Translate this page into:
Fabrication, characterization and in vitro evaluation of Melia dubia extract infused nanofibers for wound dressing
⁎Corresponding authors. kanthad.arunachalam@gmail.com (Kantha Deivi Arunachalam), sasikala@ucsiuniversity.edu.my (Sasikala Chinnappan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
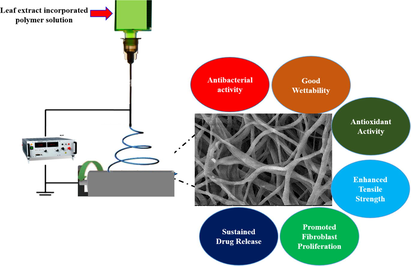
Open wounds are easily susceptible to infection, so it is necessary to develop a suitable barrier to prevent infection and enhance wound healing. The nanofibers has potential applications in regenerative medicine and tissue engineering. Certain phytochemicals are proangiogenic agents and helps in tissue remodeling. The present study focuses on the fabrication and characterization of Melia dubia (MD) leaf ethanolic extract incorporated polycaprolactone (PCL)/gelatin (Gel) electrospun nanofibers (NFs) for efficient wound dressing applications. The existence of bioactive compounds in MD leaf extract was confirmed by gas chromatography-mass spectrometry analysis (GCMS). The fabricated nanofibers are characterized by scanning electron microscope (HRSEM), fourier transform infrared spectroscopy (FTIR), X-ray crystalline diffraction analysis (XRD), contact angle and mechanical strength analysis. Antioxidant and in-vitro drug release was evaluated for NFs. Then antibacterial activity was tested against clinical pathogens and the biocompatibility of the NFs was tested on L929 fibroblast cells by MTT and confocal analysis. GCMS analysis of MD leaf extract confirms the existence of numerous bioactive compounds, that support wound healing. HRSEM analysis revealed that MD leaf extract loaded electrospun nanofibers were smooth, ultrafine, and free of beads with 256 ± 89 nm. The incorporation of the MD leaf extract into the NFs reduced their semi-crystalline nature and increased the tensile strength of the NFs. Contact angle analysis confirmed that incorporation of plant extracts enhanced the hydrophilic nature (22.93 ± 4.3°). The continuous sustained release of drug from initial concentration (∼74 %). In vitro antibacterial analysis revealed that fabricated NFs effectively inhibited the growth of Escherichia coli (18.2 ± 1.5 mm), Staphylococcus aureus (26.5 ± 2.0 mm), multidrug-resistant Staphylococcus aureus (23.0 ± 0.9 mm), and Pseudomonas aeruginosa (28.1 ± 1.2 mm). In vitro biocompatibility assays revealed that fabricated NFs are not cytotoxic and enhanced the proliferation of fibroblast. The study indicated the possible ability of Melia dubia leaf extract incorporated PCL/Gel NFs in the killing of wound pathogens with biocompatibility towards L929 cells.
Keywords
Antibacterial activity
Biocompatible
Biopolymer
Scratch assay
L929 fibroblast cells
- HRSEM
-
High resolution scanning electron microscopy
- FTIR
-
Fourier transform infrared spectroscopy
- XRD
-
X-ray crystalline diffraction analysis
- PCL
-
polycaprolactone
- Gel
-
Gelatin
- NFs
-
nanofibers
- MTT
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- TFE
-
Trifluoroethanol
- DMEM
-
Dulbecco’s Modified Eagle Medium
- MD
-
Melia dubia
- MHA
-
Muller Hinton Agar
- OD
-
optical density
- PBS
-
Phosphate-buffered saline
- GCMS
-
Gas chromatography-mass spectrometry analysis
- AO
-
Acridine orange
- EtBr
-
Ethidium bromide
- DPPH
-
Diphenyl-1-picrylhydrazyl
Abbreviations
1 Introduction
The human skin serves as an anatomical barrier, maintains body hemostasis and protects internal organs from the external damages (Yousef et al., 2021). Replacement of damaged or destroyed tissue in living organisms is referred to as wound healing and it is an intricate and complex process (Krafts, 2010). Normally, wound healing is organized as different phases such as hemostasis, inflammation, proliferation and maturation or remodelling phase. The reepithelization is a process of movement of epithelial cells from surrounding epidermis over denuded surface (Clark et al., 2007). Heavy loads of microbial infection on the injured site slowdowns the wound healing process (Alonso-Montemayor et al., 2021). Wound dressing materials like alginates, hydrogels, sponges, and films are commercially available in the market (Kim et al., 2019). Still wound care management is challenging due to the susceptible bacterial infection (Zhou et al., 2018). Aligned and nonwoven nanofibers with high porosity can be fabricated under the influence of an electric field by a method called electrospinning (Homaeigohar and Boccaccini, 2020). Electrospun nanofibers are used for various applications, such as thermal insulation and filtration, protective covering garments, conductive devices and sensors, wound dressings and tissue engineered scaffolds (Park, 2010). Poly-ɛ-caprolactone has high mechanical properties compared to other natural polymers, it has excellent processability and good biocompatibility (Unalan et al., 2019). So, the combined PCL and Gel should give improved biocompatibility with enhanced physical and mechanical properties (Sheng et al., 2019). Plant extract mediated wound dressing nowadays is gaining attention due to less toxicity and fewer side effects, inherent medicinal properties, environmentally sustainable, easily available with less cost and good alternatives for wound management (Adamu et al., 2021; Nayeem et al., 2021). Some of the plant extracts incorporated in nanofiber are Centella asiatica, Tecomella undulata, Aloe vera, Indigofera aspalathoides, Calendula officinalis, etc. (Fatehi and Abbasi, 2020).
The plant Melia dubia (MD) of the family Meliaceae is mostly known as Malabar Neem and available in the tropical moist deciduous forests. The Melia dubia has extensive medical, pharmacological and ethnomedicinal uses (Goswami et al., 2020). The ethanolic extract of MD increased the synthesis of collagen, deposition of hexosamine, wound contraction rate and tensile strength of wound in female Wistar rats (Thangavel et al., 2019). Objective of the current study is identifying bioactive compounds present in the Melia dubia ethanolic extract, fabrication and characterization of MD plant extract incorporated PCL/Gel hybrid NFs, determination of antioxidant property, in-vitro drug release of NFs and assessment of antibacterial and wound healing efficiency of NFs using L929 fibroblast.
2 Materials and methods
2.1 Chemicals
Poly-ɛ-caprolactone (Mw-80,000, Sigma Aldrich, UK), Porcine Gelatin (Gel strength 300-Type A, Sigma Aldrich, USA), Dulbecco’s Modified Eagle Medium (DMEM), Phosphate-buffered saline (PBS) (Hi-Media, India), Acetic acid, Ethanol (Analytical reagent), 2,2,2 Trifluoroethanol (TFE), Acridine Orange (AO), Ethidium bromide (EtBr) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and DPPH (diphenyl-1-picrylhydrazyl) (SRL, India).
2.2 Processing of Melia dubia leaves
MD leaves were cleaned, shade dried for 3 weeks, then made into powder using a mechanical blender. To obtain ethanolic plant extract, 25 g of leaf powder was added in 0.5 L of 70 % ethanol and stirred for 3 h in a shaker. For extraction, the stirred mixture was placed in an ultrasonic water bath at 60 ℃ for 60 min. The extract was then filtered, concentrated and stored at 4 °C. The GCMS analysis of the extract was performed to identify the presence of phytochemical using the mass spectral patterns stored in NIST library (Konappa et al., 2020).
2.3 Electrospinning of nanofiber
Nanofiber was fabricated by ESPIN-Nano, Physics Instrument Co., Chennai, India. PCL (8 %), gelatin (4 %) and plant extract (25 %) (respect to PCL Wt. %) were added to the TFE and kept in stirring condition for 14 h. The polymer mixture was taken in a plastic syringe (5 mL) and 13 kV high voltage was applied. The polymer solution at the needle orifice begun to stretch and the nanofibers were collected on the collector that was kept 14 cm away from the tip of the needle.
2.4 Characterization of synthesized nanofiber
The morphology was identified using HR-SEM (Thermo Scientific Apreo S), Functional groups of the nanofiber were identified using FTIR (Shimadzu, IR Tracer 100), crystalline nature of the compound was determined using XRD (PANalytical, Netherlands), and the wettability of NFs was determined by contact angle analysis (KYOWA DMS-401, Japan), Mechanical property of the NFs was analysed using tensile strength tester (Instron, USA).
2.5 In vitro drug release
50 mg of the plant extract incorporated nanofiber was submerged in 3 mL of PBS (pH 7.4). At different time interval, 3 mL of PBS was taken from the release medium, at the same time replaced with 3 mL of fresh PBS. The different concentration of standard was prepared using plant extract and absorbance was measured at 268 nm.
2.6 Antioxidant assay
The antioxidant activity of NF was examined at a fixed time point. For that 10 mg of PCL/Gel, PCL/Gel/MD NFs are added to 5 mL of 50 µm DPPH (dissolved in methanol) solution and kept in dark for 30 min. The antioxidant activity of the NF was analyzed using UV spectrophotometer at 514 nm. Antioxidant activity was evaluated using equation (1).
A- denotes absorbance of DPPH solution and A1-denotes absorbance of nanofibers immersed DPPH solution.
2.7 Antibacterial studies
PCL/Gel/MD NFs antibacterial activity was analyzed by disc diffusion method. The clinical pathogens (Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Methicillin-Resistant Staphylococcus aureus (multidrug-resistant S. aureus) and Pseudomonas aeruginosa (P. aeruginosa)) were obtained from Department of Microbiology, SRM Institute of Medical College Hospital and Research Center, Kattankulathur, Chengalpattu. The bacterial cultures were (1.5 × 108 CFU/mL) spread on Muller Hinton Agar (MHA). Then UV sterilized PCL/Gel and PCL/Gel/MD NFs (30 × 30 mm) were placed on MHA and incubated at 36 ± 1 °C for 24 h.
2.8 Cell culture
2.8.1 Scratch assay
The cell migration after exposing to nanofiber extracts was measured using scratch assay. L929 (fibroblast cell lines) were cultured in a 6-well culture plate containing complete media and grown up to 90% confluency. Then the cell monolayers were injured and to eliminate cell debris, wounded monolayers were washed thrice using a culture medium. By incubating the sterile mats in DMEM for 24 h, we were able to get PCL/Gel and PCL/Gel/MD NFs scaffold extracts in the medium. The extracts of the scaffold were added to the scratched cell monolayers and incubated for 1 day. Image J is used for wound area calculation. Depending on the wound closure of the control and treatment groups, the wound area percentage was calculated.
2.8.2 MTT and confocal analysis
The fibroblast cells were incubated with the media containing PCL/Gel or PCL/Gel/MD NFs extract for 24 and 48 h. MTT dye was added (5 mg/mL in PBS) to each well and incubated for about 4 h. After the incubation time, the MTT was taken out without disturbing the purple colored formazan crystals. To dissolve the insoluble crystal, solubilizing agent (DMSO) was added. Finally, the optical density (OD) was measured at 570 nm. After 48 h incubation, the live/dead staining done using AO/EtBr (1:1).
3 Results and discussion
3.1 GCMS analysis
GCMS analysis of MD plant extract demonstrated the presence of numerous bioactive compounds that enhances the wound healing process (Fig. 1). The detected compounds are presented in Table 1. The bioactive compounds like squalene, vitamin E, octadecanoic acid, ethyl ester, azafrin, heneicosane, palmitic acid, ethyl ester and 4-epi-cubedol has antioxidant, antibacterial and anti-inflammatory properties (Brigelius-Flohé, 2006; Eswaraiah et al., 2020; Islam et al., 2018; Rosselli et al., 2019; Vanitha et al., 2020). Presence of these bioactive compounds confirms that plant can be suitable for enhancing the wound healing process.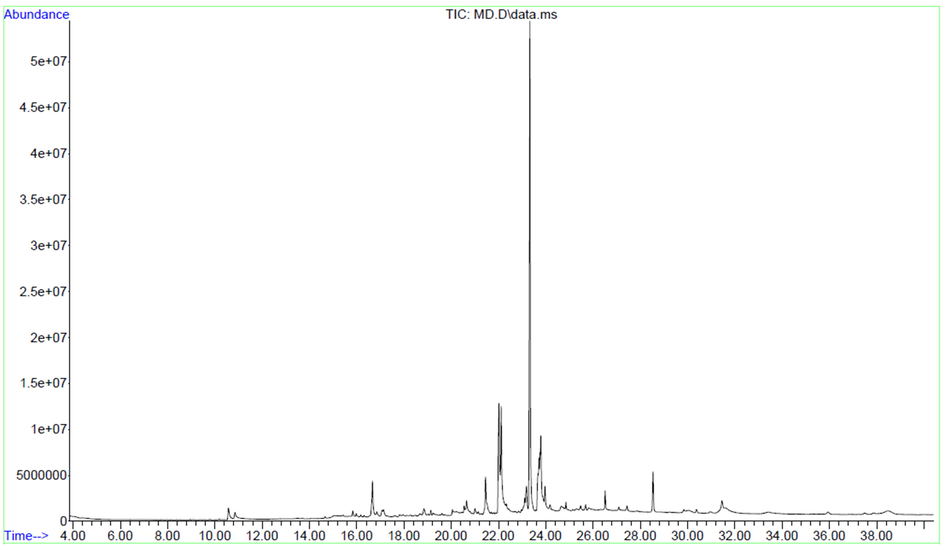
GC–MS spectrum of ethanolic extract of Melia dubia leaf extract.
S. No
Retention Time
Compound
Formula
Molecular weight
Area %
1
10.574
Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl)propan-2-yl carbonate
C13H22O4
242
1.46%
2
10.847
α-Methyl-α-[4-methyl-3-pentenyl]oxiranemethanol
C10H18O2
170
0.92%
3
14.658
Cyclohexane, 1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)-
C15H24
204
0.16%
4
15.053
6-Methyl-cyclodec-5-enol
C11H20O
168.1514
1.04%
5
15.428
Cyclohexane, 1-ethenyl-1-methyl-2,4 bis(1-methylethenyl)-, [1S-(1α,2β,4β)]-
C15H24
204.18
0.12%
6
15.829
β-ylangene
C15H24
204.1878
0.35%
7
16.172
Cedrene
C15H24
204.18
0.13%
8
16.306
β-copaene
C15H24
204.18
0.11%
9
16.662
1H Cyclopenta[1,3]cyclopropa[1,2]benzene, octahydro-7-methyl-3-methylene-4-(1-3bβ,4β,7α,7aS*)]- (β-Cuvebene)methylethyl)
C15H24
204.1878
2.91%
10
17.127
4-epi-cubedol
C15H26O
222.19
1.19%
11
17.807
Cyclotridecane
C13H26
182.2
0.48%
12
18.66
tau.-Cadinol
C15H26O
222.19
0.32%
13
18.844
2-Naphthalenemethanol, decahydro-α,α,4a-trimethyl-8-methylene-, [2R-(2α,4aα,8aβ)]-
C15H26O
222.19
0.69%
14
19.131
Eicosane, 2-methyl-
C21H44
296.34
0.22%
15
19.226
Cholestan-3-ol, 2-methylene-, (3β,5α)-
C28H48O
400.37
0.37%
16
19.608
Heneicosane
C21H44
296.34
0.13%
17
20.047
10-Heneicosane
C21H42
294.32
0.81%
18
20.543
3,7,11,15-tetramethyl-2-hexadecen-1-ol
C20H40O
296.3
0.42%
19
20.645
2-Pentadecanone, 6,10,14-trimethyl-
C18H36O
268.27
0.91%
20
20.995
Phthalic acid, 5-methylhex-2-yl isobutyl ester
C19H28O4
320.19
0.47%
21
21.141
Eicosane
C20H42
282.32
0.13%
22
21.447
Hexadecanoic acid, methyl ester
C17H34O2
270.255
3.52%
23
22.013
n-Hexadecanoic acid
C16H32O2
256.240
9.73%
24
22.108
Palmitic acid, ethyl ester
C18H36O2
284.271
7.90%
25
23.107
8,11-Octadecadienoic acid, methyl ester
C19H34O2
294.25
0.64%
26
23.177
9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-
C19H32O2
292.24
1.95%
27
23.323
Phytol
C20H40O
296.302
31.3%
28
23.667
9,12-Octadecadienoic acid (Z,Z)-
C18H32O2
280.24
2.43%
29
23.711
9,12-Octadecadienoic acid, ethyl ester
C20H36O2
308.27
2.87%
30
23.781
Ethyl 9,12,15-octadecatrienoate
C20H34O2
306.25
8.00%
31
23.966
Octadecanoic acid, ethyl ester
C20H40O2
312.3
1.69%
32
24.182
Isophytol, acetate
C22H42O2
338.31
0.40%
33
24.659
2H-1-benzopyron-2one,7-methoxy-6-(3-methyl-2-butenyl)-
C15H16O3
244.1
1.00%
34
25.289
Octadecane, 3-ethyl-5-(2-ethylbutyl)-
C26H54
366.42
0.27%
35
25.467
4,8,12,16-Tetramethylheptadecan-4-olide
C21H40O2
324.3
0.37%
36
25.817
Desoximetasone
C22H29FO4
376
0.30%
37
30.054
17-Pentatriacontene
C35H70
490.54
0.77%
38
30.366
Squalene
C30H50
410.39
0.33%
39
31.448
Heptacosane, 1-chloro-
C27H55Cl
414.39
3.137
40
33.458
Azafrin
C27H38O4
426.27
1.02%
41
35.946
Hentriacontane
C31H64
436.5
0.47%
42
37.479
Vitamin E
C29H50O2
430.38
1.47%
3.2 HR-SEM analysis
The structure and morphology of plant extract-loaded nanofiber were investigated using HRSEM. Fig. 2a & c shows the random orientation of electrospun nanofiber with a bead-free, continuous, smooth, narrow size distribution and also no particulate aggregates were found indicating no phase separation occurred during electrospinning after the incorporation of plant extract. The mean diameter for PCL/Gel and PCL/Gel/MD NFs (Fig. 2b & d) was found to be 319 ± 104 and 256 ± 89 nm, respectively. Previous study reports incorporation of Gymnema sylvestre and Henna plant extract into polymer solution decreased the fiber diameter due to higher charge density and conductivity (Ramalingam et al., 2021; Yousefi et al., 2017). PCL/Gel/MD NFs were found to be entangled compared to PCL/Gel NFs. Rosa et al. (2017) reported the entangled NFs helps in improving the nutrition infiltration, new cell integration, oxygen permeation, long-term medication release and wound exudate clearance (Rosa et al., 2017).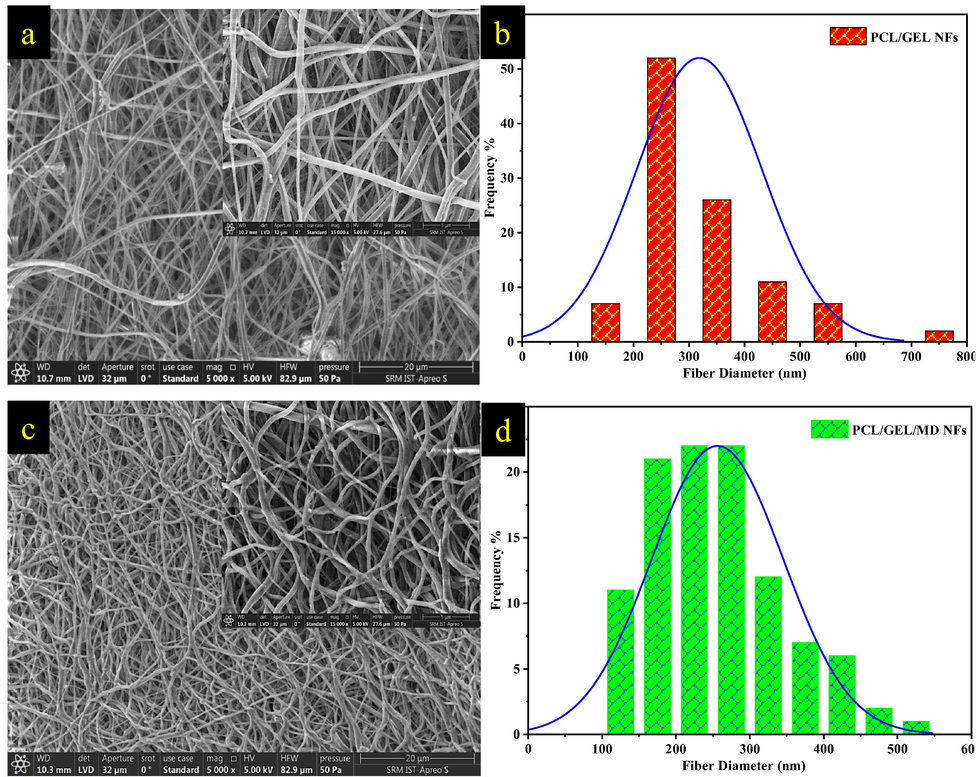
HRSEM images and histogram plot of (a&b) PCL/Gel and (c&d) PCL/Gel/MD NFs (Magnification-5000X). Insert represents higher magnification image of nanofibers ((Magnification- 15000X).
3.3 Functional group analysis NFs
The chemical bonding of PCL/Gel NFs and PCL/Gel NFs with MD plant extract was determined by FTIR analysis as given in Fig. 3a. The peaks obtained at 2942 and 1723 cm−1 in PCL/Gel nanofiber represents asymmetric –CH2 and C = O stretching vibration that corresponds to PCL. N–H stretching associated with the peaks at 3309 cm−1, C = O amide I stretching at 1648 cm−1, N–H bending associated at 1652 cm−1 and stretching at 1040 cm−1 associated with C = O corresponds to gelatin. The sole difference between PCL and gelatin is the NH2 and OH stretchings (Chong et al., 2015). In-plant extract, stretching at 3043 cm−1 represents O–H stretching and 1032 cm−1 represents the C-O stretching. In PCL/Gel/MD NFs, the plant extract band was overlapped in PCL/Gel NFs at the wavenumber of 3043, 2612, 1211, 1052, 795 and 673 cm−1.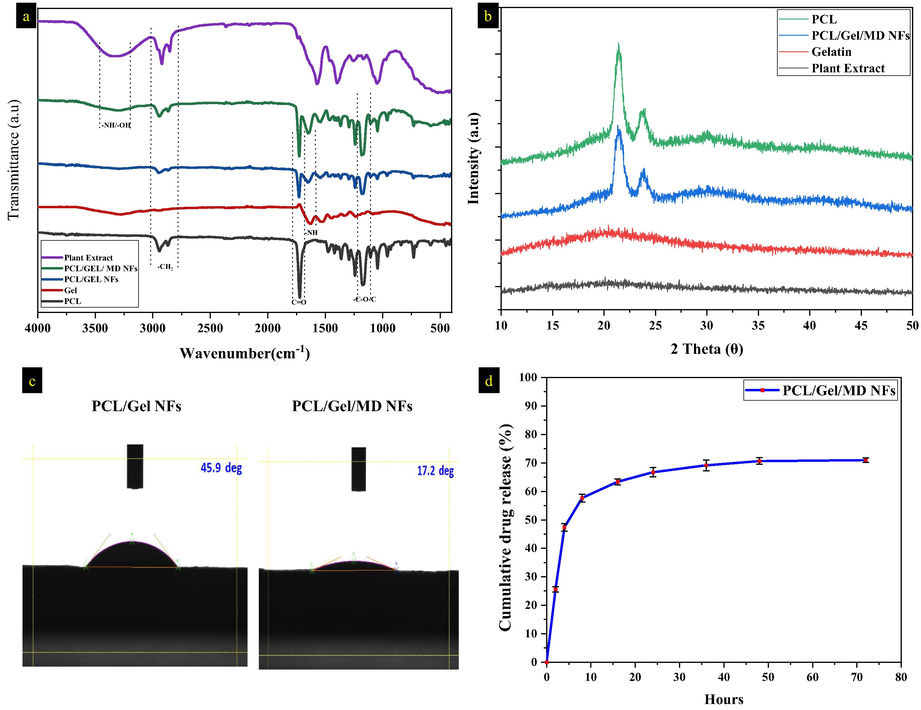
a) FTIR spectrum of plant extract, PCL/Gel/ MD NFs, PCL/Gel NFs, gelatin, and PCL, b) XRD spectrum of plant extract, gelatin, PCL and PCL/Gel/MD NFs, c) contact angle analysis of PCL/Gel and PCL/Gel/MD NFs, d) cumulative drug release percentage of PCL/Gel/MD NFs.
3.4 XRD analysis
Fig. 3b depicts the PCL/Gel & PCL/Gel/MD NFs XRD spectrum. In PCL peaks at 2θ = 21.7° and 24.8° indicate the plane of 110 and 200, respectively indicating the crystalline nature of PCL. The plant and gelatin exhibits the amorphous nature (Fig. 3b). The peak intensity of PCL/Gel/MD NFs is lesser than PCL which is due to the incorporation of plant extract. Gautam et al. (2013) stated that incorporating the amorphous components decreased the intensity and semi-crystalline nature of PCL NFs (Gautam et al., 2013).
3.5 Water contact angle analysis
The hydrophilic property of the nanofibers was analysed using water contact angle analysis. The water contact angle was found to be 51.16 ± 5.26° and 22.93 ± 4.3° for PCL/Gel and PCL/Gel/MD NFs, respectively (Fig. 3c). The wettability of MD plant extract loaded nanofibers was found to be increased when compared to PCL/Gel nanofibers; this can enhance the absorption of wound ooze outs and keeps a moist environment around the wound bed (Yeum et al., 2016). Unalan et al. (2019) reported that the existence of polar phytocomponents and hydrophilic groups in the plant enhances the hydrophilicity of the PCL/Gel NFs (Unalan et al., 2019). The wettability of NFs exhibits a dynamic role in the adhesion and proliferation of the fibroblast cells.
3.6 In-vitro drug release
Fig. 3d demonstrates the drug release profile of PCL/Gel/MD NFs. About 75.9 % of plant extract was entrapped in NFs. In the first 2 h of incubation, initial burst release was observed which might be due to the dissolution of plant extract present in the surface of the nanofibers. After that, a continuous and sustained drug release was observed up to 72 h, releasing about 74 % of the drug from the initial concentration. The extended drug release of PCL/Gel/MD NFs is due to slow degradation of gelatin entangled with PCL (Xue et al., 2014). The initial burst release of the plant extract helps to reduce the bacterial colonization (Ramalingam et al., 2019b).
3.7 Mechanical strength analysis
Mechanical properties of the NFs plays important role in tissue engineering applications. The incorporation of plant extract into polymer increased their mechanical strength (Table 2). Raghavendra et al. (2019) reported that incorporation of plant extract increased tensile strength, toughness and tensile modulus in nanofibers (Ramalingam et al., 2019a). Selvaraj et al. (2017) fabricated fenugreek incorporated nanofiber with enhanced mechanical property compared to silk fibroin (Selvaraj and Fathima, 2017). Small fiber with densely packed fibrillar structure with more entanglements enhanced the mechanical properties, in case of PCL/Gel NFs alignment of NFs with increased fiber diameter decreased the mechanical property (Lim et al., 2008).
Sample
Thickness
(mm)Maximum load (N)
Elongation at break (%)
Tensile strength (MPa)
PCL/Gel NFs
0.17 ± 0.01
4.19 ± 0.10
42.05 ± 0.79
2.23 ± 0.08
PCL/Gel/MD NFs
0.19 ± 0.08
7.01 ± 0.12
46.61 ± 2.20
7.84 ± 0.01
3.8 Antioxidant assay
The excess amount of oxidative stress leads to impaired wound healing. The antioxidants helps to control the oxidative stress in wound site thereby accelerating the wound healing process (Fitzmaurice et al., 2011; Krochmal-Marczak et al., 2021). The antioxidant property of nanofibers was evaluated by antioxidant assay (DPPH) shown in Fig. 4a & b. The DPPH solution exhibits maximum absorbance at 514 nm. PCL/Gel and PCL/Gel/MD NFs exhibited the DPPH scavenging activity of 16.65 ± 0.18 and 49.27 ± 0.53 %, respectively. The antioxidant activity of the PCL/Gel NFs, might be due to the presence of gelatin (radical scavenging peptide) (Mendis et al., 2005). GCMS and FTIR analysis of the MD extract confirms presence of phenolic compounds/groups. Kanthaswamy et al. (2019) fabricated quinone-based chromenopyrazole antioxidant-laden silk fibroin NFs, reduced oxidative stress and enhanced the dense growth of the fibroblasts (Kandhasamy et al., 2019).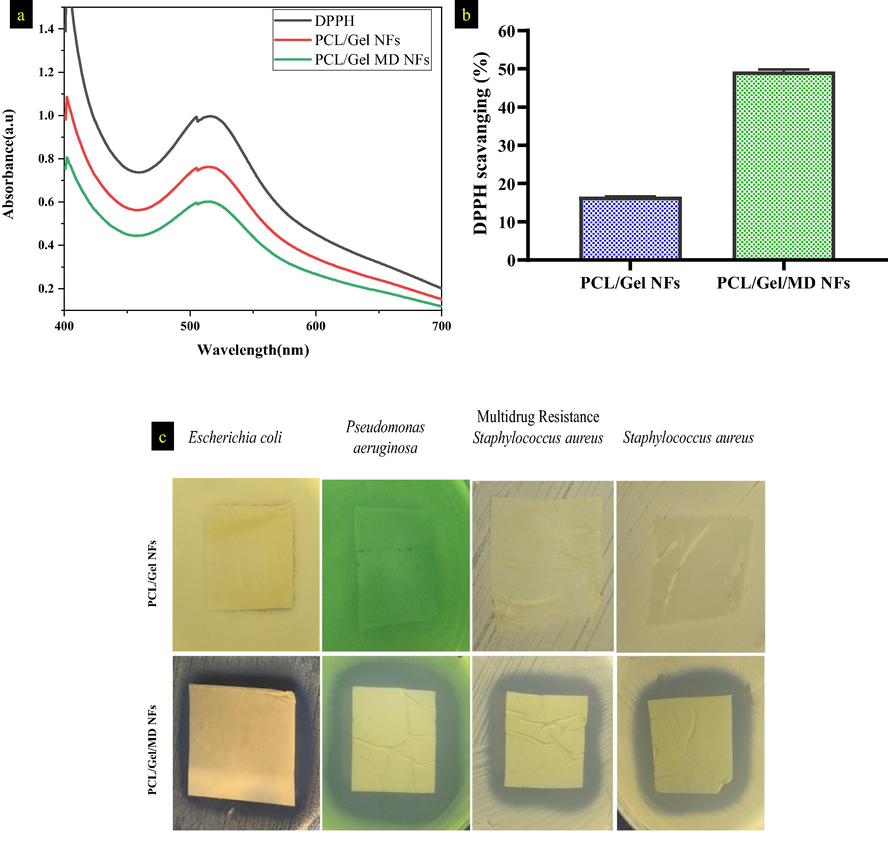
a) & b) antioxidant activity of PCL/Gel and PCL/Gel/MD NFs after 30 min incubation, c) Antibacterial activity PCL/Gel and PCL/Gel/MD NFs against Gram-positive and negative organisms.
3.9 Kirby-Bauer disc diffusion analysis
The antibacterial effect of the nanofibers was assessed using disc diffusion method (Fig. 4c). PCL/Gel NFs has no antibacterial activity against the wound pathogens. Zone of inhibition for MD plant extract incorporated NFs against E. coli, S. aureus, multidrug-resistant S. aureus, and P. aeruginosa were 18.2 ± 1.5, 26.5 ± 2.0, 23.0 ± 0.9 and 28.1 ± 1.2 mm, respectively (excluding fiber diameter). The ethanolic extract of MD leaves have terpenoids, phenolic derivatives, lipophilic compounds and limonoids as bioactive compounds which possess antibacterial activity against clinical pathogens (Goswami et al., 2020; Thangavel et al., 2019). Bioactive compounds inhibit bacterial growth by forming complex with cell wall, extracellular matrix and soluble proteins (El-Zayat et al., 2021). Zheng et al. (2005) reported unsaturated fatty acids exhibited antibacterial activity by inhibiting the bacterial enoyl-acyl carrier protein reductase (Zheng et al., 2005).
3.10 Scratch assay
The cells were scratched artificially in the center, causing the cells to migrate to the damaged location. The pace, pattern and direction of cell migration may all be analyzed using this assay (Kandhasamy et al., 2019). By densitometry analysis, the percentage of wound healing or the cell migration percentage was calculated. These values were comparable to PCL/Gel nanofiber levels. When compared to PCL/Gel NFs, nanofiber containing plant extract enhanced the healing rate, which was observed over a longer period of up to 24 h (Fig. 5a). The mean percentage of cell migration achieved for PCL/Gel was 87.74 % and 97.13 % for the PCL/Gel/MD NFs.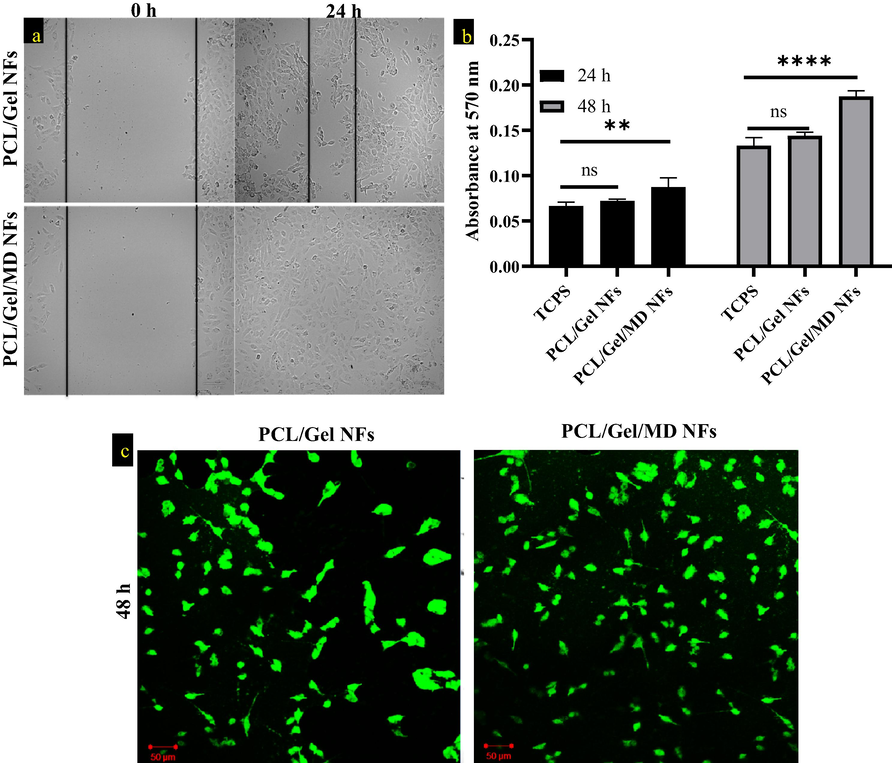
a) Wound healing assay of PCL/Gel NFs and PCL/Gel/MD NFs, b) MTT analysis of TCPS, PCL/Gel and PCL/Gel/MD NFs at 24 and 48 h. Two way ANOVA was performed. p > 0.05 is considered as nonsignificant (ns) c) Confocal image of PCL/Gel and PCL/Gel/MD NFs after 48 h (scale bar- 50 µm).
3.11 MTT assay and confocal analysis
Biocompatibility of PCL/Gel and PCL/Gel/MD NFs was assessed by MTT assay and confocal analysis. Compared to control (TCPS), the PCL/Gel and PCL/Gel/MD NFs extract-treated wells showed increased color (purple) intensity. It indicates that PCL/Gel/MD NFs positively enhance the proliferation of L929 cells at 24 and 48 h. Fig. (5b&c) indicates that the plant extract has proliferating ability and no cytotoxicity. MD ethanolic extract enhances collagen synthesis and hexosamine in wound surfaces (Thangavel et al., 2019). Ajmal et al. (2019) reported that gelatin and flavonoid content in the nanofiber enhanced fibroblast proliferation (Ajmal et al., 2019).
4 Conclusion
In this study, PCL/Gel nanofibers functionalized with Melia dubia ethanolic leaf extract has showing great potential as a wound dressing. GCMS analysis of the plant extract confirms the presence of bioactive compounds (which enhance the wound healing process). Morphological analysis showed the synthesized nanofibers are uniform, smooth, and beadless with nanometer dimension. Incorporation of the plant extract into PCL/Gel NFs decreased the crystallinity and mechanical properties. Biofunctionalized nanofiber showed hydrophilic nature and sustained release up to 72 h. Moreover, the in vitro analysis of these nanofibers exhibited potential bactericidal action against the wound pathogens; in addition to that, it also enhanced the proliferation and attachment of fibroblasts cells compared to PCL/Gel NFs. In future, the PCL/Gel/MD NFs can be used as effective wound dressing material.
Acknowledgements
The authors acknowledge the funding support from Researchers Supporting Project Number (RSP2022R465), King Saud University, Riyadh, Saudi Arabia for this research work. We would like to thank CSIR-CLRI, Chennai for helping in tensile strength analysis. The authors would like to thank Mr. Mohammed Junaid Hussain, Dr. M. R Ganesh and Ms. Shazia Anjum Musthafa, SRM IST, Chennai for helping in confocal microscopic analysis. The authors would like to sincerely thank IIISM, NRC and SCIF, SRM Institute of Science and Technology, Chennai for providing the necessary facilities required for the successful completion of the work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review of medicinal plant-based bioactive electrospun nano fibrous wound dressings. Mater. Des.. 2021;209:109942.
- [Google Scholar]
- Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm.. 2019;567:118480.
- [Google Scholar]
- A review on antibacterial and therapeutic plasma-enhanced activities of natural extracts. J. King Saud Univ. - Sci.. 2021;33(6):101513.
- [Google Scholar]
- Fabrication and evaluation of polycaprolactone/gelatin-based electrospun nanofibers with antibacterial properties. J. Nanomater.. 2015;2015:1-8.
- [CrossRef] [Google Scholar]
- Tissue engineering for cutaneous wounds. J. Invest. Dermatol.. 2007;127(5):1018-1029.
- [Google Scholar]
- Antibacterial and antioxidant potential of some Egyptian medicinal plants used in traditional medicine. J. King Saud Univ. – Sci.. 2021;33(5):101466.
- [Google Scholar]
- Identification of bioactive compounds in leaf extract of Avicennia alba by GC-MS analysis and evaluation of its in-vitro anticancer potential against MCF7 and HeLa cell lines. J. King Saud Univ. – Sci.. 2020;32(1):740-744.
- [Google Scholar]
- Medicinal plants used in wound dressings made of electrospun nanofibers. J. Tissue Eng. Regen. Med.. 2020;14(11):1527-1548.
- [Google Scholar]
- Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol. Physiol.. 2011;24(3):113-126.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of PCL / gelatin composite nano fi brous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C. 2013;33(3):1228-1235.
- [CrossRef] [Google Scholar]
- Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater.. 2020;107:25-49.
- [CrossRef] [Google Scholar]
- Phytol: a review of biomedical activities. Food Chem. Toxicol.. 2018;121:82-94.
- [CrossRef] [Google Scholar]
- Synthesis and fabrication of novel quinone-based chromenopyrazole antioxidant-laden silk fibroin nanofibers scaffold for tissue engineering applications. Mater. Sci. Eng. C. 2019;102:773-787.
- [CrossRef] [Google Scholar]
- Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev.. 2019;146:209-239.
- [CrossRef] [Google Scholar]
- GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Krafts, K.P., 2010. Tissue repair. http://dx.doi.org/10.4161/org.6.4.12555 6, 225–233. doi: 10.4161/ORG.6.4.12555.
- Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J. King Saud Univ. – Sci.. 2021;33(6):101532.
- [Google Scholar]
- Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl. Phys. Lett.. 2008;92(14):141908.
- [CrossRef] [Google Scholar]
- Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem.. 2005;53(3):581-587.
- [Google Scholar]
- Wound healing potential of Dodonaea viscosa extract formulation in experimental animals. J. King Saud Univ. – Sci.. 2021;33(5):101476.
- [Google Scholar]
- Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2010;1(4):043002.
- [CrossRef] [Google Scholar]
- Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C. 2019;98:503-514.
- [CrossRef] [Google Scholar]
- Core-shell structured antimicrobial nanofiber dressings containing herbal extract and antibiotics combination for the prevention of biofilms and promotion of cutaneous wound healing. ACS Appl. Mater. Interfaces. 2021;13(21):24356-24369.
- [Google Scholar]
- Poly-ε-caprolactone/gelatin hybrid electrospun composite nanofibrous mats containing ultrasound assisted herbal extract: antimicrobial and cell proliferation study. Nanomaterials. 2019;9:462.
- [CrossRef] [Google Scholar]
- Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett.. 2017;207:145-148.
- [CrossRef] [Google Scholar]
- Rosselli, S., Maggio, A., Formisano, C., Napolitano, F., Senatore, F., Spadaro, V., Bruno, M., 2019. Chemical Composition and Antibacterial Activity of Extracts of Helleborus bocconei Ten. subsp. Intermedius, 2, 675–679. doi: 10.1177/1934578X0700200611.
- Fenugreek incorporated silk fibroin nanofibers—a potential antioxidant scaffold for enhanced wound healing. ACS Appl. Mater. Interfaces. 2017;9(7):5916-5926.
- [Google Scholar]
- Electrospun PCL/Gel-aligned scaffolds enhance the biomechanical strength in tendon repair. J. Mater. Chem. B. 2019;7(31):4801-4810.
- [CrossRef] [Google Scholar]
- Effect of ethanolic extract of Melia dubia leaves on full-thickness cutaneous wounds in Wistar rats. Dermatol. Ther.. 2019;32(6)
- [Google Scholar]
- Unalan, I., Endlein, S.J., Slavik, B., Buettner, A., Goldmann, W.H., Detsch, R., Boccaccini, A.R., 2019. Evaluation of Electrospun Poly(ε-Caprolactone)/Gelatin Nanofiber Mats Containing Clove Essential Oil for Antibacterial Wound Dressing. Pharm. 2019, Vol. 11, Page 570 11, 570. doi: 10.3390/PHARMACEUTICS11110570.
- Heneicosane—a novel microbicidal bioactive alkane identified from Plumbago zeylanica L. Ind. Crops Prod.. 2020;154:112748.
- [Google Scholar]
- Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials. 2014;35(34):9395-9405.
- [Google Scholar]
- Novel natural polymer/medicinal plant extract electrospun nanofiber for cosmeceutical application. Nanofiber Res. – Reach. New Height. 2016
- [CrossRef] [Google Scholar]
- Yousef, H., Alhajj, M., Sharma, S., 2021. Anatomy, Skin (Integument), Epidermis. StatPearls.
- An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C. 2017;75:433-444.
- [CrossRef] [Google Scholar]
- Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett.. 2005;579:5157-5162.
- [CrossRef] [Google Scholar]
- Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials. 2018;161:11-23.
- [CrossRef] [Google Scholar]







