal ethical committee with reference number: IIUM/504/14/2/IACUC.
3.3 Animals grouping
The Sprague Dawley rats (n = 78) were acclimatised for five days and randomly divided into 13 groups (n = 6/group). The rats in the normal control group were without the development of skin infection or treatment. While the rats in the negative control groups were infected with 4 different pathogens without any treatment. For the positive control groups: the 4 groups of the infected rats were treated with antibiotics. Finally, for the treated groups: the 4 infected groups were treated with ESM formulated gel.
3.4 Experimental design
3.4.1 Strain preparation
All the fungal and bacterial strains were purchased from the American Type Culture Collection (ATCC, VA, USA). Fungal strains; Candida albicans (ATCC MYA 4901) and Microsporum gypseum (ATCC 24102) inoculum was adjusted to 0.5–2.5 × 103 CFU/mL. For bacterial strains; Staphylococcus aureus (ATCC 25923) and Streptococcus pyogenes (ATCC 19615), the turbidity of the suspensions was adjusted according to McFarland standard, i.e., approximately 5 × 108 CFU/mL (Leite et al., 2014; Petrikkou et al., 2001).
3.4.2 Induction of skin infection in the rat skin
The rats were anaesthetised using Intraperitoneal (IP) injection of 50 mg/kg pentobarbital; then the skin infections were induced by a single dose of intracutaneous injections of two fungal strains (C. albicans, M. gypseum) and two bacterial strains (S. aureus, S. pyogenes). The intracutaneous injections containing live fungi and bacteria were jabbed at 4 different sites on the shaved back of each rat. Each rat was inoculated with 0.1 mL of the pathogenic organisms (Mölne and Tarkowski, 2000).
3.4.3 Confirmation of the presence of the infections
Tissue swabs were taken from the infected skin to confirm the presence of the infections; the procedure was done by passing the swab deep into the base of the lesion to firmly sample the fresh border under the sterile condition to avoid contamination, then it was transformed to the microbiology laboratory for culture, bacterial infections were plated on Mueller Hinton agar plates and then kept under anaerobic condition at 37 ± 1 °C for a period of 24 h, and fungal infections were plated on potato dextrose agar, then the plates were incubated at 35 ± 1 °C for 48 h, the observation of visible growth of the colonies was a confirmation sign of developing the infections.
3.4.4 Preparation of ESM formulated gel
Briefly, the ESM dried powder was taken in a dry and clean beaker. In a separate beaker, the blank Carbopol 934 gel was prepared using the following procedure. Crabopol 934 was taken in a beaker, and distilled water was added slowly; it was then under the constant overhead stirrer was stirred for 2 h. To maintain the viscosity and pH, 1.0 mL Triethanolamine (TEA) was added dropwise. The pH was maintained till 5.5, which is also the skin pH range. Finally, the ESM powder was loaded to form a homogeneous gel in the amount of 2% w/w. The mixing of the gel and ESM continued for 1 h till a complete mixing was achieved without carbopol accumulation or air bubbles.
3.4.5 Treatment of bacterial skin infections with the antibiotic
Treatment of the infected rats was started 48 h post-exposure, mupirocin ointment was used to treat the groups infected with S. aureus and S. pyogenes as it was applied once daily, topical therapy with mupirocin considers equivalent to oral and systemic antimicrobial agents (Stevens and Bryant, 2016). A topical dose of 2% mupirocin ointment for 14 days applied at the infected area was reported to treat the skin infection of S. pyogenes and S. aureus (Ghaisas et al., 2014).
3.4.6 Treatment of fungal skin infections with the antibiotic and antifungal
The treatment was started 48 h post-exposure, ketoconazole ointment (2% w/w) was used to treat the groups infected with M. gypseum and C. albicans for 28 days. It was applied topically once daily. Ketoconazole is a topical antifungal ointment that has a broad-spectrum activity against systemic mycoses and superficial skin defects (Hardman Limbird and Goodman, 2001; Kumar et al., 2014). The efficacy and safety of the treatment are 4 weeks of 2% ketoconazole gel, once-daily treatment was evaluated in moderate to severe skin disease (Elewski, 2000; Elewski et al., 2006).
3.4.7 Treatment of fungal skin and bacterial infections with ESM gel
The treatment was initiated 48 h post-exposure, a thin layer of 2% of ESM formulated gel was massaged into the skin once daily until remission was obtained.
3.4.8 Histological procedure
The rats were sacrificed according to the ethical principles of research, in which the rats were given a lethal dose of 4 times the anaesthetic dose, which was the combination of Ketamine/Xylazine (Porumb et al., 2017), then the rats were laid on their abdomens, and the back hair of the rats was shaved properly, after that, the skin of the rats was dissected and cleaned from adhering fats. Then, it was quickly transferred into a jar that contained a fixative agent of 10% formalin. Then the skin tissues were cut vertically to observe the longitudinal sections and horizontally to observe the cross-sections and placed into a small plastic cassette and prepared for the next procedures, which were tissue processing, embedding, sectioning, fishing, drying, and staining using Hematoxylin and Eosin (H&E) stain, the stained slides were examined using a digital microscope at different magnification powers. The skin tissues were subjected to histological examination to check the histopathological findings in the skin tissues. Qualitative analysis was performed to assess the histopathological changes.
4 Results
Skin infections were introduced to the rats by intracutaneous injections of two bacterial strains (S. aureus and S. pyogenes) and two fungal strains (M. gypsum and C. albicans). Within 48 h, the skin infection was developed, and the clinical investigation was observed; it was confirmed by culturing the swabs, which showed growth of the infections on the agar plates. After that, the 4 infected groups of the rats were sacrificed together with the normal untreated group, and the treatment of antibiotics, as well as the formulated gel, was started; then, after a full recovery, the treated groups were sacrificed, and skin tissues were collected for histological study.
Based on the diagnosis from the clinical investigations of the infected rats, the diseases were classified for each group, as shown in Table 1.
Strain
Diagnosis
Clinical Features
histopathological Examination
S. aureus
Impetigo
Reddish spots on the skin, often clustered around the skin.The rash looks like small red pimples (papules)
.
Skin rash with honey-coloured crust.A subcorneal pustule containing numerous neutrophils.
Spongiform pustules beneath the subcorneal pustule.
S. pyogenes
M. gypsum
Tinea capitis
Multiple patches of hair loss.Black dot pattern (often with broken-off hairs)
.Neutrophils around the hair shafts
Abscesses
Dermal inflammation
C. albicans
Cutaneous candidiasis
The main symptom of candidiasis of the skin is a rash.
Bright red rash, sometimes with a breakdown of skin.Granulomas usually occur as part of Infiltrates in the dermis.
Giant cells
Neutrophils
4.1 Clinical presentation of infected rats
Infected rats with S. aureus, S. pyogenes, M. gypsum, and C. albicans are shown in Fig. 1.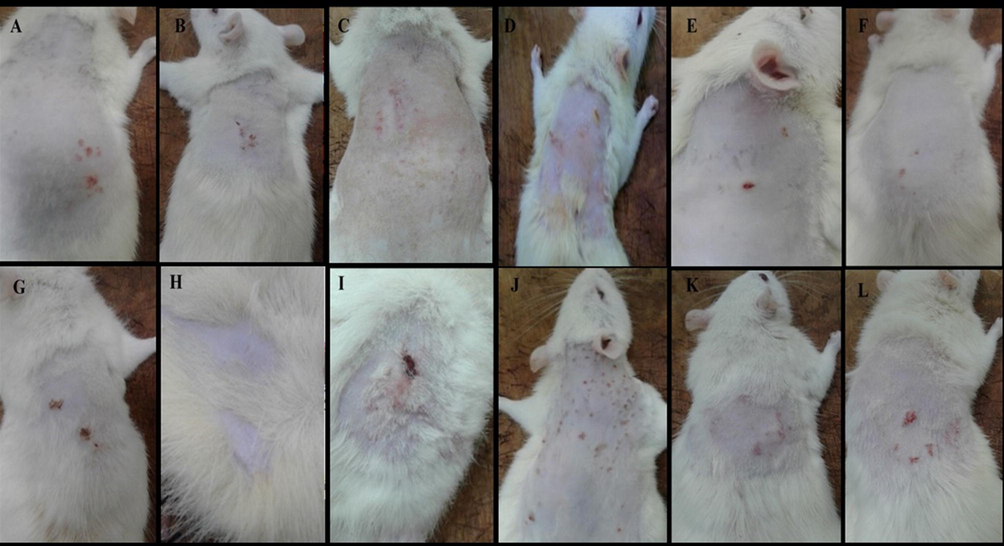
(A, B, C) Rats infected with S. aureus were shown to have reddish rashes that look like small red pimples concentrated at the injection site, and it was also shown to have honey-coloured crust as well as swelling, which is the hallmark of impetigo disease. Fig. 1(D, E, F) shows red skin rashes on the infected skin with S. pyogenes along with oedema and erythematous lesions, which represent the clinical findings of impetigo. Fig. 1(G, H, I). shows the clinical manifestations of rats infected with M. gypsum, which were shown to have black dots on the skin and patches of hair loss. These two signs are the hallmark of tinea capitis infection. Fig. 1(J, K, L), the infected rats with C. albicans were shown to display red rashes spread on the skin, mainly on the injection sites, which is a sign of cutaneous candidiasis.
4.2 Histological analysis of the infected group
Cross and longitudinal sections of the infected skin tissues with S. aureus, S. pyogenes, M. gypsum and C. albicans are shown in Fig. 2.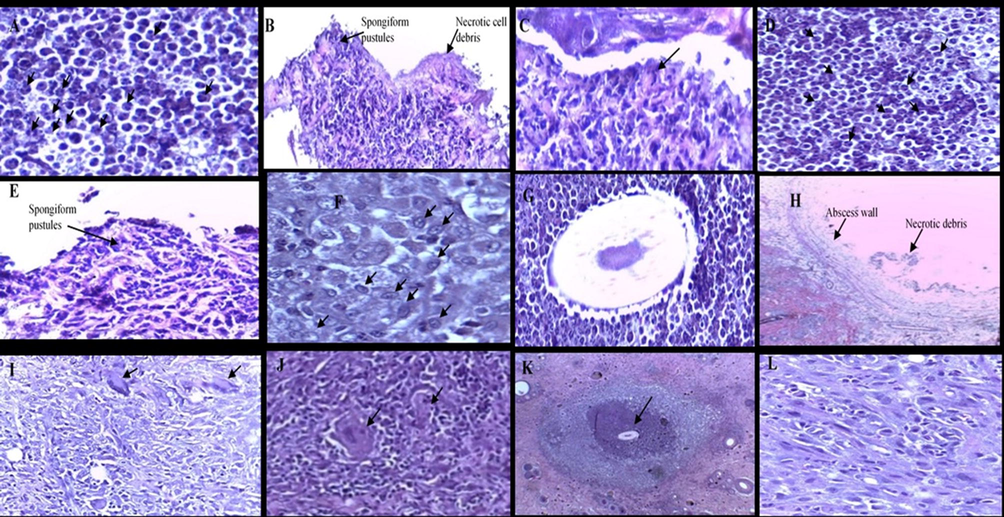
Histological features of the skin tissues infected with S. aureus (A, B and C), S. pyogenes (D, E and F), M. gypsum (G, H and I), C. albicans (J, K and L) show a longitudinal and cross-sections of skin tissues of localised infection at the injection site in the reticular dermis. (H&E, × 4). The arrows in A shows neutrophils, B shows spongiform pustules containing numerous neutrophils, C shows a cross-section of subcorneal pustule containing numerous neutrophils, D show neutrophils, E shows a cross-section of spongiform pustules which contain numerous neutrophils, F shows macrophages, G shows hair follicle surrounded with neutrophils, H shows abscess wall and necrotic debris, I shows numerous inflammatory cells including neutrophils, macrophages and giant cells, J arrows represent giant cells, K shows granuloma formation, L shows numerous inflammatory cells including neutrophils, macrophages and giant cells.
4.3 Treatment of bacterial and fungal skin infections
Rats were treated with mupirocin ointment exhibited recovery on their infected skin within 14 days against S. aureus and S. pyogenes as shown in Fig. 3(A), whereas rats treated with ketoconazole ointment disclosed a total recovery within 28 days against M. gypseum and C. albicans) as shown in Fig. 3(B). Rats that were treated with ESM formulated gel showed a full recovery after 15 days against bacterial skin infections (S. aureus and S. pyogenes) as shown in Fig. 3(C), while 32 days against fungal skin infections (M. gypseum and C. albicans) as shown in Fig. 3(D).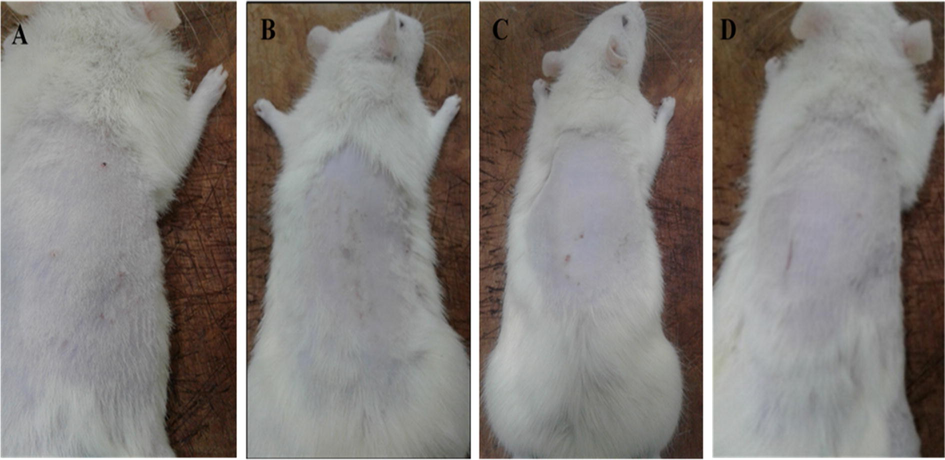
(A) shows the skin of rat recovered after treatment with mupirocin, (B) shows the skin of rat recovered after treatment with ketoconazole, (C) shows the skin of rat recovered from bacterial skin infection after treatment with ESM gel and (D) shows the skin of rat recovered from fungal skin infection after treatment with ESM gel.
4.4 Histological examination for the treated rats with the antibiotic and ESM formulated gel
The treated skin tissues with the antibiotics (ketoconazole and mupirocin ointment), as well as the skin tissues treated with ESM, formulated gel, showed a presence of numerous collagen fibres extensively and fibrosis formation, which is a remarkable sign of skin recovery process, there was a complete recovery in the skin tissues without inflammatory signs. The skin tissues were in a normal appearance when compared with the normal control group. Fig. 3 shows a normal appearance of the treated skin tissues with different magnification powers (Fig. 4).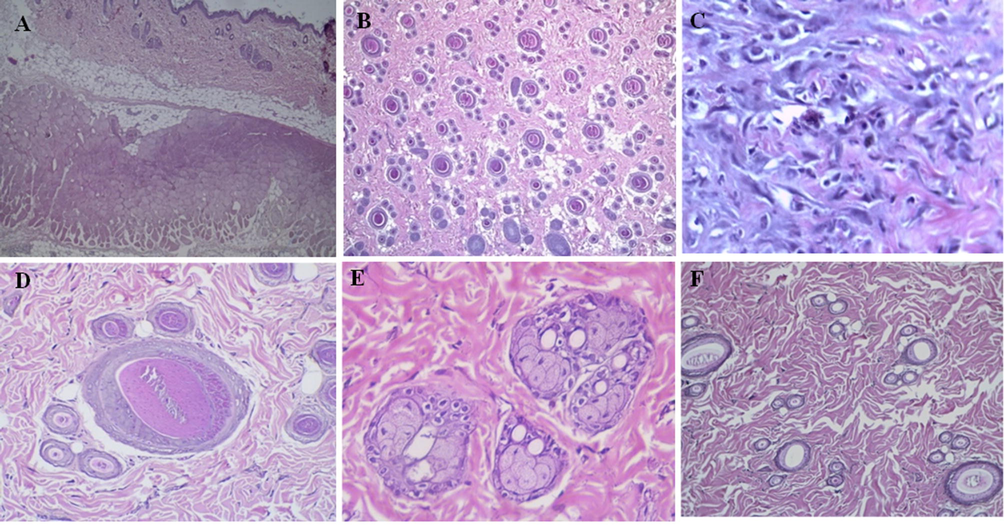
(A) shows a cross-section of untreated cell skin layers with normal appearance (H&E, × 4), (B) shows a longitudinal section of untreated cell skin tissues (H&E, × 4), (C) shows fibroblasts and fibrosis formation (H&E, × 4), (D) shows normal hair follicles (H&E, × 40), (E) shows thick collagen bundles and normal sebaceous glands (H&E, × 40) and (F) shows infection recovery by collagen fibres formation, and there is no inflammation was identified (H&E, × 20).
5 Discussion
5.1 Development of bacterial and fungal infections
The infections were developed in the rats after 48 h of bacterial and fungal intracutaneous injections, and then the treatment started immediately as it has been recorded that the timing of the start with oral or topical administration of the treatment agents is when the primary lesion begins to occur in each model or experiment. In most cases, the time is from 3 to 5 days after infection (Ghannoum et al., 2004). The formation of subcorneal pustules that occurred in both S. aureus, and S. pyogenes resembled the histology of impetigo infection, a contagious superficial pyogenic infection of the skin caused by S. aureus and S. pyogenes. Considering the histology of the infected skin tissues with M. gypsum, which was most resembled tinea capitis infection, while C. albicans pronounced acute inflammatory response characterised by the presence of neutrophils invading the epidermal layer, which represent cutaneous candidiasis infection.
5.2 Development of impetigo infection by S. aureus
Clinical investigation showed that red skin lesions and the more prominent symptoms include pustules, neutrophils which were filled in pustules. The toxins are produced by S. aureus target desmoglein, which is defined as a desmosomal cell–cell adhesion molecule in the upper of the epidermis layer; this causes subcorneal localisation of the bullae (Sternberg et al., 2004). The abscesses of neutrophils which is defined as Kogoj’s spongiform pustules were detected histologically (Sahin et al., 2002).
5.3 Development of impetigo infection by S. pyogenes
The hallmark that confirms impetigo infection was the presence of Kogoj's spongiform pustule along with neutrophil infiltration forming intraepidermal microabscesses (Kondo et al., 2013).
5.4 Development of tinea capitis by M. gypseum
Tinea capitis defined as a cutaneous fungal infection that causes dermatophytosis (Freedberg and Fitzpatrick, 2003) as it has been reported that tinea capitis described by an intense inflammatory response which is classified as tinea or ringworm to the afflicted body part (Howard, 2002). Rats infected with M. gypseum showed hairless patches of skin, and this has been reported due to the pus formation, which occurs from the hair follicles and causes hair removal (Rippon, 1982).
5.5 Development of cutaneous candidiasis by C. albicans
The presence of neutrophils, granulomas, and inflammatory infiltrate are considered remarkable signs of cutaneous candidiasis infection (Isa-Isa et al., 2010). The inflammatory response of C. albicans was characterised by neutrophils, macrophages, giant cells and granulomas, and this agreed with the previous study, which reported that whether cutaneous candidiasis was superficial or invasive, it will lead to neutrophilic inflammation (Guarner and Brandt, 2011).
5.6 Treatment of the infected rats with the antibiotic and antifungal
Mupirocin ointment was used as an antibiotic to treat bacterial skin infection as it has been proven that topical application of mupirocin cream was effective to treat skin infections induced by S. aureus and S. pyogenes (Gisby and Bryant, 2000). While ketoconazole ointment was used to treat fungal skin infections, as it has been reported that topical treatment of 2% ketoconazole successfully treated tinea capitis infection (Greer, 2000), and ketoconazole topical treatment has also been reported to be effective to treat cutaneous candidiasis (Gupta et al., 2010). In the treated groups with antibiotic and antifungal ointments, fibroblasts growth was detected in the epidermal, which is a sign of skin repair (Chhibber et al., 2015).
5.7 Treatment with ESM formulated gel
Clinically, the treated groups with ESM formulated gel exhibited a complete recovery within two weeks against bacterial infections and five weeks against fungal infections. While histologically, there was fibroblast and collagen proliferation which is a clear sign of tissue repair (Martinez et al., 2009).
6 Conclusion
In recent eras, the need for therapeutic agents from natural aquatic sources has been increased; therefore, exploratory research studies of new alternative treatments from natural sources such as eel that possesses no/minimal toxic effect is highly needed. The current study revealed that a topical application of ESM formulated gel exhibited a complete recovery in the infected rats with impetigo, tinea capitis and cutaneous candidiasis; hence it may be considered as a potential natural aquatic candidate against some skin infections caused by pathogenic bacteria (S. aureus, S. pyogenes) and pathogenic fungi (M. gypsum, C. albicans). This finding indicates that ESM formulated gel can be used to treat some skin infections almost the same as standard antibiotic and antifungal.
Acknowledgement
Authors are thankful to the Researchers supporting project number (RSP-2021/335), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J, Invest. Dermatol.. 2014;134(6):1527-1534.
- [Google Scholar]
- Antibiotic resistance in the community. J. Hosp. Infect.. 2003;55:156-157.
- [CrossRef] [Google Scholar]
- The clinician's choice of antibiotics in the treatment of bacterial skin infection. British J. Dermatol.-Supplement-. 1998;139:30-36.
- [CrossRef] [Google Scholar]
- Medically important bacterial–fungal interactions. Nat. Rev. Microbiol.. 2010;8(5):340.
- [CrossRef] [Google Scholar]
- A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples. Genome Biol.. 2012;13(7):R60.
- [Google Scholar]
- Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443(7113):818-822.
- [Google Scholar]
- A fourth neotropical species of Synbranchid eel and the phylogeny and systematics of Synbranchiform fishes. Bull. Am. Museum Natural History. 1976;157:1-69. URI
- [Google Scholar]
- Genetic diversity in a morphologically conservative invasive taxon: multiple introductions of swamp eels to the southeastern United States. Conserv. Biol.. 2002;16(4):1024-1035.
- [CrossRef] [Google Scholar]
- Salinity tolerance of non-native Asian swamp eels (Teleostei: Synbranchidae) in Florida, USA: comparison of three populations and implications for dispersal. Environ. Biol. Fishes. 2009;85(1):51-59.
- [CrossRef] [Google Scholar]
- Effects of salinity on osmoregulation, growth and survival in Asian swamp eel (Monopterus albus) (Z uiew 1793) Aquac. Res.. 2014;45(3):427-438.
- [CrossRef] [Google Scholar]
- Glutamine accumulation and up-regulation of glutamine synthetase activity in the swamp eel, Monopterus albus (Zuiew), exposed to brackish water. J. Exp. Biol.. 2009;212(9):1248-1258.
- [CrossRef] [Google Scholar]
- Gene cloning and mRNA expression of glutamate dehydrogenase in the liver, brain, and intestine of the swamp eel, Monopterus albus (Zuiew), exposed to freshwater, terrestrial conditions, environmental ammonia, or salinity stress. Front. Physiol.. 2011;2:100.
- [CrossRef] [Google Scholar]
- Non-native Asian swamp eel, Monopterus albus/javanensis (Zuiew, 1973/Lacepede, 1800), responses to low temperatures. Fish Physiol. Biochem.. 2021;47(2):465-476.
- [CrossRef] [Google Scholar]
- A preliminary screening of antifungal activity from skin mucus extract of Malaysian local swamp eel (Monopterus albus) Int. Res. J. Pharmacy Pharmacol.gy. 2013;3(1):1-8.
- [Google Scholar]
- Comparative analysis of the antibacterial, antifungal, antiproliferative and cyclic response element (CRE) induced expression of downstream luc gene activity of Monopterus albus and Channa straitus extracts. J. Appl. Pharmaceut. Sci.. 2015;5(1):42-47.
- [CrossRef] [Google Scholar]
- Induction of apoptosis and antitumor activity of Eel Skin Mucus, containing lactose-binding molecules, on human leukemic K562 cells. Mar. Drugs. 2015;13(6):3936-3949.
- [Google Scholar]
- Evaluation of the antibacterial activities of skin mucus from Asian swamp eel (Monopterusalbus) Indian J. Geo-Mar. Sci.. 2019;48(12)
- [Google Scholar]
- Evaluation of the antimicrobial properties of eel skin mucus from Monopterus albus against selected oral pathogens and identification of the anti-oral bioactive compounds using LC-QTOF-MS. J. Microbiol. Biotechnol. Food Sci.. 2019;9(1):140.
- [CrossRef] [Google Scholar]
- A preliminary screening of antifungal activities from skin mucus extract of Malaysian local swamp eel (Monopterus albus) Int. Res. J. Pharmacy Pharmacol.. 2013;3(1):1-8.
- [Google Scholar]
- In-vitro evaluation of the antifungal activities of eel skin mucus from Asian swamp eel (Monopterus albus) Fungal Territory. 2019;2(1):1-2.
- [CrossRef] [Google Scholar]
- Activation of apoptotic cell death by skin mucus from Asian swamp eel (Monopterus albus) against human lung cancer cell line. J. Agric. Mar. Sci. [JAMS]. 2020;24:39-43.
- [Google Scholar]
- Protective activity of the extracts from Japanese eel (Anguilla japonica) against zinc-induced neuronal cell death: Carnosine and an unknown substance. Trace Nutr. Res.. 2007;24:98-105. URL
- [Google Scholar]
- In vitro antimicrobial activities of an experimental dentifrice based on Ricinus communis. Brazilian Dental J.. 2014;25(3):191-196.
- [CrossRef] [Google Scholar]
- Inoculum standardisation for antifungal susceptibility testing of filamentous fungi pathogenic for humans. J. Clin. Microbiol.. 2001;39(4):1345-1347.
- [CrossRef] [Google Scholar]
- An experimental model of cutaneous infection induced by superantigen-producing Staphylococcus aureus. J. Invest. Dermatol.. 2000;114(6):1120-1125.
- [CrossRef] [Google Scholar]
- Stevens, D.L., Bryant, A.E., 2016. Impetigo, erysipelas and cellulitis. URL: https://www.ncbi.nlm.nih.gov/books/NBK333408/.
- Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J.. 2014;11(5):523-532.
- [CrossRef] [Google Scholar]
- 29. Hardman, J.G., 2001. Limbird, LE. Goodman and Gilmans. The pharmacological Basis of therapeutics, 10. DOI: https://doi.org/10.1097/00000539-200205000-00085.
- In Vitro and in Vivo evaluation of microspheres loaded topical gel delivery system of ketoconazole in male rats against Candida Glabrata. J. Pharmaceut. Sci. Res.. 2014;6(11):376.
- [Google Scholar]
- Tinea capitis: a current perspective. J. Am. Acad. Dermatol.. 2000;42(1):1-20.
- [CrossRef] [Google Scholar]
- Efficacy and safety of a new once-daily topical ketoconazole 2% gel in the treatment of seborrheic dermatitis: a phase III trial. J. Drugs Dermatol.: JDD. 2006;5(7):646-650.
- [Google Scholar]
- Design and testing of an experimental steam-induced burn model in rats. Biomed. Res. Int.. 2017;2017:1-10.
- [Google Scholar]
- Evaluation of antifungal efficacy in an optimised animal model of Trichophyton mentagrophytes dermatophytosis. J. Chemother.. 2004;16(2):139-144.
- [CrossRef] [Google Scholar]
- Sternberg, S.S., Mills, S.E., Carter, D. (Eds.), 2004. Sternberg's diagnostic surgical pathology (Vol. 1). Lippincott Williams & Wilkins.
- Recurrent impetigo herpetiformis in a pregnant adolescent: case report. Eur. J. Obstetr. Gynecol. Reprod. Biol.. 2002;101(2):201-203.
- [CrossRef] [Google Scholar]
- Pustular psoriasis of pregnancy (Impetigo herpetiformis)-Case report. Anais brasileiros de dermatologia. 2013;88(6):186-189.
- [CrossRef] [Google Scholar]
- Freedberg, I.M., Fitzpatrick, T.B. 2003. Fitzpatrick's Dermatology in General Medicine. New York: McGraw-Hill, Medical Pub. Division; p. 645. ISBN 0-07-138076-0. 1.
- Howard, D.H. (Ed.), 2002. Pathogenic fungi in humans and animals. CRC Press.
- Medical mycology; the pathogenic fungi and the pathogenic actinomycetes. Eastbourne, UK: WB Saunders Company; 1982.
- Inflammatory tinea capitis: kerion, dermatophytic granuloma, and mycetoma. Clin. Dermatol.. 2010;28(2):133-136.
- [CrossRef] [Google Scholar]
- Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev.. 2011;24(2):247-280.
- [CrossRef] [Google Scholar]
- Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob. Agents Chemother.. 2000;44(2):255-260.
- [CrossRef] [Google Scholar]
- Successful treatment of tinea capitis with 2% ketoconazole shampoo. Int. J. Dermatol.. 2000;39(4):302-304.
- [CrossRef] [Google Scholar]
- Development and characterisation of effective topical liposomal system for localised treatment of cutaneous candidiasis. J. Liposome Res.. 2010;20(4):341-350.
- [CrossRef] [Google Scholar]
- Phospholipid structured microemulsion as effective carrier system with potential in methicillin sensitive Staphylococcus aureus (MSSA) involved burn wound infection. J. Drug Target.. 2015;23(10):943-952.
- [CrossRef] [Google Scholar]
- Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J. Invest. Dermatol.. 2009;129(10):2463-2469.
- [Google Scholar]







