Translate this page into:
Green synthesis, characterization and biomedical potential of Ag@Au core–shell noble metal nanoparticles
⁎Corresponding author. ortashi9@ksu.edu.sa (Khalid M.O. Ortashi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the current times, nanotechnology has emerged as a field that promises scientific advancement with the manipulation, improvement, and application of near-atomic scale materials.Bimetallic nanoparticles (NPs) have been explored for their wide range of applications owing to their multi-functionalilty. The present study included the synthesis of bimetallic silver@gold (Ag@Au) NPs from aqueous chloroauric acid (HAuCl4) and silver nitrate (AgNO3) making use of ‘green chemistry’ with the extract of Acacia nilotica husk as a reducing and capping agent. The formed bimetallic NPs were characterized by various spectrometry and electron microscopy techniques and devices, including ultraviolet–visible spectroscopy (UV/VIS), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS) attached to scanning electron microscopy (SEM), Fourier-transform infrared spectroscopy (FTIR), and the particle size distribution was analyzed by dynamic light scattering (DLS) technique. FTIR results identified the functional groups that are involved in and interact with biomolecules and nanoparticles surfaces through extensive screening on synthesized colloidal bimetallic NPs. The synthesized core – shell had mean size and monodispersity index (PDI) values of 0.223 and 61 nm, respectively. TEM micrographs clearly indicated that the Au NPs were coating and surrounding the Ag NPs and their size, distribution and shapes were homogeneous and uniform. Anticancer activity of the synthesized Ag@Au NPs was explored using MTT assay; the recorded cell viability was 500 μg/ml and the cytotoxicity (IC50) was 74.6 ± 7.43 µg/ml for HeLa cell line. This anticancer activity of the Ag@Au NPs opens the window wide for their use in cancer therapy and other biomedical applications.
Keywords
Green synthesis
Silver@Gold
Nanoparticles
Acacia nilotica
Anticancer activity
1 Introduction
Manipulation and control of nanostructures is a key concept of nanotechnology since it aids in synthesizing nanomaterials of variable sizes and shapes with a flexible control on their physical and chemical properties. This in turn broadens their range of application for the betterment of human life (Meydan et al., 2022; Gur et al., 2022).
Nanoparticles of metals (copper, iron, gold, silver, etc) display physical and optical characteristics (surface plasmon reverberation or surface plasmon resonance) that attracted attention to them as candidates for specific applications in medicine and science (Mallick et al., 2021). Particularly, noble metals (gold and silver) are well known and have been proved for use in medicine (Behzad et al., 2020; Venditti, 2019). They are considered innocuous materials with remarkable biological characteristics to be used for several optical, electronic, electrical, catalytic, applications (Ahmed et al., 2022; Calimli et al., 2021). Silver has been widely used for its anti-carcinogenic,antimicrobial and wound healing properties (Aygun et al., 2022). Nano-gold is inert, multifunctional with low cytotoxicity; therefore, it was used as nanocarrier for drug delivery. Since it has the ability to absorb visible light within picoseconds and efficiently release wavelength- particular energy in a precise manner, it can also be used in photodynamic therapies (light-mediated clinical treatments) (Tabish et al., 2020). Merged silver and gold (Ag–Au) bimetallic nanoparticles have intriguing and unique electronic, optical, catalytic, structural and biomedical properties (Loza et al., 2020); they can be used in cosmetics and beauty care products, anticancer, cell reinforcement, and antimicrobial drugs (Seckin et al., 2022).
Green synthesis methods of NPs are environment- friendly, economic, energy- and time saving. Using plants and their parts for formulation of NPs renders them safe and stable with enhanced activity, biocompatibility, and influential penetration ability (Meydan et al., 2022; Zhang et al., 2020).
Acacia nilotica (L.) is a tree native in central and northern Sudan; it is commonly famous in Sudanese traditional herbal medicine (local name is Garad). Both fruits and bark are used for treatment of colds, bronchitis, pneumonia, diarrhoea, and dysentery (Pousset, 1989). A. nilotica is generally used in ethno- and veterinary medicine. On the other hand, in Sudanese and Indian folk medicine, its bark decoction is used as the gargled to relieve toothaches and strengthen teeth (Kabbashi et al., 2015). Many studies have shown its wide range of pharmacological properties, such as anti HIV-1 protease, antibacterial, anti-inflammatory, antioxidant, and anticarcinogenic activities (Muddathir et al., 2020).
Current trends in therapeutics research explores the use of innovative modes such as green-synthesis of NPs with phytocompounds and herbs as novel strategies in the treatment of a host of diseases, including cancer (Göl et al., 2020).
The present study describes a facile approach for the synthesis of Ag@Au NPs using A. nilotica husk extract as stabilizer- reducing agent followed by a complete characterization of the synthesized bimetallic NPs. The aim was to amalgamate the therapeutic potential of this plant with the inherent anticancer activity of the synthesized NPs to accentuate the ability of the nanostructure as an anti-carcinogenic agent. To the best of our knowledge, this is the first study on the synthesis of core @shell NPs using A. nilotica and evaluation of its anti-proliferative effect.
2 Materials and methods
2.1 Synthesis and characterization of core–shell Ag@Au NPs
All chemicals were reagent grade and used without any further purification. Silver nitrate (AgNO3) and Chloroauric acid (HAuCl4) were obtained from Techno Pharmchem (India) and, Loba Chemie (India), respectively; Acacia nilotica husk was obtained from the local market in Khartoum, Sudan.
Synthesis of core@shell NPs was achieved as follows: (1) 5 g of A. nilotica husk was washed with distilled water to remove the dust, mixed with 50 ml of boiled distilled water, incubated overnight at room temperature, filtered through filter paper (Whatman’s No. 1) and then kept at 4 °C until used; (2) 1 mM of AgNO3 (A) and 1 mM of HAuCl4 (B) were prepared as aqueous solutions; (3) core–shell nanoparticles were synthesized using a modified Abhijit Biswas’s method (Biswas et al., 2014). Briefly, 50 ml aqueous solution (A) was added to 10 ml of the extract aqueous solution. The mixture was stirred continuously at room temperature for 15 min. A very light brown colour solution indicated the reduction Ag ions to Ag NPs. Afterwards, aqueous solution (B) was added to the previous mixture, a colour change to very dark violet within 30 min was observed, indicating formation of Ag@Au core–shell nanoparticle.
2.2 Characterization of core–shell Ag@Au NPs
Ag@Au core–shell nanoparticles were analyzed for the noticeable plasmon bands by UV/VIS spectrophotometer (Perkin Elmer, Waltham, MA, USA). The mean size of the synthesized NPs was analysed by the DLS technique using Nano series (Zetasizer, HT Laser, ZEN3600 (Malvern Panalytical Ltd, Malvern, UK). Size, shape, and morphologies of NPs were characterized by JEM-1011 transmission electron microscope (TEM) (JEOL, Tokyo, Japan). Further analysis of shape and morphology of the NPs was achieved by JEOL-FE SEM scanning electron microscope (SEM), (JEOL) equipped by Energy Dispersive X-Ray Spectroscope (EDS) model INCAx-act (Oxford Instruments, Oxford, UK) for confirming the existence of silver and gold elements in the bimetallic sample. The chemical finger-printing of the extract and synthesized core–shell NPs was achieved by Infrared Spectroscopy using FTIR-Spectrum BX (Perkin-Elmer) with measurements in the range 400 to 4400 cm−1 using the potassium bromide method.
2.3 Assessment of cytotoxic effects using MTT assay
The MTT assay was carried out using a standard protocol (Awad et al., 2019). A culture of Human cervical carcinoma cells (HeLa) in DMEM supplemented with 10% heat-inactivated FBS, L-glutamine (1%), gentamycin (50 µg/ml), and buffer (HEPES), was seeded by the NPs. The cells were incubated at 37 °C in 5% CO2 and sub-cultured twice a week. Cell viability was assessed at varying concentration of the synthesized NPs.
3 Results and discussion
Nanoparticles of noble metals are unique in optical properties; they interact highly with particular light wavelengths (Zhang et al., 2016). Because of the surface plasmon resonance (SPR) of bimetallic NPs, they show good absorptions of the visible spectrum, with 400–600 nm peak intensity. SPR occurs in UV–visible spectrum as result of collective oscillations of conductive electrons (Sullivan et al., 2018). Fig. 1 presents the absorption band measurement of the Ag@Au NPs synthesized in this study. Change of band is based on the presence of another surface material. A change of sample colour was observed when silver particles were capped by gold in the reaction procedure. Here, the plasmonic absorption is governed by gold, which has a peak range 460∼550 nm. It has been demonstrated that plasmon absorption can be used for the qualitative characterization of the shell thickness of core–shell nanoparticles (Sullivan et al., 2018). The LSPR absorbance intensity of the core declined with increased shell thickness (Asadi-Aghbolaghi et al., 2021). Thus, the SPR of the Au shell is more clearly appreciable. Also, the SPR maximum intensity of Ag (makes up the core), is not remarkable, as Fig. 1 shows. Based on these observations, the shells would be fairly thick. This could be due to the direct reduction of excess HAuCl4 by the extract to produce pure core–shell NPs with small size. Also, the broad absorbance band indicates aggregation of the formed NPs (Verma et al., 2021).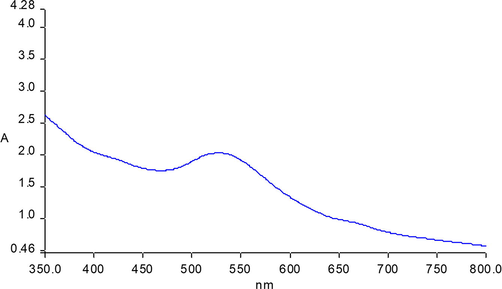
UV–visible spectrum of green synthesized Ag @Au NPs.
TEM analysis confirmed variation in size and shape of synthesized NPs by the reduction method with A. nilotica extract, which also demonstrated homogeneous size distribution. TEM micrographs of the NPs clearly indicate that Au NPs were coating and surrounding Ag NPs (Fig. 2). Apparently, the size distribution and shapes of NPs were homogeneous and uniform, with most quasi-spherical, few polygonal, and quasi rod-shaped. The isotropic feature of the formulated particles possibly reflected the efficient capping by the constituent biomolecules of the extract, that prevented the aggregation of the particles. It was suggested by Saha and colleagues that the high density of synthesiszed Ag@Au NPs had led to abundance of electromagnetic hotspots which in turn could be an efficient substrate for surface-enhanced Raman scattering (Saha et al., 2021). Likewise, the magnified TEM images reveal a nearby view of the core–shell NPs as sphere-like, polygonal, and rod-like. Additionally, TEM analysis showed increase in size of the shell layer - clearly due to the thickness of the metallic gold layer- in agreement with previous reports (Verma et al., 2021; Pham et al., 2021).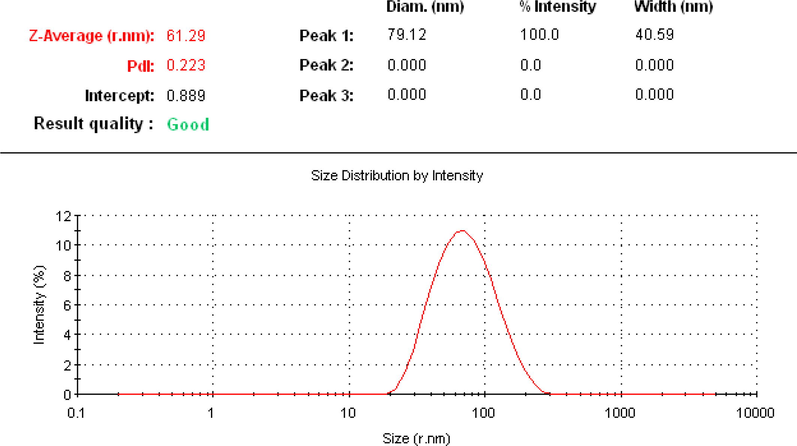
DLS measurement of produced core–shell nanoparticles.
Average size and shape of synthesized nanoparticles could be analyzed by SEM as an analytical imaging technique and confirmation of existence of elements in NPs could be achieved by EDS mapping technique (Nayem et al., 2020; Umamaheswari et al., 2018). The SEM image shows slight aggregation of Ag@Au NPs with spherical and a few polygonal shapes, as shown in Fig. 3 (left). The gold element fixed the elevated absorption peak at 2.15 keV and in the range between 8 and 14 keV, which is the same as that of metallic Au nanocrystallites (Ramezani et al., 2008). On the other hand, due to the SPR, the absorption peak noticeable at 3.0 keV in EDS spectrum indicates and confirms the presence of silver element (Fig. 3 - right) (Shaik et al., 2018). Other peaks appearing in Ag@Au NPs EDS spectrum in the range 0–0.5 keV show occupancy of C and O2 elements (Naraginti and Li, 2017). Accordingly, the intensity of signals of Au and Ag vary with the molar ratio of each in the sample. As a result, there were no impurity peaks observable in the samples, confirming their high purity. The EDS spectrum disclosed the presence of Au element peaks in five different positions and one peak of Ag. The EDS spectrum clearly showed 43.82% and 56.18% yields for silver and gold, respectively. These results indicate that the mixture of gold and silver atoms exist in the solution and the gold exist as shell.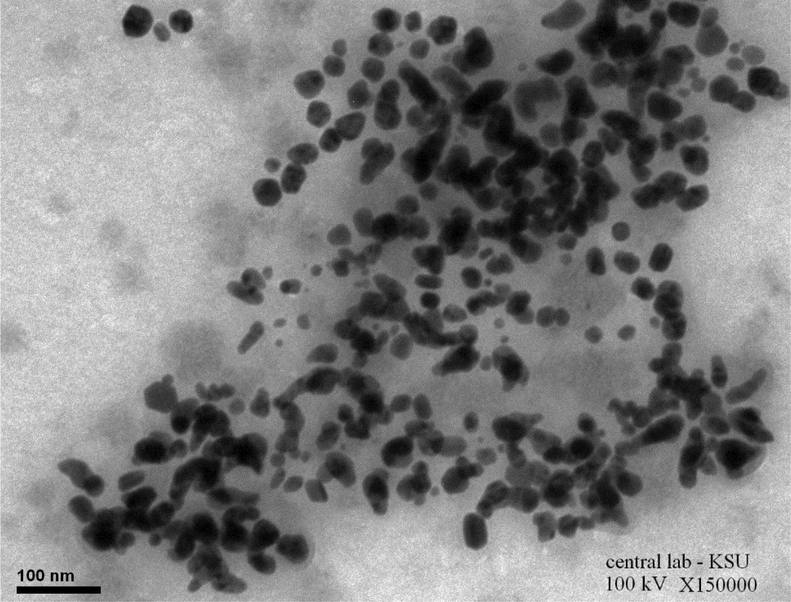
TEM micrographs of the formed Ag@ Au core -shell NPs.
To gain further information about the presence of bio-molecules capping layer on the synthesized NPs sample, FTIR analysis of A. nilotica extract and synthesized NPs were carried out and overall similarities between the two samples were found. In the spectra of A. nilotica and silver core- gold shell NPs (Fig. 4), the chemical constituent and functional groups reflected their comparable peaks corresponding to carboxylic acid and ketones (1724–1700 cm−1), NH3+ in NH4OH due to NH3 deformation (1600–1520 cm−1). Also, a N-O symmetric stretch (1360–1290 cm−1) was caused due to the compounds of N-O nitro, aliphatic ethers (C-O-C), silicates (1040–1030 cm−1), NH2 in NH4OH; secondary alcohols (1095–1074 cm−1) (C–OH) group frequencies reflected NH2 groups in-plane. In addition, peaks (900–700 cm−1) =CH of aromatic hydrocarbons, corresponds to =C–H. Out-of-plane bending and (700–610 cm−1) vibration modes were relevant to alkynes –C≡ C– H: C– H bond, also been reported in previous studies (D’Angelo and Zodrow, 2011; Sadiq et al., 2015). The peaks (1124.99 and 865.64 cm−1) seen in the extract indicating possible presence of biomolecule were absent in Ag@Au NPs spectrum, which might be responsible for the formulation of core - shell NPs (Aadil et al., 2014; Singh et al., 2018).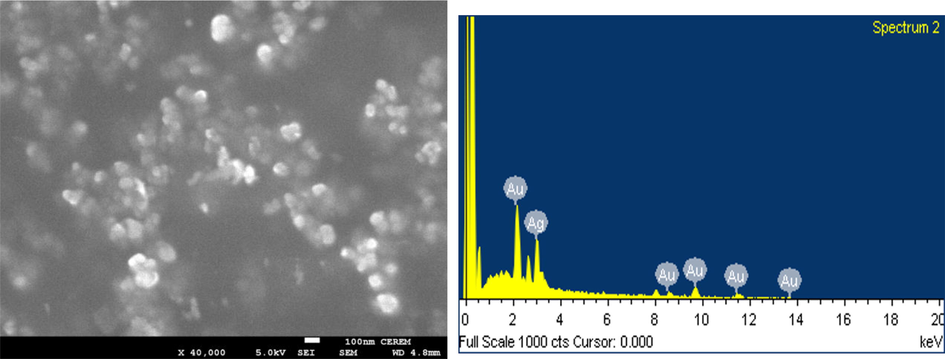
SEM image (Left) EDS spectrum (Right) of green Ag@Au core–shell NPs.
Hydrodynamic size, polydispersity index, and surface zeta potential of synthesized NPs were determined by dynamic light scattering technique (Raj et al., 2018). Fig. 5 shows that the average size of the synthesized core–shell was 61 (r.nm) and monodispersity index (PDI) was 0.223. In general, nanoparticles that have PDI values less than 0.5 are monodisperse (Costa et al., 2020).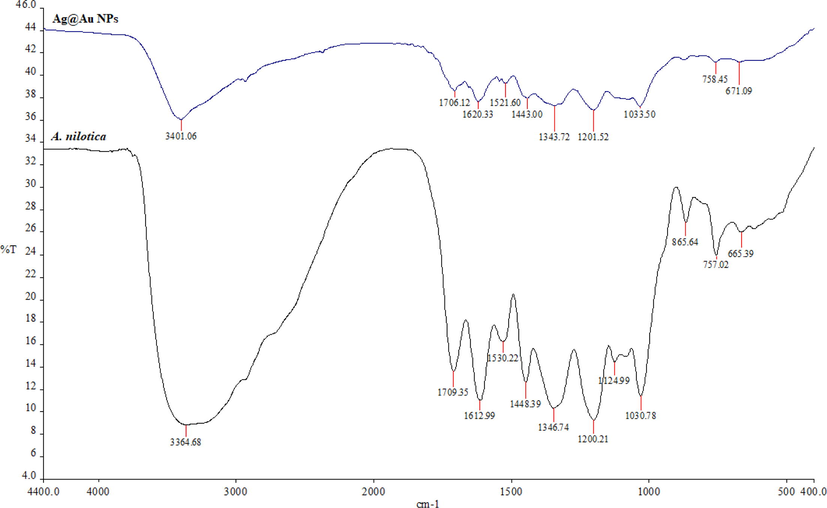
FTIR spectra analysis of synthesized Ag@Au NPs and extract to identify functional groups (chemical constituents) and interactions between biomolecules and nanoparticle surfaces.
There have been very few studies that have reported the synthesis of core@ shell noble metal nanoparticles using plant extracts. Botha et al. (2019) synthesized bimetallic nanoparticles with a diameter of 186.3 nm using the leaf extract of Sambucus canadensis and evaluated their cytotoxicity. Furthermore, Ahmed and his colleagues synthesized Au-Ag NPs of about 26–90 nm using bio-waste extract of Trapa natans and also examined their cytotoxicity for various cancer cells (HCT116, MDA-MB-231, and HeLa) (Botha et al., 2019; Ahmad et al., 2019). Anti-carcinogenic activity and behaviour of NPs in biological fluids is attributed to their structure, size, shape and distribution (Zhang et al., 2020; Botha et al., 2019). The present study, assessed the anticancer activity of Ag@Au NPs in the cervical carcinoma (HeLa) cell line. The growth (viability) of HeLa cells was inhibited to 6.31 ± 1.36% at the concentration 500 µg/ml with IC50 at 74.6 ± 7.43 µg/ml (Fig. 6). The MTT assay assesses the activity of succinate dehydrogenase, therefore, the observed effect could be explained based on the cytostatic or cytotoxic effect of the NPs. However, results revealed a significant toxic potency of the synthesized Ag core @Au shell NPs. Previous studies have reported the states of NPs in bio-media as accumulation into and clearance from cells. The Trojan horse effect could be possibly the reason for the accumulation of NPs into cells which may be aided by the gold shell surface coating. It was also stated that once the Ag@Au NPs enter the cells, cells are ruptured with consequent release of silver, which eventually results in greater toxicity (Singh et al., 2018). In addition, the cytotoxic effect of nanoparticles could also be explained based on the oxidative damage to cells and tissues via generation of free radicals, disruption of mitochondrial electron transfer chain by diffusion into cells or endocytosis. Further, effects on intracellular calcium concentration and activation of transcription factors by the production of reactive oxygen species (ROS), which are also implicated in causing DNA damage, disarray in several cellular processes, such as cellular signalling pathways, gene expression, and inflammatory and apoptotic pathways (Singh et al., 2018).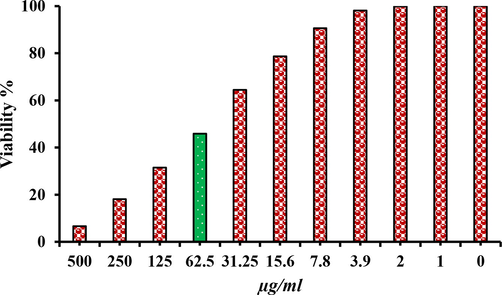
MTT assay result confirm the cytotoxic effect of green synthesized core@shell NPs against Hela cell lines.
However, further in vitro and in vivo studies are imperative for an insightful understanding of the mechanisms and modes of clearance of nanoparticles to broaden the existing knowledge that is the cornerstone of development and improvement of novel anticancer drugs in the fileld of nanomedicine.
4 Conclusion
In conclusion, the present study reported green formulation of silver@ gold core–shell nanoparticles using the husk extract of the medicinal plant Acacia nilotica. This green method is non-toxic, eco-friendly, simple to apply, economical, and uses non-hazardous materials. The results showed that A. nilotica acts as a significant reducing and stabilizing agent in the synthesis of NPs. The formulated core- shell NPs were described and characterized: the Au NPs were surrounding the Ag NPs with homogeneous and uniform size and shapes. The synthesized Ag@Au NPs proved significantly high cytotoxicity against HeLa cancer cells at 500 μg/mL with IC50 values noted at 74.6 ± 7.43 µg/ml, thus demonstrating potential use in cancer therapeutics and other applications in pharmaceutical industry and nanomedicine.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors extend their appreciation to the Researchers Support Project (number RSP-2021/173) of King Saud University.
Disclosure statement
There were no conflicts of interest reported by the authors.
References
- Novel eco-synthesis of PD silver nanoparticles: characterization, assessment of its antimicrobial and cytotoxicity properties. Materials. 2019;12(23):3890.
- [Google Scholar]
- Free radical scavenging activity and reducing power of Acacia nilotica wood lignin. Int. J. Biol. Macromol.. 2014;67:220-227.
- [Google Scholar]
- Biosynthesized composites of Au-Ag nanoparticles using Trapa peel extract induced ROS-mediated p53 independent apoptosis in cancer cells. Drug Chem. Toxicol.. 2019;42(1):43-53.
- [Google Scholar]
- Green approaches in synthesizing nanomaterials for environmental nanobioremediation: technological advancements, applications, benefits and challenges. Environ. Res.. 2022;204:111967
- [Google Scholar]
- Effects of ligands on (de-) enhancement of plasmonic excitations of silver, gold and bimetallic nanoclusters: TD-DFT+ TB calculations. PCCP.. 2021;23(33):17929-17938.
- [Google Scholar]
- Highly active PdPt bimetallic nanoparticles synthesized by one-step bioreduction method: characterizations, anticancer, antibacterial activities and evaluation of their catalytic effect for hydrogen generation. Int. J. Hydrog. Energy. 2022
- [Google Scholar]
- An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem.. 2021;94:92-104.
- [Google Scholar]
- (Ed.). Nanotechnology in Sustainable Agriculture. Crc Press; 2021.
- Peptide stabilized Ag@Au core-shell nanoparticles: synthesis, variation of shell thickness, and catalysis. Zeitschrift für anorganische und allgemeine Chemie.. 2014;640(6):1205-1211.
- [Google Scholar]
- Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep.. 2019;9(1):1-8.
- [Google Scholar]
- General synthesis methods of inorganic materials for supercapacitors. In: Khan A., Asiri A.M., Boddula R., Kolosov A., eds. Handbook of Supercapacitor Materials: Synthesis, Characterization, and Applications. Wiley; 2021. p. :187-203.
- [Google Scholar]
- Green synthesis of gold nanoparticles obtained from algae sargassum cymosum: optimization, characterization and stability. BioNanoScience.. 2020;10(4):1049-1062.
- [Google Scholar]
- Chemometric study of functional groups in different layers of Trigonocarpus grandis ovules (Pennsylvanian seed fern, Canada) Org. Geochem.. 2011;42(9):1039-1054.
- [Google Scholar]
- Green synthesis and characterization of Camellia sinensis mediated silver nanoparticles for antibacterial ceramic applications. Mater. Chem. Phys.. 2020;250
- [Google Scholar]
- Green synthesis, characterization and bioactivity of biogenic zinc oxide nanoparticles. Environ. Res.. 2022;204
- [Google Scholar]
- Antigiardial, antioxidant activities and cytotoxicity of ethanolic extract of leaves of Acacia nilotica (L) Adv Med Plant Res.. 2015;3:33-38.
- [Google Scholar]
- Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater.. 2020;30(21):1909260.
- [Google Scholar]
- Nanotechnology in Sustainable AgricultureNanotechnology in Sustainable Agriculture. CRC Press 2021
- [Google Scholar]
- Characterization of Rheum ribes with ZnO nanoparticle and its antidiabetic, antibacterial, DNA damage prevention and lipid peroxidation prevention activity of in vitro. Environ. Res.. 2022;204
- [Google Scholar]
- Arum italicum mediated silver nanoparticles: synthesis and investigation of some biochemical parameters. Environ. Res.. 2022;204
- [Google Scholar]
- In vitro activities of Acacia nilotica (L.) Delile bark fractions against oral bacteria, glucosyltransferase and as antioxidant. BMC Complement. Med. Ther.. 2020;20(1):1-9.
- [Google Scholar]
- Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J. Photochem. Photobiol. B: Biol.. 2017;170:225-234.
- [Google Scholar]
- Green synthesis of gold and silver nanoparticles by using Amorphophallus paeoniifolius tuber extract and evaluation of their antibacterial activity. Molecules. 2020;25(20):4773.
- [Google Scholar]
- Facile synthesis of silver/gold alloy nanoparticles for ultra-sensitive rhodamine B detection. RSC Adv.. 2021;11(35):21475-21488.
- [Google Scholar]
- Pousset, J.L., 1989. Plantes médicinales africaines: Utilisations pratiques. Ellipses, editor. Paris, .95.
- Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun.. 2018;503(4):2814-2819.
- [Google Scholar]
- Screening of medicinal plant methanol extracts for the synthesis of gold nanoparticles by their reducing potential. Zeitschrift für Naturforschung B. 2008;63(7):903-908.
- [Google Scholar]
- Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Ind. Crop. Prod.. 2015;77:873-882.
- [Google Scholar]
- Au–Ag core–shell composite nanoparticles as a selective and sensitive plasmonic chemical probe for l-cysteine detection in Lens culinaris (lentils) RSC Adv.. 2021;11(33):20380-20390.
- [Google Scholar]
- An environmental approach for the photodegradation of toxic pollutants from wastewater using Pt–Pd nanoparticles: antioxidant, antibacterial and lipid peroxidation inhibition applications. Environ. Res. 2022:112708.
- [Google Scholar]
- Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability.. 2018;10(4):913.
- [Google Scholar]
- Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. artificial cells. Nanomed. Biotechnol.. 2018;46(6):1163-1170.
- [Google Scholar]
- Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed.. 2018;13:3571.
- [Google Scholar]
- Synthesis of monodisperse chitosan nanoparticles. Food Hydrocoll.. 2018;83:355-364.
- [Google Scholar]
- Smart gold nanostructures for light mediated cancer theranostics: combining optical diagnostics with photothermal therapy. Adv. Sci.. 2020;7(15):1903441.
- [Google Scholar]
- Green synthesis, characterization and catalytic degradation studies of gold nanoparticles against congo red and methyl orange. J. Photochem. Photobiol. B: Biol.. 2018;178:33-39.
- [Google Scholar]
- Nanostructured materials based on noble metals for advanced biological applications. Nanomaterials. 2019;9(11):1593.
- [Google Scholar]
- Visible light enhanced p-nitrophenol reduction by glycerol over Ag/Cu core-shell bimetallic nanocatalysts. J. Environ. Chem. Eng.. 2021;9(4):105655
- [Google Scholar]
- Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020:799.
- [Google Scholar]
- Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci.. 2016;17(9):1534.
- [Google Scholar]







