Translate this page into:
Acute and subacute toxicity assessment of liquid CO2 extract of Phaleria macrocarpa fruits flesh in mice model
⁎Corresponding authors. quahmed@iium.edu.my (Qamar Uddin Ahmed), mdzaidul.sarker@marianas.edu (Md. Zaidul Islam Sarker)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Phaleria macrocarpa (Scheff.) Boerl., an evergreen tree is found in Indonesia, Malaysia and Oceania, explicitly Northern Territory of Australia and Papua New Guinea. The fruits are traditionally used to treat various ailments including diabetes, psoriasis, looseness of the bowels, skin inflammation, malignancy, kidney, liver and heart sicknesses. However, to date, no comprehensive study investigating its toxicity profile utilizing liquid CO2 extract (LCE) has been reported in ICR mice. Hence, this research was performed to investigate the both acute and sub-acute toxicities of the liquid carbon dioxide extract from ripe fruit flesh of the P. macrocarpa in mice.
Methods
Acute toxicity was assessed at a single dose of 3000 mg/kg bw for 2 weeks according to the up and down method of OECD 425 guideline, while the sub-acute test was assessed by liquid carbon dioxide extract at doses of 250, 500, 1000, and 2000 mg/kg bw for 28 days. The animals’ general behaviour, food intake, body weight, organ coefficients, biochemical and histological morphology were observed and analysed.
Results
After acute exposure to LCE, there was no evidence of any severe or fatal effects in the mice. An acute toxicity result exhibited that LD50 of LCE was > 3000 mg/kg bw. The findings of subacute toxicity evaluation revealed that LCE at doses of 250 and 500 mg/kg bw had no significant detrimental impact. Moreover, LCE at 1000 and 2000 mg/kg bw demonstrated toxicity to the heart, liver, kidney and lung in mice identified by histological and biochemical investigation.
Conclusions
The results confirmed that the LCE dosage at 500 mg/kg bw is considered a safe dose that can provide a better therapeutic effect without eliciting any adverse side effects.
Keywords
P. macrocarpa
Liquid carbon dioxide extract
Acute toxicity
Sub-acute toxicity
Biochemical analysis
Histopathology
1 Introduction
Traditional medicinal plants play a crucial role in our everyday life, and they are commonly used as healing hotspots to cure a variety of ailments. It was assessed that about 80% of the total populace relies upon customary medication for essential medical care, particularly in underdeveloped nations and regions (Mintah et al., 2019). More recently, the interest in regular herbal plant treatments has expanded surprisingly in America and European countries (World Health Organization. WHO Global Report on Traditional and Complementary Medicine, 2019). The broad acknowledgment of the customary medication can be ascribed to its history of experimentation, accessibility, and affordability (Salmerón-Manzano et al., 2020). The dependency on herbal plants with possible therapeutic benefits stimulates phytochemical and pharmacological studies to evaluate their chemical components, pharmaceutical value, and potential for drug discovery. During recent years, a variety of clinical medicinal products and functional meals foods, such as herbal drinks, teas and beverages, were found based on indigenous ethics and scientific pharmacological research on medicinal plants (Pereira et al., 2017). The World Health Organization regulatory authorities are increasingly concerned about the safety and efficacy of medicinal products produced from the traditional medicinal plants throughout their use (World Health Organization, 2004). According to the reports, besides the hazardous secondary metabolites present, some plant extracts may be contaminated with air pollutants, particularly heavy metals, which can further cause significant health problems (Rodríguez and Mandalunis, 2018). As a result, it is essential to examine the safety of plant extracts for human ingestion before evaluating their possible therapeutic functions. Conducting acute and subacute oral toxicity studies in vivo is one of the most effective approaches to achieve these objectives (Pritchard et al., 2003).
In tropical circumstances, Phaleria macrocarpa blooms all year long in the Southeastern Asian area, especially Malaysia, Indonesia and Oceania (Mia et al., 2021). The fruit of P. macrocarpa grows on the stems and branches of the trees, hanging from short stalks that are attached to the trunk. The fruit of Phaleria macrocarpa is well-known for its use as a healthy drink or tea, as well as in medications and cosmetics (Zhang et al., 2012). Traditionally, the fruits are eaten fresh, like guava, or combined with other relishes, like chili, to improve their flavour (Easmin et al., 2015). The fruits of P. macrocarpa have been claimed to treat diabetes, rheumatism, fever, heart disease, and cancer (Easmin et al., 2015). Soeksmanto (Soeksmanto, 2006) observed that when mice were administered a dose of 170 mg/kg bw of butanol extract, they had moderate necrosis of the proximal convoluted tubules (Soeksmanto, 2006). Armenia et al. (Armenia et al., 2006) performed similar research in which they induced P. macrocarpa extract at doses 200, 100, 50 mg/kg bw in Japanese Quail for eight weeks and observed elevated serum glutamate level as well as mild hepatic hypertrophy (Armenia et al., 2006).
Plant materials can be extracted in many ways, both conventional and non-conventional. The toxicity of plant extract or compounds depends on the different factors such as extraction procedure, temperature, pressure, nature of the solvent, and their ratio (Soquetta et al., 2018). LCE is a non-conventional approach that is more ecologically friendly than traditional methods due to the reduced use of synthetic and organic chemicals, shorter operating time, and improved extract quality. In this technique, they replace the organic solvents used in conventional extraction with CO2, which is non-toxic, non-flammable, recyclable, and cheap. It is also easier to remove from the extracts since it is non-reactive with the extracts' organic constituents (Rout et al., 2011; Aiello et al., 2020). The major benefits of this technique are the recovery of thermally labile and volatile compounds from residues using low temperature and pressure (Azmir et al., 2013).
However, the non-conventional, liquid CO2 extraction method of P. macrocarpa fruit flesh has yet to be tested for its toxicity effects. Thus, the current research focuses on the safety of LCE of P. macrocarpa fruit via acute and subacute toxicity assessments in mice through oral administration.
2 Results
2.1 Acute toxicity study
The acute toxicity investigation found that a single dose of 3000 mg/kg treated orally resulted in no indication of morbidity or death in experimental mice during the two-week study period of the experiment. No behavioural alterations in mice were observed during the entire treatment period. The body weight increase of the treated experimental groups was somewhat lower than that of the normal control group, but the difference was not statistically significant (Table 1). The organ exhibited that coefficients of the heart, kidney, brain, and lung were all within the normal range at the end of the experiment, with the exception of the liver, which was significantly decreased (Table 2). Furthermore, no histological alterations were identified in the tissues of the LCE-treated and control groups, with the exception of the liver and kidney. During the 14-day monitoring period, no animals died. As a consequence, the LD50 of LCE was estimated to be >3000 mg/kg bw. Values are means ± SEM (n = 4). *p < 0.05 and **p < 0.01 significantly different from the control group. Values are means ± SD (n = 4).
Weeks
Acute toxicity
Sub-acute toxicity
Control
3000
Control
250
500
1000
2000
Initial weight (g)
30.20 ± 0.464
31.35 ± 0.366
30.53 ± 0.575
31.60 ± 0.722
30.60 ± 0.999
31.05 ± 0.951
30.80 ± 0.778
One week (g)
33.05 ± 0.533
33.45 ± 0.202
33.23 ± 0.545
33.03 ± 0.560
33.25 ± 0.777
31.95 ± 1.090
31.25 ± 0.702
Two weeks (g)
36.60 ± 0.564
35.90 ± 0.108
35.90 ± 0.367
35.10 ± 0.639
34.30 ± 0.656
32.85 ± 1.144
31.50 ± 0.607
Three weeks (g)
–
–
37.60 ± 0.286
36.90 ± 0.715
35.70 ± 0.863
33.90 ± 0.991
31.83 ± 0.657
Fourth (week)
–
–
39.43 ± 0.263
38.68 ± 0.872
37.25 ± 0.655
34.80 ± 1.150
32.43 ± 0.544
BWG
6.4
4.65
8.90
7.05
6.65
3.75*
1.63**
Food intake (g/W)
–
–
236.3 ± 4.813
198.3 ± 5.452
191.5 ± 4.975
154.8 ± 9.578*
140.8 ± 6.945*
Water intake (mL/W)
–
–
314 ± 3.416
286.5 ± 4.349
282.5 ± 3.227
216 ± 12.06*
205.3 ± 6.799*
Parameters
Acute toxicity
Sub-acute toxicity
Control
3000
Control
250
500
1000
2000
Heart (g/100 g)
0.59 ± 0.019
0.59 ± 0.012
0.60 ± 0.021
0.61 ± 0.011
0.62 ± 0.020
0.62 ± 0.026
0.63 ± 0.014
Liver (g/100 g)
4.93 ± 0.014
4.81 ± 0.005
4.95 ± 0.059
4.91 ± 0.009
4.90 ± 0.051
4.92 ± 0.09
4.80 ± 0.011
Kidney (g/100 g)
1.41 ± 0.006
1.47 ± 0.017
1.52 ± 0.009
1.53 ± 0.010
1.52 ± 0.09
1.53 ± 0.010
1.58 ± 0.008
Lung (g/100 g)
0.64 ± 0.003
0.63 ± 0.006
0.68 ± 0.006
0.64 ± 0.011
0.66 ± 0.008
0.67 ± 0.012
0.68 ± 0.006
Brain (g/100 g)
1.20 ± 0.005
1.21 ± 0.006
1.22 ± 0.009
1.23 ± 0.011
1.25 ± 0.009
1.26 ± 0.09
1.25 ± 0.013
2.2 Subacute toxicity study
2.2.1 Water intake, food consumption and body weight
There were no death or behavioural changes detected in the LCE-treated groups compared to the control group throughout the subacute trial. As displayed in Table 1, after the fourth week, body weight gains were marginally lower in the experiment group receiving doses of 500 and 250 mg/kg bw compared to the control group with no significant difference. However, body weight gains were significantly lower at the doses of 1000 (*p < 0.05) and 2000 (**p < 0.01) mg/kg bw which were 3.75 g and 1.63 g respectively compared to the control group (8.90 g). Food intake reduced substantially at dosages of 1000 mg/kg bw and 2000 mg/kg bw when compared to the control (p < 0.05). Furthermore, food and water intake were found to be lower in the 2000 and 1000 mg/kg bw mice groups than the control group of mice (**p < 0.01).
2.2.2 Organ coefficient
The organ coefficient’s results are shown in Table 2. The organ coefficients of the heart, lungs, and brain in treated groups ranging from 250 to 2000 mg/kg bw did not vary significantly from their control groups. However, the LCE dosage of 2000 mg/kg bw exhibited a slight difference in the kidney and liver, compared to the other groups.
2.3 Serum biochemistry analysis
LCE at 1000 and 2000 mg/kg bw revealed that TP and ALB significantly decreased (Figure-1A.a) while ALP, CRE, BUN, ALT and AST levels increased significantly (Fig. 1Ab, Fig. 1Ba and 1Bb) in contrast to the control group. As far as the lipid profile is concerned, TC, HDL-C and LDL-C were slightly increased at doses of 1000, 2000 and 1000 mg/kg bw respectively, whereas LDL-C and TG had significantly decreased at higher dose and the rest of the groups in lipid profiles were non-significant compared to the control group (1B.c). The GLU values (Fig. 1A.a) presented no significant changes when compared, at different doses, to the control group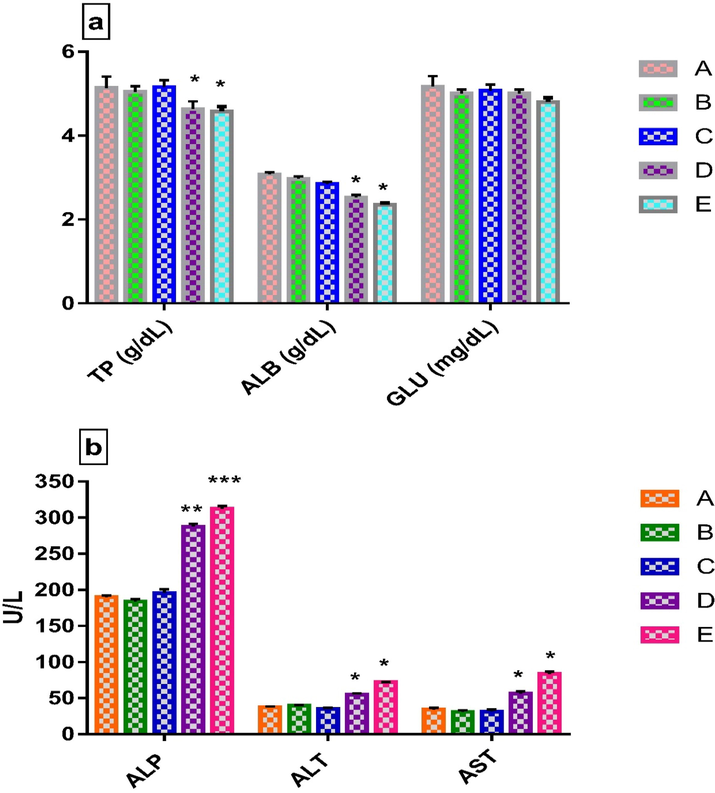
a. Biochemical analysis of TP, ALB and GLU, Values are means ± SEM (n = 4). *p < 0.05 significantly different from control group (two-way ANOVA followed by Tukey’s multiple comparisons test) b. Liver function test (ALP, ALT and AST). Values are means ± SEM (n = 4). *p < 0.05, **p < 0.01 and ***p < 0.001 significantly different from control group (two-way ANOVA followed by Tukey’s multiple comparisons test). Legends: A-Normal control (without LCE extract), B-250 mg/kg bw, C-500 mg/kg bw, D-1000 mg/kg bw, E-2000 mg/kg bw.
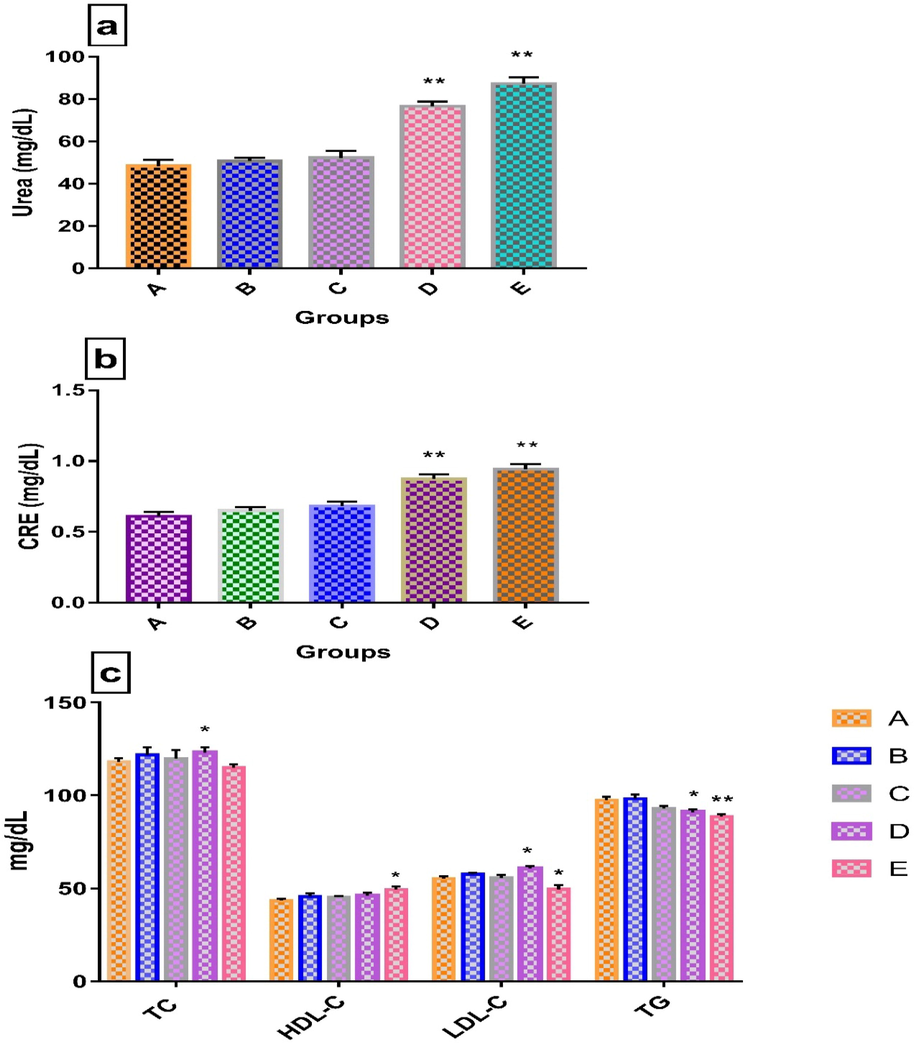
a. Urea, Values are means ± SEM (n = 4). **p < 0.01 significantly different from control group (two-way ANOVA followed by Dunnett’s multiple comparisons test). Legends: A-Normal control (without LCE extract), B-250 mg/kg bw, C-500 mg/kg bw, D-1000 mg/kg bw, E-2000 mg/kg bw. b. CRE Values are means ± SEM (n = 4). **p < 0.01 significantly different from control group (two-way ANOVA followed by Dunnett’s multiple comparisons test) c. Biochemical analysis of lipid profile (TC, HDL-C, LDL-C and TG). Values are means ± SEM (n = 4). *p < 0.05 and **p < 0.01 significantly different from control group (two-way ANOVA followed by Dunnett’s multiple comparisons test).
2.4 Histopathological analysis
In the histological analysis, Groups (A-E) were for subacute toxicity and Group E was high dose (3000 mg/kg bw) of acute toxicity. In acute toxicity, histological analysis of normal control mice has not been shown here due to the normal findings of cellular structure similar to the sub-acute control mice. At the end of the experiment period to ascertain toxicity elicited, important organs kidney, including liver, lung, brain and heart of the mice in the control group and rest of the groups treated with different doses (250, 500, 1000 to 2000 mg/kg bw) in subacute and acute toxicity (3000 mg/kg bw) were analysed for histopathological examination. As shown in Fig. 2 to Fig. 6, the microscopic findings exhibited that slight pathological alteration in liver, kidney, lung, and heart were observed in the treated animals after receiving the higher dose from 1000 to 2000 mg/kg bw of LCE, whereas another vital organ in the form of the brain showed no notable pathological alterations (Fig. 2, from A–F).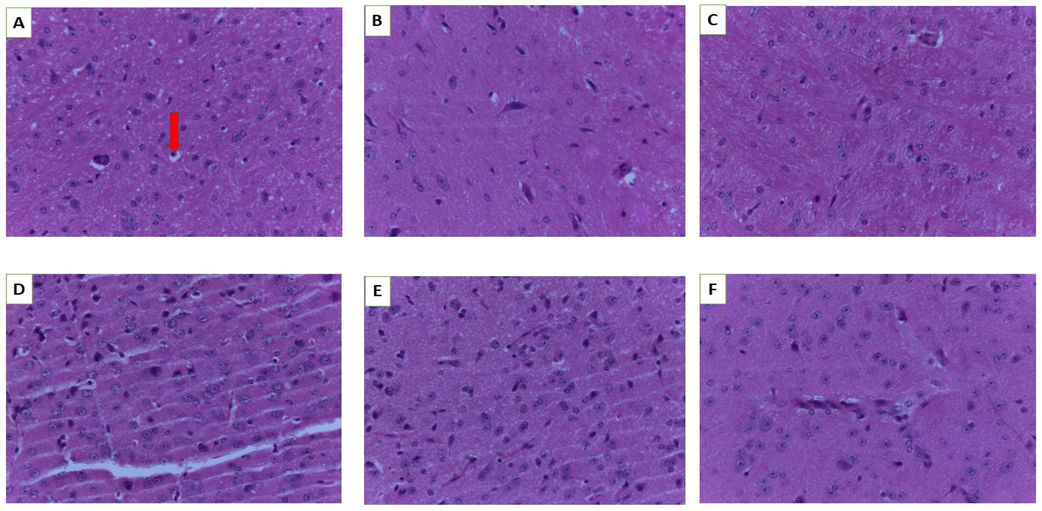
Histology of the brain. A (Normal control), B (250 mg/kg bw), C (500 mg/kg bw), D (1000 mg/kg bw), E (2000 mg/kg bw) and F (3000 mg/kg bw). Red arrow-oligodendroglia (magnification 40×).
The microscopic observation of the liver structure of animals that were administered at 500 and 250 mg/kg bw was comparable to the control (Fig. 3A–C) and no indication of any alteration and apoptosis was noticed. On the other hand, animals receiving the highest dose at 1000 and 2000 mg/kg bw (Fig. 3D and E) displayed certain toxicities in the form of necrotic hepatocytes, dilated central vail and lymphocytic infiltration when these groups were compared to the control group. However, during the acute toxicity study, only necrotic hepatocytes toxicity effect was observed at a dose of 3000 mg/kg bw.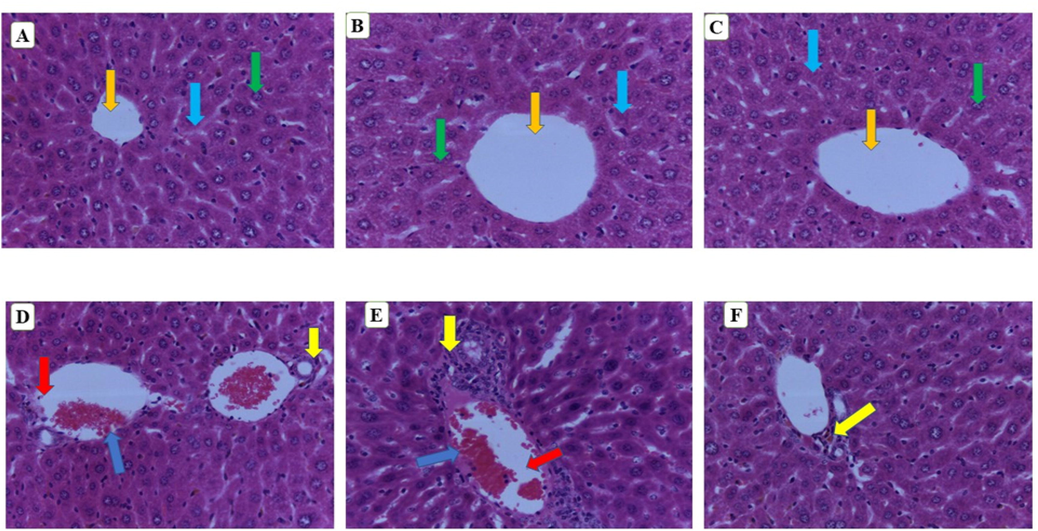
Histology of liver. A (Normal control), B (250 mg/kg bw), C (500 mg/kg bw), D (1000 mg/kg bw), E (2000 mg/kg bw) and F (3000 mg/kg bw). Green arrow-nuclei of hepatocytes, orange arrow- central vain, light blue- sinusoids, blue arrow-lymphocytic infiltration, yellow arrow-necrotic hepatocytes, red arrow-dilated central vein. (magnification 40×).
The kidney’s histological assessment is shown in Fig. 4(A–F). Normal glomeruli and tubules were observed at the doses of 250 and 500 mg/k g bw and control groups. However, in the other high dose treatment groups, dilated tubules, focal degeneration, apoptosis, renal lesions and tissue haemorrhages were observed (Fig. 4D–F).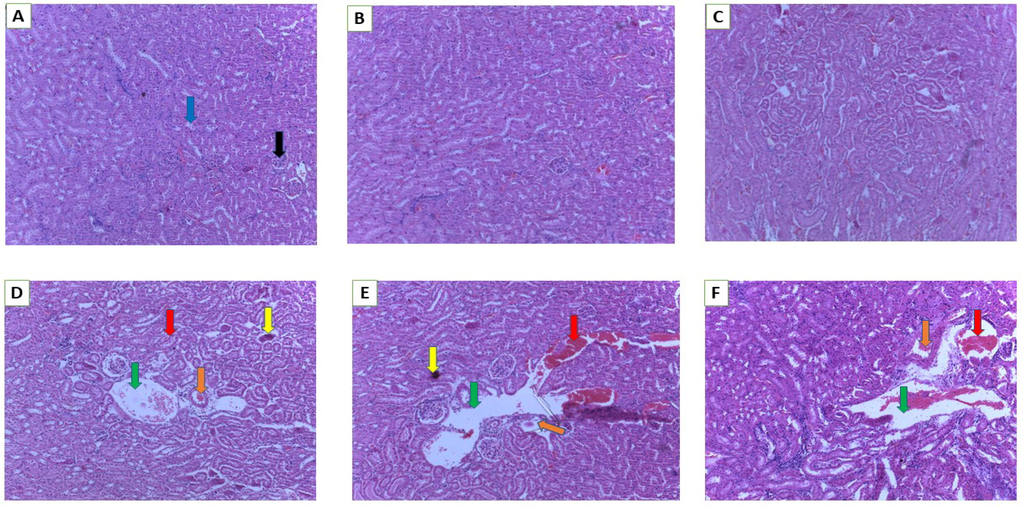
Histology of kidney. A (Normal control), B (250 mg/kg bw), C (500 mg/kg bw), D (1000 mg/kg bw), E (2000 mg/kg bw) and F (3000 mg/kg bw). Blue arrow- distal convoluted tubules, black arrow -glomerulus, green arrow-dilated tubules, orange arrow- focal degeneration, yellow arrow-apoptosis, red arrow-renal lesions/tissue haemorrhages (magnification 10×).
In Fig. 5, a number of pathological alterations in the lung were detected in the 2000 mg/kg bw treated group, including increased thickness of the edematous capillary endothelium cells, alveolar-capillary wall, dissolution of partial structure, breakdown of pulmonary alveoli and disruption of the structure of cell membrane (Fig. 5E).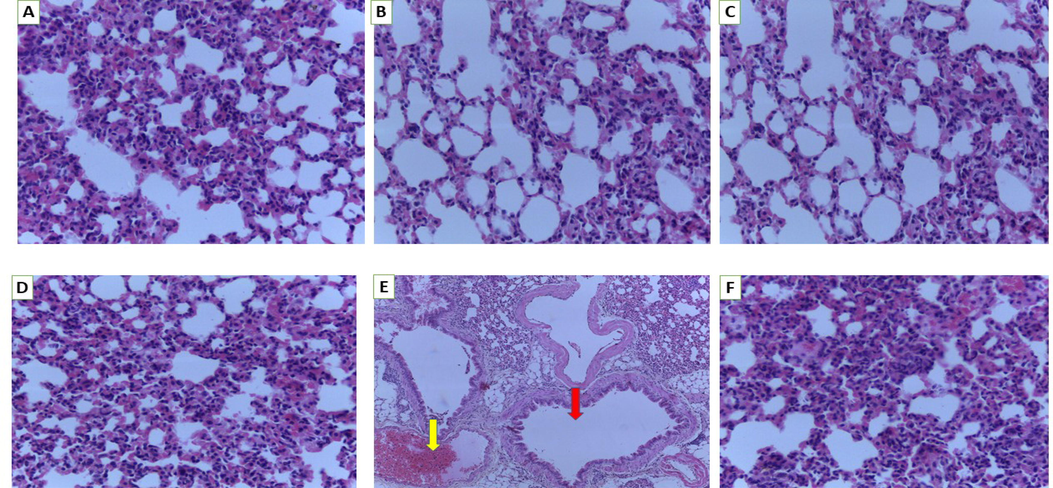
Histology of lung. A (Normal control), B (250 mg/kg bw), C (500 mg/kg bw), D (1000 mg/kg bw), E (2000 mg/kg bw) and F (3000 mg/kg bw). Red arrow-lung showing extensive emphysema and focal fibrosis of the alveoli with ruptured alveolar walls, yellow arrow-haemorrhage with degeneration of alveoli (magnification 40×).
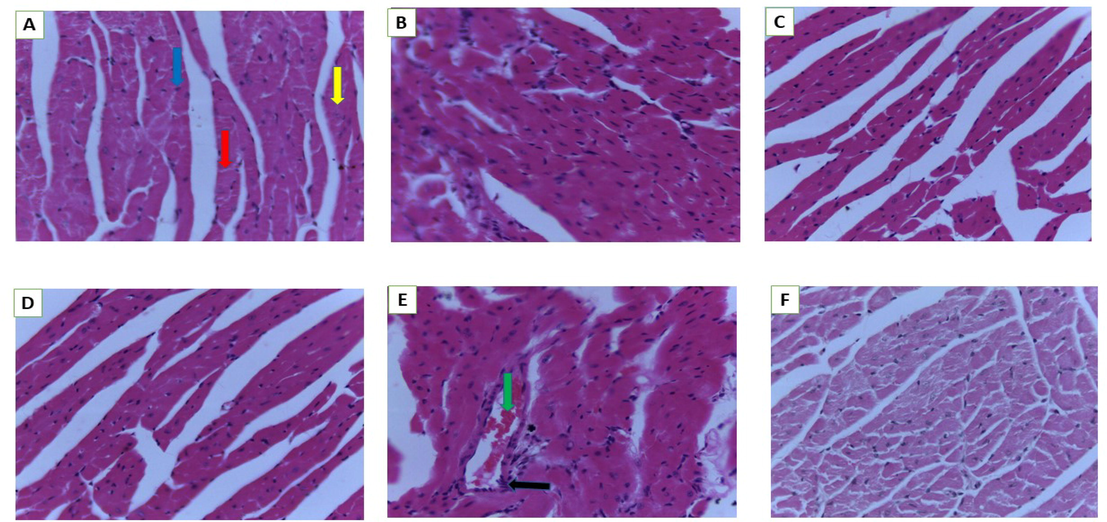
Histology of heart. A (Normal control), B (250 mg/kg bw), C (500 mg/kg bw), D (1000 mg/kg bw), E (2000 mg/kg bw) and F (3000 mg/kg bw). Blue arrow- nuclei of myocytes, yellow arrow-muscle fibre, red arrow-intercalated disc, black arrow -aggregation, green arrow-blood vessel (magnification 40×).
A control mouse's heart had normal myocardial fibre architecture (Fig. 6A). Extensive hyalinization, fragmentation and degeneration of cardiac fibres with congested blood vessels were seen in mice treated with 2000 mg/kg bw (Fig. 6E); similar effects were not detected in animals treated with lower dosages.
3 Discussion
Traditional herbs have been used for millennia to cure a variety of illnesses (Newman, 2020). Phytotherapy is becoming more popular as the WHO promotes suitable ethnomedicinal usage and indicates the safety evaluation of herbal medications. The FDA and WHO both highlight the need of conducting scientific research to validate the efficacy and safety of herbal treatments (Avila et al., 2020; World Health Organization, 1993). A preliminary toxicological study is required to verify the safety of herbal medicines before they may be recommended for the treatment of various communicable and non-communicable syndromes. The fruits of P. macrocarpa are rich in flavonoids and have long been used in folk medicine to treat certain chronic illnesses. However, no data on the toxicity of P. macrocarpa fruit flesh using non-conventional methods have been published so far. As a result, toxicological analyses are necessary to assess safety, emphasizing the importance of evaluating the toxicological profile when determining a safe dose (Mia et al., 2021). In this research, oral acute and sub-acute toxicity effects of P. macrocarpa fruits liquid carbon dioxide extract were investigated using a mice model. Mice are one of the most commonly used mammalian species in preclinical investigations spanning from pharmacology to safety evaluation. Mice are genetically quite similar to humans. Moreover, one year lifespan of mice is considered equivalent to approximately 30 years lifespan of human beings (Hsu et al., 2011).
The acute toxicity result revealed that no toxic symptoms, mortality, or apparent behavioural abnormalities were seen in any animals at the tested doses. As a result, the P. macrocarpa fruits liquid carbon dioxide extract (LCE) may be classified as non-toxic using the acute toxicity classification technique (Duan and Liang, 2011). However, the histological alterations were observed in the liver and kidney at 3000 mg/kg bw. To confirm the safe dose, subacute toxicity assessments were carried out with four different LCE doses (250, 500, 1000, and 2000 mg/kg bw) for consecutive 28 days. The intermediate and high doses of the LCE (1000 mg/kg and 2000 mg/kg, respectively) significantly decreased food consumption, weight gain and water intake in mice during the testing phase, indicating that high doses of the LCE had negative side effects. The body weight changes might be attributable either to reductions in food and water consumption or to organ damage induced by the test plant extract (Deyno et al., 2020). A similar finding was also previously revealed by Yang et al. (2019) and Lazic et al. (2020) in which it was indicated that the significant reduction in body weight gain of the treated group was most likely caused by increasing the dose and duration of exposure to test substances. The weight of the organ coefficient is useful in determining whether the organ is normal or has become pathological (Oyenihi et al., 2021). At the end of the subacute period, there were significant differences in the relative organ weight of liver and kidney at doses of 1000 and 2000 mg/kg bw treated groups (p < 0.05). Changes in the weight of an internal organ may be the only evidence to show that an organ is not normal. Similar phenomenon was also revealed by Aboryag et al. (2017) and Traesel et al. (2014) in which the kidney weight had been reported to be changed due to morphological abnormalities such as tubular hypertrophy. Therefore, serum biochemical and histopathological parameters were evaluated for the toxicity of LCE and determined major toxic effects on organs.
The liver's activity can be determined by biliary secretion, protein synthesis, glucose metabolism, or by examining aberrant protein levels in the blood to see if the liver cells viz., hepatocytes have been destroyed (Peng et al., 2016). AST, ALP and ALT are important parameters which are increased in the bloodstream when the liver is damaged or injured (Yalçın et al., 2020). Furthermore, elevated levels of these enzymes are linked to hepatitis, liver necrosis and liver toxicity, making them useful in the diagnosis of liver illness (Khaoula and Ali, 2020). In this research, the ALT, AST, and ALP levels in the 1000 mg/kg and 2000 mg/kg LCE treated groups increased significantly (p < 0.05), indicating that large doses may be toxic to the liver tissue and result in hepatic damage. The serum value of TP and ALB at doses of 1000 and 2000 mg/kg bw was substantially lower than those of the control group, showing a decrease in liver capability to synthesize proteins while protein excretion increased in renal capacity (Yun et al., 2018). At high doses, the substantial decrease in food and water consumption in mice may have resulted in reduced protein synthesis. Urea and creatinine are the most vital parameters involved in renal function which damage glomeruli of the kidney due to elevating their level in the blood (Abouelghar et al., 2020). The urea and creatinine levels of mice in the group of 1000 mg/kg and 2000 mg/kg bw. exhibited a significant increase in comparison to those of the control group (p < 0.05) which indicated impairment of renal function. These findings were also confirmed by the microscopic observation of the kidney which showed tissue edema, congestion of the vessels at dosages of 2000 mg/kg bw. All these findings were steady with the reduction in TP and ALB, which was further verified by histological examination.
However, there was a significant difference in lipid profile at high doses, implying that LCE influenced lipid metabolism in mice. Although, the glucose level was decreased at high doses but it still remained in the normal range.
The histological investigations supported the biochemical analyses. Differences in histopathology occurred predominantly in the lung, heart, liver and kidney (Yang et al., 2019). In the microscopic observation, hyperemia was also found in the lungs at 2000 mg/kg bw. High doses of LCE could lead to alterations in different indices in mice. TP and ALB biochemical indexes were also considerably less, according to blood and biochemical analyses. The ALT, AST and BUN, CRE were substantially increased and resulted in serum biochemical disturbance with different ions (Zhou et al., 2017; Zhao et al., 2020). These findings indicated that higher doses of LCE might cause heart disease, glomerular enlargement, partial apoptosis, alveolar congestion and heart cell damage to mouse organs. The histological slices can confirm all lesions. However, additional research is still needed on the precise mechanism elicited for harmful activity.
4 Materials and methods
4.1 Sample preparation for extraction
P. macrocarpa’s fruits were initially washed with the clean water and subsequently dried with the help of tissue papers to eliminate any debris. The fruits were then split into flesh and seed parts. After that, the fruit’s flesh was chopped into small pieces and put in the oven at 30–35 °C until complete dryness. The completely dried samples were crushed to a fine powdered form using a Fritsch Universal Cutting Mill-115 (Pulverisette 19-Germany) (Azmi et al., 2021) and carefully kept at room temperature until further usage to carry out other experiments.
4.2 Liquid carbon dioxide extract preparation
With certain modifications, the LCE method was carried out according to the approach outlined by Aiello et al. (Aiello et al., 2020). P. macrocarpa fruits powder (250 gm) was soaked in ethanol at a sample: ethanol (co-solvent) ratio of 1:2 and put in an extraction vessel. The ratio of carbon dioxide solvent to feed was 24:1. Extraction of feed materials was carried out at 6.9 to 7.0 MPa and temperatures ranging from 29 to 30 °C. The liquid carbon dioxide then flowed into the vessel to begin the smooth process of extraction. By vaporization in the reboiler, the extracts were separated from the liquid CO2. The extracts were then left at the bottom of the reboiler. Extraction was carried out until there was no more solution flowing out of the system. The extract from the cyclone separator was collected using the blue-top Schott container. Following collection, the ethanol was evaporated using a rotary evaporator and stored at −80 °C until further analysis.
4.3 Animals
In this research, twenty-eight male pathogen-free ICR experimental mice (28–34 g), 6–7 weeks old as well as the animal feed were purchased from the local animals’ provider “A-sapphire enterprise Ltd., Kuala Lumpur, Malaysia”. The animal feed was composed of fat (5%), carbohydrates (60%), fiber (5%) and protein (20%). All the experimental mice were kept in an SPF grade laboratory at constant humidity (40%–70%) and room temperature (22 ± 2 °C) on a 12-h light–dark cycle. All animals were kept in sterile polypropylene cages and provided a basic diet as well as filter water. Prior to treatment, the mice were acclimatized for 7 days.
4.4 Acute oral toxicity
The acute toxicity evaluation was done according to the Organization for Economic Cooperation and Development (OECD) guidelines 425 (Organisation for Economic Co-operation and Development (OECD)/OCDE, 2008). Animals were dosed one at a time by following the up-and-down process. Eight mice having their weight ranging from 28 to 32 g were divided randomly into two different groups having four mice in each group (control and 3000 mg/kg). The LCE dose was chosen by the guidance based on the substance's hazardous potential. The control group was given the same amount of distilled water as the experimental group in order to create a comparison. Mortality and general behaviour alterations were observed at 30 min, 2 h, 4 h, 6 h, 10 h, and 24 h after administering the extract on the starting day and then daily for a total of fourteen consecutive days. The animals’ general behaviours including salivation, breathing difficulty, tremors, faeces consistency, itching, fur & skin, hypo-activity, sleep, convulsion, coma, and mortality were observed daily for a total of 2 weeks. Food and water, given to mice, were measured every day at the same time. The difference was used to determine the amount of food (water) consumed. During therapy, water intake, and food consumption were monitored daily and body weight variations were reported weekly. In the meantime, mice's deaths and abnormalities were noted.
4.5 Subacute oral toxicity
The subacute toxicity assay was carried out for 4 weeks according to the OECD 407 guidelines (OECD (Organization for Economic Cooperation and Development), 1995) Briefly, twenty male mice were randomly divided into five groups comprising four mice each. The LD50 in this research was found to be >3000 mg/kg bw. Based on that dose, the treated groups were orally administered LCE at doses of 2000, 1000, 500, and 250 mg/kg bw via oral gavage for 28 days, whereas the control group was given distilled water. Throughout the treatment, the toxicity indicators and abnormal behaviours were all monitored on a regular basis. The weight was kept track of on a weekly basis. All animals were anaesthetized with pentobarbital at the end of the study, and blood samples were taken immediately for biochemical examinations. The specific vital organs namely liver, brain, heart, lung, and kidney were taken and weighed in order to determine organ coefficients, while tissues were collected and used for histological examination (Porwal et al., 2017). Using the following equation, the relative organ weight was determined in relation to the body weight of the mouse (Betti et al., 2012).
4.6 Histological observation
The experimental mice were carefully euthanized, and subsequently their vital organs (liver, kidney, brain, lung, and heart) were preserved in 10% buffered formalin followed by a standard histological procedure that included tissue processing and paraffin embedding. Subsequently, these tissues were sliced at 5 µm using a microtome blade and all sections were meticulously stained with hematoxylin and eosin. All slides were then viewed under light electron microscope (LEICA 3 MSCOPE MODEL).
4.7 Biochemical analysis
For evaluating the biochemical parameters, the whole blood was taken in a plan tube, serum separator, from mice and put around 20 °C until blood clotting, then it was centrifuged at 3000 rpm for 15 min to get the serum. Some biochemical parameters such as glucose (GLU), total protein (TP), alkaline phosphatase (ALP), creatinine (CRE), alanine aminotransferase (ALT), albumin (ALB), aspartate aminotransferase (AST), triglycerides (TG), urea, high-density lipoprotein cholesterol (HDL-C) and total cholesterol (TC) were analysed using Backman coulter AU 480. Low density lipoprotein (LDL)-Cholesterol was calculated by following the Friedewald equation (Friedewald et al., 1972).
LDL-Cholesterol = TC-(TG/5 + HDL). All units here are mg/dL.
4.8 Statistical analysis
Data have been represented as mean ± standard error of mean (SEM). In order to assess the significant differences (p < 0.05, p < 0.01, p < 0.001), the values were examined using one-way and two-way ANOVA followed by Dunnet’s, Tukey’s and Sidak’s multiple comparison tests (GraphPad Prism 7.00).
5 Conclusions
The acute and subacute toxicity of P. macrocarpa fruits liquid carbon dioxide extract was studied via oral administration in mice in this research study. The results revealed no mortality among mice after single oral administration of the tested doses. The LD50 of LCE for mice was found to be more than 3000 mg/kg bw and the LCE showed slight toxicity in liver histology when compared to the control group. According to the findings of the subacute toxicity test, it was discovered that the LCE caused alterations in several parameters, which were shown to be dose–response related. As a result, at a dose 1000 mg/kg bw, the extract exhibited modest toxicity, albeit it was not substantial during daily oral administration. As a result, it should be used with caution when administered. Mice exposed to higher dosages (2000 mg/kg bw) of the extract showed signs of toxic effects on their liver, spleen, kidney, and lungs. In summary, the dose of up to 500 mg/kg bw liquid carbon dioxide extract of P. macrocarpa fruit can be considered a safe dose. So, this safe dose is much higher in liquid carbon dioxide extraction than the previously reported safe dose using conventional extraction methods. These findings give important early information on the toxicity profile of P macrocarpa fruits. As a result, more evaluations (such as investigations of genotoxicity, sub-chronic toxicity) are necessary before this plant may be recommended to be used in clinical trials. It is essential to realize that medicinal plants should be studied and assessed in terms of their toxicity and safety based on their solvent and extraction technique. So, while developing an LCE-based phytomedicine, the dose must be chosen carefully to maximize efficacy while minimizing adverse side effects.
6 Institutional Review Board Statement
The animal handling research protocol was initially approved by the IIUM ANIMAL CARE AND USE COMMITTEE (I-ACUC), with the ethical clearance reference number IIUM/504/14/2/I-ACUC.
7 Informed Consent Statement
Not applicable.
8 Data Availability Statement
Data is available in the article.
CRediT authorship contribution statement
Md. Abdur Rashid Mia: Investigation, Writing – original draft. Qamar Uddin Ahmed: Conceptualization, Writing – review & editing, Resources, Supervision, Project administration, Funding acquisition. Abul Bashar Mohammed Helaluddin: Supervision. Sahena Ferdosh: Supervision. Monowarul Mobin Siddique: Methodology, Supervision. Syed Najmul Hejaz Azmi: Methodology, Writing – review & editing, Supervision. Jahangeer Ahmed: Conceptualization, Writing – review & editing, Resources, Supervision, Project administration, Funding acquisition. Md. Zaidul Islam Sarker: Conceptualization, Resources, Supervision.
Acknowledgments
The authors also extend their sincere appreciation to the Researchers Supporting Project (RSP-2021/391) at the King Saud University, Riyadh, Saudi Arabia. The authors are also grateful to the University Malaysia Pahang, Malaysia and the Research Management Centre, IIUM, for providing financial assistance through Joint Research Project (ID: SRCG20-002-0002; UMP-IIUM-UiTM Sustainable Research Collaboration Grant 2020) to accomplish this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Histopathological changes in the kidney following congestive heart failure by volume overload in rats. Oxid. Med. Cell Longev.. 2017;2017:6894040.
- [Google Scholar]
- Oxidative stress, hematological and biochemical alterations induced by sub-acute exposure to fipronil (COACH) in albino mice and ameliorative effect of selenium plus vitamin E. Environ. Sci. Pollut. Res. Int.. 2020;27:7886-7900.
- [Google Scholar]
- Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol.. 2020;55:2472-2480.
- [Google Scholar]
- Armenia, E.F.; Widya, R.M.; Rusdi, D.J.; Netty, M. Anti-Atherosclerotic effect and liver toxicity of ethanolic extract of Phaleria macrocarpa (Scheff. Boerl) fruit on Japanese Quail. Asian Symposium on Medicinal Plants, Spices and other natural product XII (ASOMP), Padang, Indonesia, 2006, 13-18.
- An FDA/CDER perspective on nonclinical testing strategies: Classical toxicology approaches and new approach methodologies (NAMs) Regul. Toxicol. Pharmacol.. 2020;114:104662
- [Google Scholar]
- Optimization for synthesis of silver nanoparticles through response surface methodology using leaf extract of Boswellia sacra and its application in antimicrobial activity. Environ. Monit. Assess.. 2021;193(8):497.
- [Google Scholar]
- Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng.. 2013;117:426-436.
- [Google Scholar]
- Acute and repeated-doses (28 days) toxicity study of Hypericum polyanthemum Klotzsch ex Reichardt (Guttiferare) in mice. Food Chem. Toxicol.. 2012;50:2349-2355.
- [Google Scholar]
- Acute and sub-acute toxicity of Echinops kebericho decoction in rats. BMC Complement. Med. Ther.. 2020;20:1-11.
- [Google Scholar]
- Technical Guidelines Assembly of Veterinary Medicine Research. Beijing: Chemical Industry Press; 2011.
- Bioactive compounds and advanced processing technology: Phaleria macrocarpa (Sheff.) Boerl, a review. J. Chem. Technol. Biot.. 2015;90:981-991.
- [Google Scholar]
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem.. 1972;18:499-502.
- [Google Scholar]
- A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food Chem. Toxicol.. 2011;49:2624-2630.
- [Google Scholar]
- Preventive and curative effects of Atriplex Halimus L. aqueous extract on benzene provoked hepatic injury in rats. J. Drug Deliv. Ther.. 2020;10:217-222.
- [Google Scholar]
- Determining organ weight toxicity with Bayesian causal models: Improving on the analysis of relative organ weights. Sci. Rep.. 2020;10:1-12.
- [Google Scholar]
- Md. Abdur Rashid Mia, Sahena Ferdosh, Qamar Uddin Ahmed, Abul Bashar Mohammed Helaluddin, Md. Zaidul Islam Sarker (2021). Bridging indigenous knowledge and scientific evidence for pharmacological studies of Phaleria macrocarpa: A review. Nat. Prod. J. 11. Doi: 10.2174/2210315511666210322161112.
- Medicinal Plants for Treatment of Prevalent Diseases. In: Pharmacognosy-Medicinal Plants. London, UK: IntechOpen; 2019.
- [Google Scholar]
- Modern traditional Chinese medicine: Identifying, defining and usage of TCM components. Adv. Pharmacol.. 2020;87:113-158.
- [Google Scholar]
- OECD (Organization for Economic Cooperation and Development), Guidelines for Testing of Chemicals. No 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents; Paris, France, 27 July 1995.
- Organisation for Economic Co-operation and Development (OECD)/OCDE. Acute Oral Toxicity-Up and Down Procedure; OECD Guidelines for the testing of Chemicals 425; Organisation for Economic Co-operation and Development: Paris, France, 2008; pp. 1–27.
- Toxicity assessment of watermelon seed supplemented diet in rats. Drug Chem. Toxicol.. 2021;1–8
- [Google Scholar]
- Evaluation of the acute and subchronic toxicity of Aster tataricus L. F. Afr. J. Tradit., Complementary Altern. Med.. 2016;13:38-53.
- [Google Scholar]
- Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol.. 2017;107:581-589.
- [Google Scholar]
- Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci. Pharm.. 2017;85:29.
- [Google Scholar]
- Making better drugs: decision gates in non-clinical drug development. Nat. Rev. Drug Discov.. 2003;2:542-553.
- [Google Scholar]
- A review of metal exposure and its effects on bone health. J. Toxicol.. 2018;2018:1-11.
- [Google Scholar]
- Liquid CO2 extraction of flowers and fractionation of floral concrete of Michelia champaca Linn. J. Supercrit. Fluids. 2011;56:249-252.
- [Google Scholar]
- Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health. 2020;17:3376.
- [Google Scholar]
- Effect of butanol extract of maturated mahkota dewa (Phaleria macrocarpa) fruit on kidney tissue of Mice (Mus musculus) Biodiversitas J. Bio. Div.. 2006;7:278-281.
- [Google Scholar]
- Green technologies for the extraction of bioactive compounds in fruits and vegetables. CYTA J. Food. 2018;16:400-412.
- [Google Scholar]
- Acute and subacute (28 days) oral toxicity assessment of the oil extracted from Acrocomia aculeata pulp in rats. Food Chem. Toxicol.. 2014;74:320-325.
- [Google Scholar]
- Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines. Manila, Philippines: World Health Organization; 1993.
- WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems. Geneva, Switzerland: WHO; 2004.
- WHO Global Report on Traditional and Complementary Medicine 2019. Geneva, Switzerland: World Health Organization; 2019. p. :10-80.
- In vivo protective effects of Ginkgo biloba L. leaf extract against hydrogen peroxide toxicity: cytogenetic and biochemical evaluation. Environ. Sci. Pollut. Res.. 2020;27:3156-3164.
- [Google Scholar]
- Acute and subacute toxicity evaluation of ethanol extract from aerial parts of Epigynum auritum in mice. Food Chem. Toxicol.. 2019;131:110534
- [Google Scholar]
- Enzymatic extract from Ecklonia cava: acute and subchronic oral toxicity and genotoxicity studies. Regul. Toxicol. Pharmacol.. 2018;92:46-54.
- [Google Scholar]
- Isolation, characterization and cytotoxic activity of benzophenone glucopyranosides from Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl) Bioorg. Med. Chem. Lett.. 2012;22:6862-6866.
- [Google Scholar]
- Toxicological safety evaluation in acute and 28-day studies of aqueous extract from Bei-Qi-Wu-Jia formula. J. Ethnopharmacol.. 2020;248:112324
- [Google Scholar]
- The etiology of basal vacuolizations in renal tubular epithelial cells evaluated in an isolated perfused kidney model. J. Forensic Sci.. 2017;62:915-920.
- [Google Scholar]







