Translate this page into:
Association of oral candidal carriage, candidal density and CD4 count among normal, HIV patients with HAART and without HAART
⁎Corresponding author. sshahabe@kku.edu.sa (Shahabe Saquib Abullais)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Oral candidiasis is considered one of the most frequent opportunistic infections in Human Immunodeficiency Virus (HIV) individuals. Symptomatic candida colonization of the oral and vaginal mucosal surfaces is closely correlated with the severity of cellular immunodeficiency in HIV infection. HAART is considered as the mainstay treatment that shows a decrease in the incidence of opportunistic diseases, including oral lesions. The current study was designed to analyse the candidal carriage rate and candidal density in HIV patients undergoing HAART and without HAART.
Methods
The study population comprised of 120 subjects and was equally distributed into 3 groups: Group 1 (HIV patients with HAART), Group 2 (HIV patients without HAART), and Group 3 (healthy subjects as controls). The oral rinse samples from all the groups were collected and incubated for candidal growth. The complete growth of any candidal colonies on the culture plates was recorded as a positive candidal carriage. The number of colonies forming units per mL was calculated for candidal density.
Results
The candidal carriage rate in the HIV patients undergoing HAART was higher than in subjects naïve to HAART. A higher number of patients with HAART therapy showed candidal density <2000 CFUs/mL. There was a reduction of oral candidiasis seen in patients with non-protease inhibitor HAART therapy. There was no significant association found between the candidal carriage rates and candidal density with CD4 lymphocyte count. HIV individuals undergoing HAART therapy showed higher candidal carriage rate and lower candidal density than the non-HAART group.
Conclusion
Candidal density is a more valuable marker in predicting the development of oral candidiasis than candidal carriage rate. Reduction in the occurrence of oral candidiasis is also seen in patients who are on NNRTI HAART therapy.
Keywords
Oral candidiasis
HAART
Candidal carriage
Candidal density
CD4 T cell
- HIV
-
human immunodeficiency virus
- HAART
-
highly active antiretroviral therapy
- AIDS
-
acquired immunodeficiency syndrome
- NNRTI
-
Non-nucleoside reverse transcriptase inhibitors
- ART
-
anti-retroviral therapy
- SDA
-
Sabouraud’s Dextrose Agar
- SPSS
-
statistical package for social sciences
- ELISA
-
enzyme-linked immunosorbent assay
- CFUs
-
colony-forming units
- IRB
-
institutional ethics review board
- CD4
-
cluster of differentiation 4
Abbreviations
1 Introduction
Acquired Immunodeficiency Syndrome (AIDS) is caused by Human Immunodeficiency Virus (HIV), which causes life-threatening opportunistic diseases (Douek et al., 2009). According to WHO data, approximately 38.0 million individuals around the world were living with HIV infection till 2019. In the year 2019, about 1.7 million new individuals got infected with HIV, whereas 0.7 million individuals lost their life due to HIV-related causes. Approximately 81% of individuals with HIV infection are aware of their HIV status in 2019. The remaining 19% of the individuals need access to HIV testing facilities.
In a healthy subject, a precise equilibrium exists between the multifaceted oral microbial ecology and the body’s immune response. HIV infects the human immune system causing reduction of CD4 T cells by direct viral killing, apoptosis and by CD8 cytotoxic lymphocytes following immune system failure resulting in opportunistic infections (Cunningham et al., 2010).
Yeasts are opportunistic pathogens that cause disease in the host compromised by underlying local or systemic pathological processes (Alrayyes et al., 2019). C. albicans are considered harmless oral microflora in the normal condition but the predominant causative organism involved in mucocutaneous candidiasis (70%) (Annapurna et al., 2012). Oral candidiasis may be a sentinel event and an independent predictor of immunodeficiency in patients with AIDS (Dodd et al., 1991). Prompt diagnosis and adequate antiretroviral therapy can decrease morbidity and mortality among such patients (Kantheti et al., 2012).
The introduction of Highly Active Anti-Retroviral Therapy (HAART) decreases morbidity and mortality by the increase in the CD4 count and reduction in the viral load (Nittayananta et al., 2010, Patton et al., 2000, Perla et al., 2021). In HAART therapy, drug resistance can be minimized by using combinations of drugs to restore immune function. Oral colonization of inherently drug-resistant organisms is more common in HIV infections (Leigh et al., 2001). Hence the experiment was designed to assess the oral carriage rate of the Candidal species and their density in HIV patients with and without HAART.
2 Materials and methods
2.1 Patient selection
The design employed for the current study was a descriptive cross-sectional multicentric. The patients attending the Voluntary Counselling and Confidential Testing Centre, Anti-retroviral Therapy (ART) center who were positive for HIV by tests [enzyme-linked immunosorbent assay (ELISA) and Western blot analysis] were included in the study. Institutional Ethics Review Board (IRB) approval was obtained after a thorough evaluation of the experimental protocol (MGV/092/11-14). Based on the inclusion criteria a total of 120 individuals (73 males and 47 females, aged 19–66 years) were selected for the study and divided into 3 groups. Group 1 consisted of 40 HIV-positive patients being treated with HAART regime and with a known CD4 T-lymphocyte count. Whereas, 40 HIV-positive patients not yet initiated with the HAART regime with a known CD4 T-lymphocyte count were included in Group 2. Group 3 included 40 HIV seronegative healthy subjects as controls. All the patients received NRTI + NNRTI combination, while none received (protease inhibitor) PI based drug regimen. HIV patients with HAART duration less than a month, patients with systemic ailments, pregnant patients and patients wearing prosthetic dentures were excluded from the study. The study protocol was explained to the patients and an informed consent form was obtained in writing before enrolment in the experiment.
2.2 Collection of samples
The patients were asked to rinse with normal saline (10 mL) for 60 s and expectorate into the sterile container. The rinse was collected into a sterile container, and was centrifuged for 15 min at 1700 rpm. 0.1 mL of undiluted and diluted oral rinse sample was immediately inoculated on two plates of Sabouraud’s Dextrose Agar (SDA) containing chloramphenicol. Dilution of 10:1 of the oral rinse sample was prepared by mixing 0.1 mL of oral rinse sample of HIV patients with 0.9 mL of sterile normal saline. The above plates were incubated aerobically at 37 °C for 48 to 72 h. Creamy white, smooth, pasty colonies of candidal growth have appeared in 3–4 days and some growth was seen on overnight incubation.
Positive candidal carriage rate was considered by complete growth of any candidal colonies on the culture plates (Fig. 1a and 1b) and negative by the absence of growth (Fig. 2a and 2b). The candidal density was calculated by counting the number of candidal colonies present in the plate manually. Surface count technique was employed to count the colonies. In this method, 0.1 mL of the sample was kept in the center of a dried plate and spread with a spreader all over the surface of the plate. The plate was then incubated, and the colonies were counted. The calculation was as follows: N no of colonies in 0.1 mL of 10-1dilution of the normal saline (since 0.1 mL of 10-1was spread on the agar plate): 10 N no of colonies in1mL of10-1dilution of the normal saline: 100 N colonies in 1 mL of sterile saline which gives the CFU’s/mL.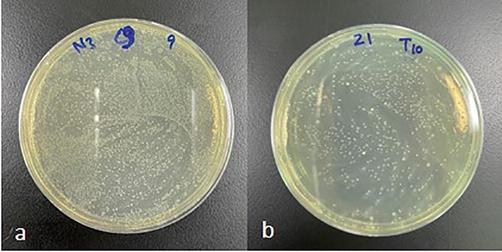
Positive growth of candida species in (a) undiluted and (b)diluted (10−1) plates.
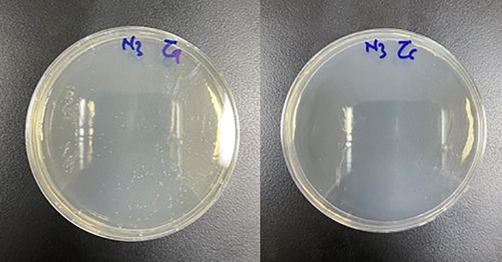
Negative growth of candida species in (a) undiluted and (b)diluted (10−1) plates.
2.3 Statistical analysis
The statistical analyses were done using the Statistical Package for Social Sciences (SPSS) version 21.0 (IBM Corporation, USA) for Microsoft Windows. A p-value < 0.05 was considered statistically significant under 95% confidence interval. A Chi-square test was applied to test the association between the candidal carriage rate and candidal density among the HIV-positive individuals with and without HAART. The same was applied to test the association between the candidal carriage rate and Candidal density with the CD4 count ≤ 200 and > 200 cells/mm3 and duration of the HAART.
3 Results
The comparison of candidal carriage rate between normal individuals and HIV patients showed a statistically significant difference (p < 0.05), indicating that there was positive a correlation between the two attributes. HIV patients showed a 60% of candidal carriage rate, whereas among the normal individuals it was found to be 37.5% (Table 1). *: P-value < 0.05 is considered to be statistically significant, **: P-value < 0.001 is considered to be highly statistically significant.
Individuals
Candida species
(Present)Candida species
(Absent)Total
P-value
%
n
%
n
%
n
HIV
60.0%
48
40.0%
32
100%
80
0.020*
Normal
37.5%
15
62.5%
25
100%
40
Total
52.5%
63
47.5%
57
100%
120
With HAART
77.5%
31
22.5%
09
100%
(40)
0.001**
Without HAART
42.5%
17
57.5%
23
100%
(40)
Total
60.0%
(48)
40.0%
(32)
100%
(80)
The distribution of candidal carriage rate between HAART and without HAART group showed a highly significant difference (p < 0.001). Regarding the patients with the HAART regime, 77.5% showed the presence of candida. Analysis of patients without HAART regime revealed that 42.5% had candidal species. Thus, it can be concluded that the prevalence of candida was more in patients receiving HAART than those who were naive to HAART therapy (Table 1).
Candidal density of 1–2000 CFU’s/mL and >2000 CFU’s/mL in healthy individuals and HIV patients revealed no significant relationship (p > 0.05). Out of 63 patients who showed a positive candidal carriage rate, 48 were HIV patients and 15 were healthy individuals. It was found that 41.7% of HIV patients had candidal density > 2000 CFU’s/mL compared to 26.7% of healthy individuals (Table 2). NS: P-value > 0.05 is considered to be non-significant, *: P-value < 0.05 is considered to be statistically significant.
Individuals
1–2000
(CFU’s/mL)>2000
(CFU’s/mL)Total
P-value
%
N
%
n
%
n
HIV
58.3%
(28)
41.7%
(20)
100%
(48)
0.371NS
Normal
73.3%
(11)
26.7%
(04)
100%
(15)
Total
61.9%
(39)
38.1%
(24)
100%
(63)
With HAART
70.9%
(22)
29.0%
(09)
100%
(31)
0.017*
Without HAART
35.3%
(06)
64.7%
(11)
100%
(17)
Total
58.3%
(28)
41.7%
(20)
100%
(48)
The association between the HAART regime and the candidal density count showed a statistically significant relationship (p < 0.05). It was observed that 70.9% of the subjects with HAART regime had a candidal density count of 1–2000 CFU’s/mL, compared to 35.3% of subjects without HAART regime. However, candidal density counts of > 2000 CFU’s/mL were presented in 29.0% of the subjects with HAART regime compared to 64.7% of subjects without HAART regime (Table 2).
The standard classification of CD4 count ≤ 200 and > 200 cells/mm3 was used for HIV patients. A non-significant correlation was seen between the HAART and without HAART group associated with CD4 count. Furthermore, the other studied parameters such as the prevalence of candidal carriage rate and candidal density revealed a non-significant correlation with a CD4 count of HIV patients (p > 0.05) (Table 3). NS: P-value > 0.05 is considered to be non-significant.
CD4 COUNT (cells/mm3)
Parameters
Total
≤200
>200
P-value
%
n
%
N
%
n
HIV individuals
With HAART
50%
40
57.5%
23
42.5%
17
0.117 NS
Without HAART
50%
40
40.0%
16
60.0%
24
Candidal Carriage Rate
Candida species (Present)
60.0%
48
66.7%
(26)
53.6%
(22)
0.235 NS
Candida species (Absent)
40.0%
32
33.3%
(13)
46.4%
(19)
Candidal density (CFUs/ml)
1–2000 (CFU’s/ml)
57.7%
46
51.3%
20
63.4%
26
0.273 NS
>2000 (CFU’s/ml)
42.3%
34
48.7%
19
36.6%
15
The association between the duration of HAART (<12 months and ≥ 12 months) with CD4 count and with candidal density. Patients with CD4 count ≤ 200 showed no difference with the duration of the HAART. Whereas, the patients < 12 months of HAART therapy showed a higher prevalence of CD4 count > 200 compared to ≥ 12 months HAART therapy. The duration of the HAART therapy showed no effect on the candidal density above 2000 (CFUs/mL). However, an increased number of patients with candidal density <2000 (CFUs/mL) were reported in ≥ 12 months HAART therapy group compared to < 12 months of HAART therapy group, but the values were statistically non-significant (p > 0.05) (Table 4). NS: P-value > 0.05 is considered to be non-significant.
Duration of HAART therapy
Total
<12 month
≥12 month
%
n
%
N
%
n
P-value
≤200
57.5%
23
43.5%
10
56.5%
13
0.184 NS
>200 1–2000
42.5%
17
65.5%
11
35.0%
6
Candidal density (CFUs/ml)
(CFU’s/mL)
67.5%
21
43.0%
9
57.0%
12
0.709 NS
>2000 (CFU’s/mL)
35.5%
10
50.0%
5
50.0%
5
4 Discussion
Human immunodeficiency virus infection remains a threat to global health and its oral manifestation is an important indicator for the disease progression (Ranganathan and Hemalatha, 2006). Common species isolated from the oral cavity of patients with HIV infection is C. albicans. Antifungal drugs are effective in treating mucosal candidiasis can lead to colonization with less susceptible species among normal susceptible strains (Badiee et al., 2010). Due to the rapid global spread of resistant clinical isolates of candida species, accurate identification of candidal species is therefore crucial for successful clinical management in patients with HIV.
Nucleoside Reverse Transcriptase Inhibitors (NRTIs), Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs), and Protease Inhibitors (PIs) are the groups of drugs used in the HAART regime. These drugs interfere the viral replication and reduce the viral load to undetectable levels to allow immune restoration and decrease the incidence of opportunistic disease occurring in the oral cavity (Greenspan et al., 2001, Ceballos-Saloberna et al., 2004).
The candidal carriage rate in HIV-infected patients was significantly higher than healthy groups in the present study. This indicates that the presence of cellular immunodeficiency in HIV infection is closely related to candidal colonization of oral mucosal surfaces and the development of symptomatic Candidiasis (Teanpaison and Nittyananta, 1998). The candidal carriage rate in the HIV patients undergoing HAART and those who were not on HAART therapy demonstrated an increase in candidal carriage rate in the former group. This correlation could be explained in literature emphasizing that HAART reduces incidences of oral candidiasis. Candidal species oral colonization is defined as the acquisition and maintenance of yeast cells without clinical signs. Therefore, dissociation needs to be made between colonization and infection. Other possible explanations for this colonization include poor compliance to HAART therapy, failure to reduce the viral load, and an inadequate rise in the CD4 lymphocyte count (Ananthalakshmi et al., 2011).
Our findings were in contrast to the studies reported in the literature that showed an increase in the candidal carriage rate in HIV individuals not undergoing HAART therapy compared to patients under HAART therapy (Martins et al., 2010, Lar et al., 2012). The presence of candidal species can be a risk factor as they overcome the host clearance mechanism. Therefore, candida density (expressed in the number of CFU’s/mL) is directly correlated with the presence of clinical candidiasis. Other factors like production of adhesins, secreted aspartyl proteinase and host factors will also influence the presence or absence of the clinical disease (Jain et al., 2010). Host local predisposing conditions like reduced saliva secretion, epithelial change, altered commensal flora, high carbohydrate diet, denture wearing and host systemic factors such as age, tobacco, smoking, endocrine disorders, nutritional deficiencies, immunosuppressive conditions and drugs have also been associated with oral candidiasis colonization (Patel et al., 2006).
We found a significantly higher number of patients not receiving HAART having a candidal density above 2000 CFU’s/ml compared to those who were on HAART. Candidal densities in individuals with HAART and normal individuals were almost the same. This indicates that HAART has a role in controlling candidal density and hence candidal infection (Soares et al., 2004). There is an increase in number of patients with HAART having density < 5000 CFU’s/ml compared to patients without HAART. HAART reduces the frequency and severity of oral opportunistic diseases in HIV-infected patients (Umadevi et al., 2007, Greenspan et al., 2004, Wray et al., 1990, Perla et al., 2021).
Hyphal form of C. albicans penetrates the deep tissue by Secreted Aspartic Proteases (SAP’s) in HIV patients. These proteases were thought to be inhibited by the HAART-based protease inhibitors (Sono et al., 1992, Fusek et al., 1994). Hence a decreased prevalence was found to be associated with the advent of HAART which included the use of Protease Inhibitors. PI based HAART group showed a significant decrease in episodes of oral candidiasis and candidal carriage than NNRTI group (Cauda et al., 1999, Cassone et al., 2002).
In our study, all the patients received NRTI + NNRTI combination while none received PI based drug regimen. Based on our results, we can conclude that reduction in the occurrence of oral candidiasis is also seen in patients who are not on PI- included HAART therapy.
The duration of the HAART on candidal density and CD4 count was not statistically significant in our study. The candidal density showed similar irrespective of the duration. The CD4 count also showed more count in the initiation of HAART treatment. More scientific studies are recommended in correlation with the duration of HAART and candida.
We observed no significant association was seen between candidal carriage rate and CD4 lymphocyte count. The vital host defense mechanisms against candidal infection are governed by T lymphocyte dependent cell-mediated immune response. There are two diverse subgroups of T helper cells (Th1 and Th2) with antagonistic functions. Furthermore, Th1 secretes IFN-α and IL-2 which are responsible for protection against oral candidiasis, whereas Th2 is responsible for IL-4 and IL-10 production has been linked to candidal reactivity (McMichael and Rowland-Jones, 2000).
Mucocutaneous candidiasis comparatively is more common in HIV-infected individuals than other immunosuppressive conditions and be seen when HIV viral load is >10,000 copies/mL. This can be attributed to the fact that the switch from Th1 cells to Th2 cells probably progresses the HIV infection with a concomitant increase in the HIV viral load. Hence HIV viral load is currently considered an important progressive indicator of HIV-induced immune depression and a good predictive indicator than CD4 cell counter activity (McMichael and Rowland-Jones, 2000).
5 Conclusion
The candidal carriage rate is higher in and candidal density is lower in HIV patients undergoing HAART therapy. Candidal density is a more valuable marker in predicting the development of oral candidiasis than the candidal carriage rate. Reduction in the occurrence of oral candidiasis is also seen in patients who are not on PI-included HAART therapy. HIV viral load can consider the main indicator of the progression of HIV-induced immune depression and is thought to be a better predictive indicator than CD4+ cell count.
Author contribution
Shahabe Saquib Abullais, Nitish Perla: designed and coordinated the research, Shahabe Saquib Abullais, Nitish Perla, Shaik Mohamed Shamsudeen: carried out sampling, Nitish Perla, Shaik Mohamed Shamsudeen, Mohammad Yahya AlShahrani: carried out laboratory procedures, Mohammad Yahya AlShahrani, Suheel Manzoor Baba, Shafait Ullh Khateeb, Nabeeh Abdullah AlQahtani: helped in data analysis, Shahabe Saquib Abullais, Nitish Perla, Mohammad Yahya AlShahrani, Suheel Manzoor Baba, Shafait Ullh Khateeb, Nabeeh Abdullah AlQahtani: helped in drafting, review and editing the manuscript. All authors have gone through the final manuscript and approved it.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group Project under grant number (RGP-1/247/1442).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Oral Candidal carriage and associated risk indicators among adults in Sakaka, Saudi Arabia. BMC Oral Health. 2019;19:86.
- [CrossRef] [Google Scholar]
- Oral manifestations of HIV patients in South Indian population. J. Pharm. Bio. Allied. Sci.. 2012;4:364-368.
- [CrossRef] [Google Scholar]
- Distribution and antifungal susceptibility of candida species from mucosal sites in HIV positive patients. Arch. Iran. Med.. 2010;13:282-287.
- [CrossRef] [Google Scholar]
- The effect of highly active antiretroviral therapy on the prevalence of HIV associated oral candidiasis in a Spanish cohort. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod.. 2004;97:345-350.
- [CrossRef] [Google Scholar]
- Antiretroviral therapy with protease inhibitors has an early, immune reconstitution-independent beneficial effect on Candida virulence and oral candidiasis in human immunodeficiency virus infected subjects. J. Infect. Dis.. 2002;185(2):188-195.
- [Google Scholar]
- Role of protease inhibitors in preventing recurrent oral candidosis in patients with HIV infection: a prospective case-control study. J. Acquir. Immune. Defic. Syndr.. 1999;21:20-25.
- [CrossRef] [Google Scholar]
- Manipulation of dendritic cell function by viruses. Curr. Opin. Microbiol.. 2010;13:524-529.
- [CrossRef] [Google Scholar]
- Oral Candidiasis in HIV infection: Pseudomembranous and erythematous candidiasis show similar rates of progression in AIDS. AIDS.. 1991;5(11):1339-1344.
- [Google Scholar]
- Emerging concepts in immune-pathogenesis of AIDS. Annu. Rev. Med.. 2009;60:471-484.
- [CrossRef] [Google Scholar]
- Extracellular Aspartic Proteinases from Candida albicans, Candida tropicalis and Candida parapsilosis yeasts differ substantially in their specificities. Biochem.. 1994;33:791-799.
- [CrossRef] [Google Scholar]
- Effect of Highly Active Antiretroviral therapy on frequency of oral warts. Lancet.. 2001;357:1411-1412.
- [CrossRef] [Google Scholar]
- Incidence of oral lesions in HIV-1-infected women reduction with HAART. J. Dent. Res.. 2004;83:145-150.
- [CrossRef] [Google Scholar]
- Comparative study of adherence of oral candida albicans isolates from HIV sero-positive individuals and HIV sero-negative individuals to human buccal epithelial cells. Indian. J. Pathol. Microbiol.. 2010;53:513-517.
- [CrossRef] [Google Scholar]
- Oral lesions in HIV infection in developing countries: An overview. Adv. Dent. Res.. 2006;19:63-68.
- [CrossRef] [Google Scholar]
- Isolation, identification, and carriage of candidal species in PHLAs and their correlation and immunological status in cases with and without HAART. J. Oral. Maxillofac. Pathol.. 2012;16:38-44.
- [CrossRef] [Google Scholar]
- Prevalence and distribution of Candida Species in HIV infected persons on antiretroviral therapy in Jos. J. Med. Med. Sci.. 2012;3:254-259.
- [Google Scholar]
- Candida-specific systemic cell-mediated immune reactivities in human immunodeficiency virus–positive persons with mucosal candidiasis. J. Infect. Dis.. 2001;183(2):277-285.
- [Google Scholar]
- Oral Candia carriage of patients attending a dental clinic in Braga, Portugal. Rev. Iberoam. Micol.. 2010;27:119-124.
- [CrossRef] [Google Scholar]
- Effects of long-term use of HAART on oral health status of HIV-infected subjects. J. Oral. Pathol. Med.. 2010;39:397-406.
- [CrossRef] [Google Scholar]
- Effect of antifungal treatment on the prevalence of yeast in HIV infected subjects. J. Med. Microbiol.. 2006;55:127-184.
- [CrossRef] [Google Scholar]
- Changing prevalence of oral manifestation of human immunodeficiency virus in the era of protease inhibitor therapy. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod.. 2000;89:299-304.
- [CrossRef] [Google Scholar]
- Quantification of oral candidal carriage rate and prevalence of oral candidal species in HIV patients with and without highly active antiretroviral therapy. J. Microsc. Ultrastruct.. 2021;9(4):145-153.
- [CrossRef] [Google Scholar]
- Pediatric HIV-related oral manifestations: a five-year retrospective study. Braz. Oral. Res.. 2004;18:6-11.
- [CrossRef] [Google Scholar]
- Comparison of secretary acid proteinases from Candida tropicalis, Candida parapsilosis, Candida albicans. Microbiol. Immunol.. 1992;36:1099-1104.
- [CrossRef] [Google Scholar]
- Prevalence of Candida species in AIDS patients and HIV free subjects in Thailand. J. Oral. Pathol. Med.. 1998;27:4-7.
- [CrossRef] [Google Scholar]
- Oral lesions among persons with HIV disease with and without highly active antiretroviral therapy in southern India. J. Oral. Pathol. Med.. 2007;36:136-141.
- [CrossRef] [Google Scholar]
- Alteration of humoral responses to Candida in HIV infection. Br. Dent. J.. 1990;168:326-329.
- [CrossRef] [Google Scholar]







