Translate this page into:
Phytochemical screening and GC-MS chemical profiling of an innovative anti-cancer herbal formula (PHF6)
⁎Corresponding author. mmyalzahrani@imamu.edu.sa (Mohammed Al-Zharani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present investigation assessed the anticancer potential and chemical profile of the polyherbal formulation (PHF6) against human breast cancer cells. The plants (Alchemilla vulgaris, Cichorium pumilum, Crataegus azarolus, Eruca sativa, Ferula hermonis, and Hypericum triquetrifolium) were combined in equal quantities and extracted using soxhlet apparatus. The total polyphenolic (TPC) of the hexane and chloroform extract of PHF6 were 3.11 ± 0.004 and 7.88 ± 0.004 µg/mL (as gallic acid equivalent), respectively. The radical scavenging activity (RSA) values for the hexane and chloroform extract were 35.1 % ±0.01 and 36.9 % ±0.06, respectively. For hexane extract, the IC50 values were found to be 82.8 and 48.7 µg/mL against MCF-7ER(+)/PR(+) and MDA MB-231ER(−)/PR(−), respectively. Similarly, the IC50 value of chloroform extract was 44.4 µg/mL against both cell lines. The MDA MB-231ER(−)/PR(−) cells stained with DAPI post-treatment with hexane and chloroform extract showed cell shrinkage, nuclear fragmentation, and chromatin condensation. Moreover, GC–MS screening of the PHF6 revealed several compounds with different reported biological activities, such as phytol and octadecanoic acid. PHF6 showed promising anticancer activity against cancer cells tested, thus deserving more clinical studies in the future.
Keywords
Antioxidant
Apoptosis
GC-MS
Polyherbal formulation
1 Introduction
Breast cancer (BC) is the leading cause of cancer among Saudi females (Asiri et al., 2020).
In Saudi Arabia, the incidence of BC increased around threefold from 2004 (783 cases) to 2016 (2240 cases). The data revealed that 70 % of the total BC cases were found in Riyadh (25.2 %), Makkah (24.1 %) and the eastern region (21.2 %). The author attributed the increase to many factors, including aging, socioeconomic and traditional lifestyles such as unhealthy diet, lack of exercise, smoking and genetic predisposition (Albeshan and Alashban, 2021).
Although chemotherapies are effective, target specific, and improve the survival of BC patients (Holohan et al., 2013). However, treatment failure remains a major issue worldwide (Dasari and Tchounwou, 2014) due to intrinsic and acquired chemo-resistance in the tumour cell. There has been a rise in botanical formulations used as an adjunct to cancer therapy (Chattopadhyaya et al., 2011). For many years, the decoction of a polyherbal mixture has been used to treat cancer by Ayurvedic physicians (Pathiranage et al., 2020). Polyherbal formulations play an important role in the regulation of different disease targets in various pathways hence neutralising adverse effects, enhancing efficacy, overcoming drug resistance mechanisms, regulating transporters and enzymes to improve drug bioavailability (Amin et al., 2015; Zimmermann et al., 2007; Aziz et al., 2013). Murunikkara and Rasool 2014 reported that these herbal remedies are promising anticancer potential, that improve the immune system, reduce the toxicity of chemotherapy, and improve the patients' survival rates (Nagla et al., 2010). In traditional medicine, herbal mixtures have been used for a long time because of their mythological belief and historical heritage. As per traditional belief, diseases can be cured by a multidrug approach, unlike Western medicine, which commonly uses single-drug therapy.
PHF6 is an innovative polyherbal formulation consisting of equal quantities of six medicinal plants that have been reported individually against different cancer types. Several experimental studies have illustrated the cytotoxic activities of Alchemilla vulgaris L. (Rosaceae) against prostate PC-3, breast MCF7, neuroblastoma H-SY5Y and ovarian A2780, cancer cell lines (Vlaisavljević et al., 2019; Moqidem, 2021). Likewise, the extract of Cichorium pumilum Jacq. (chicory) (Asteraceae) are reported to have significant cytotoxic activities against Lung cancer WI38, VA13, A549, and liver cancer HepG2 cell lines (Zhang et al., 2006). Crataegus Azarolus L. (Rosaceae) is also reported for its cytotoxic activities against HCT-116 and HT-29 human colorectal cancer cell lines (Mustapha et al., 2016).
There are several reports of cytotoxic activities of Hypericum triquetrifolium Turra. (Hypericaceae) against COR-L23 (lung carcinoma), HepG-2 (hepatocellular carcinoma), ACHN (renal cell adenocarcinoma), and the amelanotic melanoma C32 cell lines (Conforti et al., 2007). Similarly, Eruca sativa LINN. (Brassicaceae) and Ferula hermonis Boiss. (Apiaceae) were also having s significant anticancer activity against HCT116 (colon carcinoma), Hela (Cervix carcinoma), HepG2 (liver carcinoma), MCF7 (breast carcinoma), U251 (brain carcinoma), MDA-MB-231(breast carcinoma) and LoVo (colon cancer) cell lines (Nazif et al., 2010; Abutaha et al., 2019).
The above-selected plants were individually reported for their anticancer potential. However, no single polyherbal formulation has been developed for anticancer activity. Therefore, taking leads from the available investigation, we aimed to develop a polyherbal formulation from these plants and assess their anticancer and apoptotic potential in two different breast cancer cells.
2 Materials and methods
2.1 Reagents
DMEM (Dulbecco's Modified Eagle Medium), fetal bovine serum, penicillin/streptomycin antibiotic, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) and trypsin/EDTA were purchased from Invitrogen (Carlsbad, CA). Hexane, chloroform, ethyl acetate, methanol, and DMSO, were purchased from Sigma-Aldrich (St-Louis, MO). DAPI (4′,6-Diamidino-2-Phenylindole, Dihydrochloride) were obtained from Life Technologies (Carlsbad, CA). 2,2-diphenyl-1-picrylhydrazyl (DPPH), lactate dehydrogenase (LDH) kit, Acridine orange (AO) and ethidium bromide (EB) were purchased from Sigma-Aldrich (St-Louis, MO).
2.2 Extraction
The dried leaves of A. vulgaris, C. pumilum,C. azarolus, E. sativa, F. hermonis, and H. triquetrifolium were purchased in a single lot from herbal suppliers in Riyadh. The polyherbal formula (PHF6) was prepared by mixing equal quantities of the 6 plant powders (2 g each). The PHF6 (12 g) was placed in a thimble (37 × 130 mm), then inserted into the Soxhlet apparatus with an extractor column and collecting bottom flask of 500 mL. The heating mantle was used to heat the collecting bottom flask. The PHF6 was extracted using different solvents (Hexane, chloroform, ethyl acetate, and methanol) of increasing polarity for 20 h. The temperature used to extract the solvent was set to 70 °C for all the solvents used. Whatman No 1 filter paper was used to filter the extract and later centrifuged and vacuum evaporated using rotary apparatus.
2.3 GC–MS analysis
The chemical composition of PHF6 chloroform extract was assessed using gas chromatography and a mass spectrometer (Turbomass, PerkinElmer, Inc., Waltham, MA, USA). The method adopted for GC–MS analysis was conducted as reported earlier (Nasr et al., 2020). The chemical constituents were determined by comparing the mass spectra obtained using the National Institute of Standard and Technology (NIST) and WILEY Spectral libraries.
2.4 Total polyphenolic content (TPC)
Folin-Ciocalteu (FC) phenol colorimetric test was used to calculate the TPC of PHF6. PHF6 active extract (12.5 µL) was added to 125 µL of FC reagent. After 5 min of dark incubation, 12.5 µL of sodium carbonate (7.5 %) was pipetted to the mixture and incubated for 1.5 h at 25 °C. The standard curve was constructed using different concentrations of Gallic acid (0–100 µg/ml), and the TPC was recorded in micrograms per millilitre of gallic acid equivalents (μg/mL of GAE). All the analysis was conducted in triplicate (Abutaha et al., 2020).
2.5 Total flavonoid content (TFC)
The TFC of PHF6 active extract was calculated using the Aluminium chloride (AlCl3) colourimetric method. A hundred microliters of PHF6 was mixed with 100 µL of AlCl3 (2 %), and 10 min (25 °C) later, the absorbance (Abs) was read by spectrophotometry at 368 nm. The standard curve was constructed using different concentrations of Quercetin (0.1–0.9) to assess the TFC (Abutaha et al., 2020). TFC was expressed as micrograms per millilitre of Quercetin equivalents (μg/mL of QUE).
2.6 DPPH assay
Ten microliters of 1 mg/mL of different solvent extracts were mixed separately with 190 µL of 2,2-diphenyl-1-picrylhydrazyl (DPPH) (100 mM) in a 96-well- plate, and the Abs was read at 515 nm after 30 min (25 °C) in the dark. Dimethyl sulfoxide (DMSO) and Ascorbic acid were used as the negative and positive control, respectively. The experiment was performed in triplicate (Abutaha et al., 2020).
The radical scavenging activity was estimated as follows:
2.7 Cytotoxicity assay
MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) were maintained in high glucose DMEM medium supplemented with 10 % fetal bovine serum and streptomycin / penicillin (100 µg/ml) at 37 °C and 5 % CO2. These cell lines had been originally obtained from the German Cell Culture Collection (DSMZ), Leibniz, Germany. The cells grow as adherent cells in T25 tissue culture flasks. When confluent, the cells were harvested with trypsin/EDTA, washed with the DMEM medium, and then used as described previously (Abutaha et al., 2020).
2.7.1 MTT assay
Two human breast cancer cells, namely MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+), were seeded (at a density of 5 × 104 cells per mL) into a 24-well tissue culture plate overnight. Later, cells were treated with PHF6 extracts (0–250 μg/mL) and 0.01 % DMSO as a vehicle control. The plates were incubated for 24 h using CO2 incubator (5 % CO2, at 37 °C) for 24 h. Then, 100 μL of MTT (5 mg/ml) solution was pipetted to each well and incubated for 2 h. The formazan crystal formed was dissolved in HCl-isopropanol (0.01 %). Finally, a microplate reader (ChroMate, USA) was used to read the Abs (550 nm) (Abutaha et al., 2020).
2.7.2 Cytosolic lactate dehydrogenase (LDH) assay
Cytosolic LDH release was assessed using LDH kit as recommended by the manufacturer. Cells were treated as in the previous section, and the release of LDH was measured. The increase in Abs at 490 nm is proportional to LDH release into the extracellular medium. Wells treated with 0.01 % DMSO were considered as a vehicle control.
2.8 Morphology assay
Cells were treated (at IC50 concentration) in 24-well plates as reported in 2.5.1 section and then imaged directly using a fluorescence microscope (EVOS, USA) After 24 h of exposure.
2.8.1 Apoptosis assays
To assess the apoptotic potential of PHF6 against MDA-MB-231 cells, DAPI (4,6-diamidino-2-phenylindole) staining and Acridine Orange (AO) / Ethidium Bromide (EB) staining were carried out as reported previously (Abutaha, 2021). In brief, cells were cultured in 24-well plates and treated with PHF6 (IC50) at 37 °C for 24 h. Post-treatment, 1 μL of AO/EB (1:1) was added to each well and imaged directly. For DAPI staining, treated cells were washed with PBS, fixed with iced cold ethanol, washed with PBS, and then stained with DAPI (1 µg/ml) for 30 min. Finally, cells were imaged under a fluorescence microscope (EVOS, USA) for apoptosis assessment
3 Results
3.1 Total phenolic and flavonoid contents of PHF6 extracts
The total polyphenolic contents in hexane and chloroform extracts were 3.11 ± 0.004 and 7.88 ± 0.004 µg/mL (as gallic acid equivalent), respectively. No flavonoid was detected in both extracts.
3.2 Antioxidant activity of PHF6 extracts using DPPH assay
We conducted DPPH assay to assess antioxidant potential in the PHF6 extracts. The mechanism for investigating the antioxidant potential consists of the reduction reaction of the violet-colored DPPH• free radical forming the yellow-colored DPPH-H. The elimination of DPPH• results in a reduction of absorbance at 515 nm (Oleinik et al., 2022). The radical scavenging activity values for the hexane and chloroform extract were 35.1 % ±0.01 and 36.9 % ±0.006, respectively.
3.3 GC–MS profile of the active extract
The main constituents identified in the PHF6 chloroform extract are reported in Table 1 and Fig. 1. Some of the main compounds detected in the herbal formula are 4-Methyl-2,6-dihydroxyquinoline Quinoline, 2-butyl- (14.81 %), Phenol, 2-cyclohexyl-(9.26 %), Pyridin-4-amine, N-cyclohexyl- (7.78 %), 2,3,4-Tetrahydro-3-isopropyl-5-methyl-1-oxonaphthalene (5.94 %), Naphthalene, 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl)- (95.41 %), and alpha-Calacorene (4.06 %).
RT (min)
% Area
Name
Molecular Weight (amu)
Compound nature
5.053
0.31
Octane, 2-methyl-
128.157
alkane
5.657
0.20
Butanal, 2-ethyl-3-methyl-
114.104
aldehyde
6.16
0.71
Bicyclo[3.1.1]hept-2-ene, 2,6,6-trimethyl
136.125
Terpene
6.503
0.50
2(5H)-Furanone, 5,5-dimethyl-
112.052
ketone
7.33
0.68
Phenol
94.042
phenol
8.145
0.13
Decane, 2-methyl-
156.188
alkane
8.386
0.17
Ethane, hexachloro-
233.813
haloalkane
8.609
0.12
Benzene, 1-methyl-4-(1-methylethenyl)-
132.094
–
10.085
0.26
5-Tetradecene, (E)-
196.219
–
10.289
0.26
Benzoic acid
122.037
acid
10.505
0.31
Bicyclo[3.1.1]hept-3-en-2-one, 4,6,6-trimethyl-, (1S)-
150.104
Terpene
10.823
1.16
Benzofuran, 2,3-dihydro-
120.058
–
10.931
1.80
Propane, 1-ethoxy-2-methyl-
102.104
–
11.707
0.13
Phenol, 2-(1,1-dimethylethyl)-
150.104
–
12.954
0.45
1-Tetradecene
196.219
–
13.062
0.12
Hydroxy-.alpha.-terpenyl acetate
212.141
Terpene
13.705
4.06
alpha.-Calacorene
200.157
Sesquiterpenoids
14.182
5.94
1,2,3,4-Tetrahydro-3-isopropyl-5-methyl-1-oxonaphthalene
202.136
Terpene
14.437
3.90
Quinoline, 2-butyl-
185.12
–
14.672
5.41
Naphthalene, 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl)-, (1S-cis)-
202.172
Terpene
14.971
2.38
Phenol, 2,4-bis(1,1-dimethylethyl)-
206.167
–
15.162
0.12
Carbonic acid, 4-isopropylphenyl propargyl ester
218.094
–
15.359
0.22
2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)-
180.115
Terpene
15.429
0.17
Indan, 1,1,6,7-tetramethyl-
174.141
Terpene
15.607
0.37
2,3,4,5,6-Pentamethyl acetophenone
190.136
ketone
15.683
0.50
2-Methyl-6-propylpyridine
135.105
–
15.779
1.54
2-Butanone, 4-(4-hydroxyphenyl)-
164.084
Ketone
15.932
7.78
Pyridin-4-amine, N-cyclohexyl-
176.131
–
15.989
9.26
Phenol, 2-cyclohexyl-
176.12
–
16.396
2.14
Cyclohexanol, 1-(4-fluorophenyl)-4-hexyl-
278.205
alcohol
16.625
1.57
2H-1-Benzopyran-2-one, 3-amino-4-hydroxy-
177.043
cyclic ester
16.829
5.44
Benzaldehyde, 3-methyl-
120.058
aldehyde
17.013
3.32
Benzene, 1-cyclopenten-1-yl-
144.094
–
17.223
0.65
Benzaldehyde, 2,4-dihydroxy-
138.032
aldehyde
17.369
2.54
Naphthalene, 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methylethyl)-, (1S-cis)-
202.172
–
17.427
3.41
5(1H)-Azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8S-cis)-
218.167
ketone
17.796
2.87
4(1H)-Pteridinone, 2-amino-6,7-dimethyl-
191.081
Ketone
17.923
0.18
Benzene, 1-methyl-2-nitroso-
121.053
–
18.018
0.33
Phenol, 2-ethyl-4,5-dimethyl-
150.104
–
18.298
2.09
Ethanone, 1-(6,6-dimethylbicyclo[3.1.0]hex-2-en-2-yl)-
150.104
Ketone
18.769
14.81
4-Methyl-2,6-dihydroxyquinoline
175.063
–
18.934
5.57
1,9-Tetradecadiene
194.203
fatty acid
19.036
0.62
2-Pentadecanone, 6,10,14-trimethyl-
268.277
Ketone
19.208
0.44
6-Octen-1-ol, 3,7-dimethyl-, acetate
198.162
ester
19.405
1.04
1,4-Eicosadiene
278.297
alkene
19.628
0.23
4,6,10,10-Tetramethyl-5-oxatricyclo[4.4.0.0(1,4)]dec-2-en-7-ol
208.146
Terpene
19.851
0.28
Isoaromadendrene epoxide
220.183
Terpene
19.933
0.12
7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione
276.173
Ketone
20.531
0.94
Cycloeicosane
280.313
alkene
20.595
0.27
Octadecanoic acid
284.272
fatty acid
20.818
0.20
Isoaromadendrene epoxide
220.183
–
20.932
1.66
7H-Furo[3,2-g][1]benzopyran-7-one, 4-methoxy-
216.042
ester
21.74
0.28
Phytol
296.308
Terpene
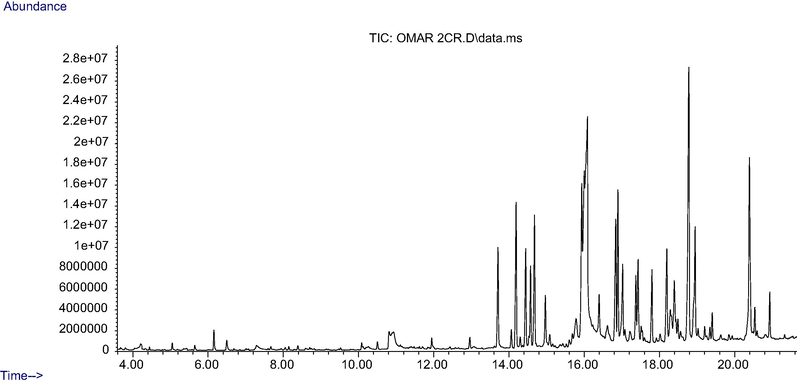
Chromatogram of PHF6 chloroform extract using gas chromatography-mass spectrometry.
3.4 PHF6 extracts induce cytotoxicity in cancer cell lines
All cell lines tested were incubated (24 h) with four increasing concentrations of solvent extracts. The concentrations were 250, 125, 62.5, 25 µg/mL and 0.01 % DMSO as a vehicle control. Breast cancer cells were not sensitive to the ethyl acetate and methanol extracts, where the different concentrations tested failed to induce IC50. The results showed that the decrease of cell viability by hexane and chloroform extract was dose-dependent for both the cell lines tested. The IC50 concentration was found to be 48.7 and 82.8 µg/mL against MCF7 and MDA-MB-231, respectively, for hexane extract (Fig. 2A); similarly, the chloroform extract revealed IC50 values of 44.4 µg/mL against MCF7 and MDA-MB-231 (Fig. 3A). To further confirm the toxicity of the extracts, LDH assay was carried out. LDH is another indicator of cytotoxicity. The hexane and chloroform extracts caused a significant LDH release at 250 and 125 μg/mL, confirming the cytotoxicity on the cell lines tested (Figs. 2 and 3).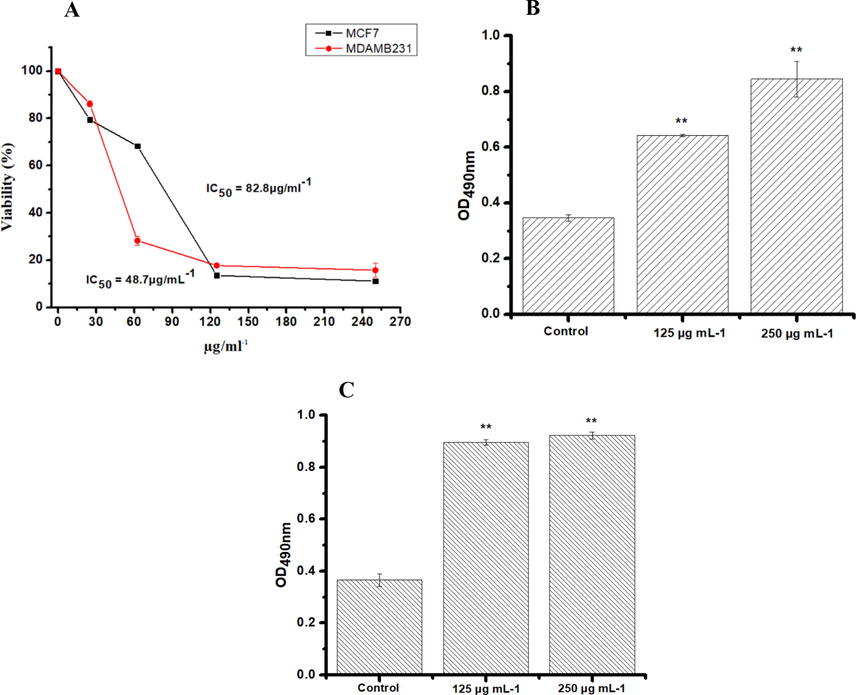
MTT and LDH Assay in MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) post 24 h treatment with increased concentrations of PHF6 hexane extract. The absorbance was measured at 550 nm and 490 nm using a plate reader for MTT (A) and LDH assay (B and D) respectively. Three independent experiments were carried out in duplicate. Values represent as means ± SD. The result were considered significant at **P < 0.05.
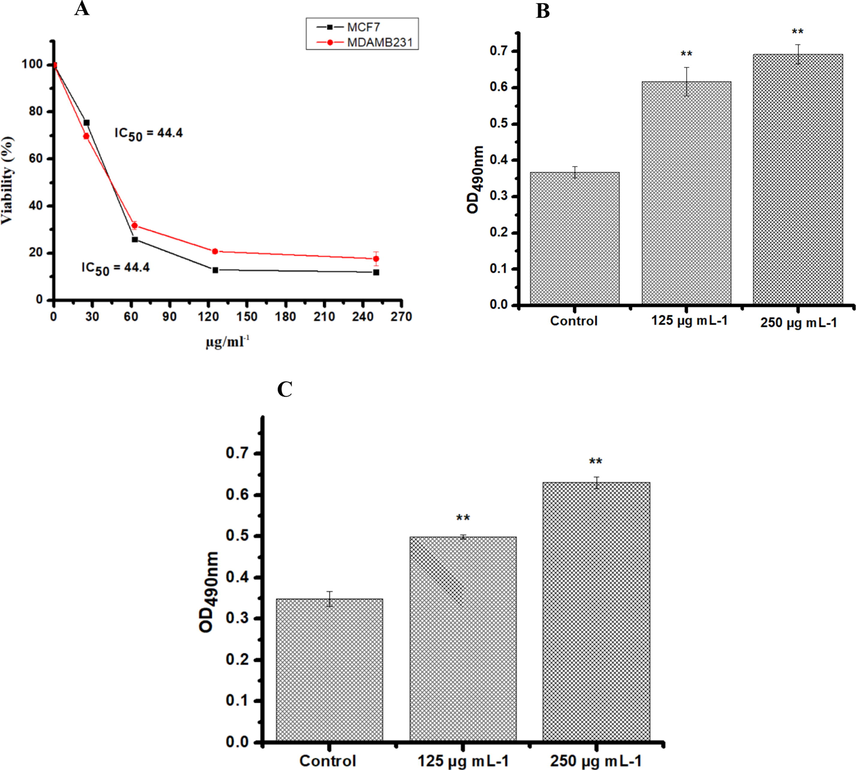
MTT and LDH Assay in MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) post 24 h treatment with increased concentrations of PHF6 chloroform extract. The absorbance was measured at 550 nm and 490 nm using a plate reader for MTT (A) and LDH assay (B and D) respectively. Three independent experiments were carried out in duplicate. Values represent as means ± SD. The result were considered significant at **P < 0.05.
3.5 PHF6 extracts induce apoptosis-related morphological changes in cancer cell lines
The MDA-MB-231 ER(−)/PR(−) cells were incubated with hexane extract, stained with DAPI, and imaged to investigate the resulting morphological changes. The control cells exhibited a normal nuclear morphology, but hexane-treated cells showed shrinkage, margination of the nucleus, nuclear fragmentation, and chromatin condensation (Fig. 4). To further investigate the apoptosis potential of the hexane and chloroform extracts on MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) cells, the cell morphology was assessed using AO/EB staining. AO stains both dead and live cells, whereas EB stains only cells that have lost the integrity of their membrane. Cells that appeared green in colour with intact nuclei indicate viable cells, green cells with fragmented nuclei indicate early apoptosis and red indicate late apoptosis and necrosis. The control cells were viable cells with large green nuclei, representing the intact cell membranes (Fig. 4D). However, when treated with 44.4 and 48.7 µg/mL of hexane and chloroform extracts, the number of cells with green nuclei were reduced, and all cells exhibited signs of fragmentation of chromatins and nuclear condensation (white arrows; Fig. 4). These results confirmed that the extract induced apoptosis in MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) cells.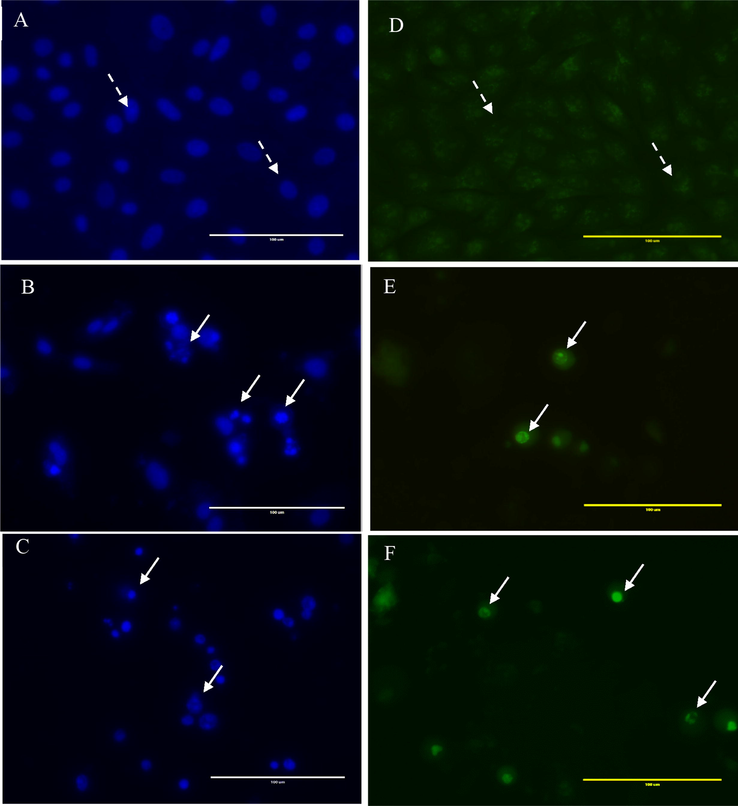
DAPI and AO/EB staining of MDA MB-231ER(−)/PR(−) cells treated with PHF6 chloroform extract at 48.7 µg/mL and PHF6 hexane extract at 44.4 µg/mL for 24 h. DAPI staining; A: control (DMSO 0.01 %), B treated with chloroform extract and C treated with hexane extract. AO/EB staining; (D): control (DMSO 0.01 %), E treated with chloroform extract and F treated with hexane extract. The dashed arrow shows live cells, and the White arrow indicates apoptotic cells. The images were captured using a fluorescence microscope (40×). Scale bar 100 μm.
4 Discussion
Secondary metabolites obtained from plants are generally involved in treating several diseases. The phytochemical and GC–MS analysis of PHF6 revealed the presence of several types of secondary metabolites such as phenols, esters, terpenes, aldehyde and ketones with several known biological activities. For instance, phenol, 2-(1,1-dimethylethyl)- is a phenol with antifungal activity (Ren et al., 2019). Octadecanoic acid is a fatty acid with anticancer and anti-inflammatory effects (Manivannan et al., 2017).
Hydroxy-alpha-terpenyl acetate binds to many drug targets, reducing oxidative stress and neurotoxicity. It also has anti-amyloidogenic properties, antioxidant capacity, acetylcholinesterase, and butyrylcholinesterase enzyme inhibition (Chowdhury and Kumar, 2020). Phytol has been reported to have anti-nociceptive and antioxidant potential (Santos et al., 2013). Other compounds have also been detected in the PHF6, such as naphthalene, phenol, 2-cyclohexyl-, and octadecanoic acid. It is reported that these compounds induce apoptosis in different cancer cells (Evans et al., 2009; Beretta et al., 2017; Liu et al., 2010). The tentatively identified phytochemical compounds would require additional characterization using different chromatographic and spectroscopic techniques, and experts assessment, for the elucidation of structures found in PHF6 extract.
Several studies have shown the correlation between the consumption of herbal-derived antioxidants and many health benefits in humans (Pandey and Rizvi, 2009). The effect of antioxidants on DPPH is attributed to hydrogen donating ability (Baumann, 1979). The results of DPPH assay revealed that the PHF6 extract have compounds capable of free radical scavenging by their hydrogen donating ability. Polyphenol contents scavenge the radicals of DPPH by their hydrogen donating ability (Baumann, 1979; Huang et al., 2005). The radical scavenging activity values for the hexane and chloroform extracts were 35.1 % ±0.01 (TPC = 3.11 ± 0.004 µg/mL) and 36.9 % ±0.006 (TPC = 7.88 ± 0.004 µg/mL), respectively. Antioxidants are very important to prevent the toxic effect of free radicals in different illnesses, including cancer (Rahman et al., 2015; Huang et al., 2005). This assay show that PHF6 extract is a source of antioxidants, which could inhibit the progression of different illnesses caused by free radicals, such as cancers. However, the components responsible for the anti-oxidative potential are unclear. Therefore, further study is required to isolate the antioxidant botanical compounds present in PHF6 extract. The phenolic contents found in many herbal formulations, such as THP-R019, THP-R017, THP-R016, THP-R015, THP-R014, and THP-R010, indicate their antioxidant potentials and support their acclaim as anticancer agents (Chanthasri et al., 2018).
In the present investigation, we studied the anticancer effect of PHF6 using cell viability assay using MTT, LDH release and fluorescence microscopy.
Breast cancer cells were not sensitive to the ethyl acetate and methanol extracts. This could be attributed to the nature of the chemical compounds extracted based on the low (hexane and chloroform) and high polarity (ethyl aceate and methnaol) of the solvents used. Non polar copmunds are soluble in the lipid bilayer and thus freely cross cell membrane which could justify the cytotoxicity of hexane and chloroform extracts in this study.
Results of the MTT assay showed a variation in response to hexane extract. The difference in response could be attributed to the differences in the metabolic genotype. Progesterone (PR) and estrogen (ER) receptors are prognostic markers of BC (Baumgarten and Frasor, 2012). Therefore, MDA MB-231ER(−)/PR(−) and MCF-7ER(+)/PR(+) were employed to assess the cytotoxic impact of PHF6. Cytotoxicity assay revealed that MDA MB-231cells are more sensitive to the PHF6 treatment than MCF-7. This finding agrees with the study of Yang et al., who reported that MDA MB-231ER(−)/PR(−) are more sensitive than MCF-7ER(+)/PR(+) when treated with Cynanchum paniculatum aqueous extract. The authors attributed these cytotoxic differences in the extract to different mutational types (Sun et al., 2021).
Most chemotherapeutic drugs induce apoptosis in tumour cells such as cisplatin, doxorubicin, fluorouracil and vincristine, therefore, they are used for cancer treatment (Gavamukulya et al., 2014; Milner et al., 2002). Fluorescence microscopy was used to investigate the mechanisms involved in the growth inhibition of MDA-MB-231ER(−)/PR(−) cells treated with PHF6 extract. As part of the confirmation of apoptosis induction, the AO/EB apoptosis detection assay is widely used to detect apoptosis qualitatively and quantitatively (Biffl et al., 1996; Demchenko, 2013). AO stains both dead and live cells, whereas EB stains only cells that have lost the integrity of their membrane. Cells that appeared green in colour with intact nuclei indicate viable cells, green cells with fragmented nuclei indicate early apoptosis and red indicate late apoptosis and necrosis. The single (DAPI), dual staining (AO/EB) and the morphological changes that occurred during the cell death after treatment with PHF6 extract indicate that the cells are undergoing apoptosis. This result is further supported by the GC–MS result that confirmed the presence of several compounds that was reported to induce apoptosis. Therefore, it can be infered that the anticancer potenitals observed in the PHF6 extract could be attributed to the presence of these compounds.
5 Conclusion
In conclusion, our data revealed that PHF6 extract could induce cell death via apoptosis. This is promising since the PHF6 extract contains compounds that can be used in apoptosis-resistant cells. Also, it showed more toxicity toward triple negative breast cancer cells. There may be some limitations in this study. The first is that no investigation was carried out for invasion, migration and colonisation of the cells upon treatment with PHF6 extracsts. It is significant because most cancer-related deaths are attributed to metastasis. The second limitation is the lack of toxicological studies of PHF6 extract using in vivo animal model.
Acknowledgement
This research was supported by the Deanship of Scientific Research, Imam Mohammad Ibn Saud Islamic University, Saudi Arabia, Grant No. (19-12-12-007).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of hexane root extract of ferula hermonis boiss. On human breast and colon cancer cells: An in vitro and in vivo study. BioMed Re. Int.. 2019;2019
- [Google Scholar]
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chem.. 2020;18(1):472-481.
- [Google Scholar]
- Incidence trends of breast cancer in Saudi Arabia: A joinpoint regression analysis (2004–2016) J. King Saud Univ.-Sci.. 2021;33(7):101578
- [Google Scholar]
- Coadministration of black seeds and turmeric shows enhanced efficacy in preventing metabolic syndrome in fructose-fed rats. J. Cardiovasc. Pharmacol.. 2015;65(2):176-183.
- [Google Scholar]
- Incidence rates of breast cancer by age and tumor characteristics among Saudi women: recent trends. Cureus.. 2020;12(1)
- [Google Scholar]
- Studies on two polyherbal formulations (ZPTO and ZTO) for comparison of their antidyslipidemic, antihypertensive and endothelial modulating activities. BMC Complement. Altern. Med.. 2013;13(1):1-9.
- [Google Scholar]
- Prostaglandin synthetase inhibiting O_2-radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn-Schmiedebergs Arch Pharmacol.. 1979;308:27-32.
- [Google Scholar]
- Minireview: inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol. Endocrinol.. 2012;26(3):360-371.
- [Google Scholar]
- Synthesis and evaluation of new naphthalene and naphthoquinone derivatives as anticancer agents. Arch. Pharm.. 2017;350(1):e1600286.
- [Google Scholar]
- Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J. Trauma Acute Care Surg.. 1996;40(4):575-579.
- [Google Scholar]
- Antioxidant capacities and total phenolic contents of 20 polyherbal remedies used as tonics by folk healers in Phatthalung and Songkhla provinces, Thailand. BMC Complement. Alternat. Med.. 2018;18(1):1-11.
- [Google Scholar]
- The concept of Antimicrobial Activity in Ayurveda and the effect of some indigenous drugs on Gram-Negative Bacteria. Int. J. Ayurvedic Herbal Med.. 2011;1(01)
- [Google Scholar]
- Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods. 2020;68:103892
- [Google Scholar]
- Cytotoxic activity of antioxidant constituents from Hypericum triquetrifolium Turra. Nat. Prod. Res.. 2007;21(1):42-46.
- [Google Scholar]
- Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol.. 2014;740:364-378.
- [Google Scholar]
- Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65(2):157-172.
- [Google Scholar]
- Stearate preferentially induces apoptosis in human breast cancer cells. Nutr. Cancer. 2009;61(5):746-753.
- [Google Scholar]
- Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola) Asian Pacific J. Trop. Med.. 2014;7:S355-S363.
- [Google Scholar]
- Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13(10):714-726.
- [Google Scholar]
- The chemistry behind antioxidant capacity assays. J. Agric. Food Chem.. 2005;53(6):1841-1856.
- [Google Scholar]
- Synthesis and inhibitory evaluation of cyclohexen-2-yl-and cyclohexyl-substituted phenols and quinones to endothelial cell and cancer cells. Eur. J. Med. Chem.. 2010;45(6):2147-2153.
- [Google Scholar]
- Prediction aided in vitro analysis of octa-decanoic acid from Cyanobacterium Lyngbya sp. as a proapoptotic factor in eliciting anti-inflammatory properties. Bioinformation. 2017;13(9):301.
- [Google Scholar]
- Induction of apoptosis by chemotherapeutic drugs: the role of FADD in activation of caspase-8 and synergy with death receptor ligands in ovarian carcinoma cells. Cell Death Differ.. 2002;9(3):287-300.
- [Google Scholar]
- Moqidem, Y., 2021. Evaluation of the Anticancer Potential of Alchemilla vulgaris Extract Against Human Neuroblastoma Cells.
- Crataegus azarolus leaves induce antiproliferative activity, cell cycle arrest, and apoptosis in human HT-29 and HCT-116 colorectal cancer cells. J. Cell. Biochem.. 2016;117(5):1262-1272.
- [Google Scholar]
- The effect of combining herbal therapy with conventional chemotherapy on the incidence of chemotherapy side effects in 2nd stage breast cancer patients. J. Am. Sci. Medical Surg. Nurs.. 2010;11(6):748-801.
- [Google Scholar]
- Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharmaceut. J.. 2020;28(11):1474-1480.
- [Google Scholar]
- Chemical composition and cytotoxic activity of Eruca sativa L. Seeds cultivated in Egypt. Asian J. Chem.. 2010;22(3):2407.
- [Google Scholar]
- In vitro antioxidant extracts evaluation from the residue of the Hevea brasiliensis seed. Sci. Rep.. 2022;12(1):1-14.
- [Google Scholar]
- Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev.. 2009;2(5):270-278.
- [Google Scholar]
- Evaluation of anticancer effects of a pharmaceutically viable extract of a traditional polyherbal mixture against non-small-cell lung cancer cells. J. Integrat. Med.. 2020;18(3):242-252.
- [Google Scholar]
- In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC. Res. Notes. 2015;8(1):1-9.
- [Google Scholar]
- Ren, J., Wang, J., Karthikeyan, S., et al., 2019. Natural anti-phytopathogenic fungi compound phenol, 2, 4-bis (1, 1-dimethylethyl) from Pseudomonas fluorescens TL-1.
- Santos, C.C. d. M. P., Salvadori, M.S., Mota, V.G. et al., 2013. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J.. 2013.
- The Influence of cell cycle regulation on chemotherapy. Int. J. Mol. Sci.. 2021;22(13):6923.
- [CrossRef] [Google Scholar]
- Alchemilla vulgaris agg. (Lady's mantle) from central Balkan: antioxidant, anticancer and enzyme inhibition properties. RSC Adv.. 2019;9(64):37474-37483.
- [Google Scholar]
- Bioactive guaianolides from siyekucai (Ixeris chinensis) J. Nat. Prod.. 2006;69(10):1425-1428.
- [Google Scholar]
- Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov. Today. 2007;12(1–2):34-42.
- [Google Scholar]







