Translate this page into:
Study of influence of metal ions in the diagnosis of recombinant hepatitis B surface antigen (HBsAg) using ELISA technique
⁎Corresponding author. wmohammad@jazanu.edu.sa (Waquar Ahsan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Preliminary diagnosis of hepatitis B is performed by measuring the levels of serological markers using enzyme-linked immunosorbent assay (ELISA). Errors in diagnosis of the disease using serum markers might occur due to the presence of metallic salts in diagnostic samples. The present study was designed to evaluate the effects of various metal ions on the diagnosis of recombinant hepatitis B surface antigen (HBsAg).
Methods
A simple step sandwich ELISA technique was used to evaluate the effects of metal ions on the diagnosis of HBsAg. Further, the zeta potential, electrical conductivity and redox potential of solutions with proteins and metal salts were measured to evaluate the interaction of these metal ions with the protein.
Results
The results showed that recombinant HBsAg significantly interacted with the tested metal ions, with lithium ion showing the highest interaction, followed by barium, aluminium, and nickel as the percent decrease in the free HBsAg concentration in presence of these metal ions were found to be 95.5, 94.1, 93.5, and 91.6%, respectively. Zeta potential of the protein in reaction mixture also changed considerably and marked decrease in the concentration of free HBsAg was observed. This decrease in the concentration of free HBsAg resulted into error in the ELISA test which was negative in presence of these metal ions due to strong interaction with the HBsAg protein.
Conclusions
The findings of this study clearly demonstrated that the presence of metal ions in pathological samples can result in interaction with the antigen leading to possible diagnostic errors.
Keywords
ELISA
Diagnostic errors
Hepatitis B antigen
Metal ions
Interaction
1 Introduction
Metals and metallic compounds are present abundantly in nature. Metal ions play a crucial role in sustaining life by imparting unique catalytic properties to proteins. The amino acids of proteins provide the functional groups that form active ligands for a metallic cation, resulting in the overall structural stability of the molecule. In general, these metalloproteins formed by the complexation of proteins and metal cofactor, are essential for normal cellular physiology to maintain viability (Alhazmi et al., 2015; Chen et al., 2019). Metal ions also play a vital role in cellular and subcellular functions by means of various regulatory biochemical interactions. Additionally, metal-based therapeutic agents are prepared via the complexation of metal ions which are being utilized for the treatment and diagnosis of various diseases. However, metal ions are known to interact with the biological proteins leading to change in the protein conformation, partial unfolding and even aggregation in some cases (Alhazmi, 2019; Alhazmi et al. 2021).
Rapid industrialization, unregulated application of chemicals in agriculture, and unmonitored wastewater discharge has resulted in extensive pollution due to the release of various heavy metals and metal salts into the environment. Heavy metal ions are highly toxic and heavy metal pollution in the environment is day by day increasing due to human activities such as industrial effluents, sewage discharge, and insecticides used in the crop growth (Briffa et al., 2020; Teschke, 2022). Recent studies demonstrated that the wastewater from different cities discharged into the sea caused widespread chemical and metal ion contamination that imparted toxic effects to the marine ecosystem (Patel et al., 2019). Consuming sea foods in excess including fishes can lead to exposure of heavy metals such as arsenic, mercury, lead, cadmium, chromium, and nickel which are deposited in the liver, heart, and tissues of fish (Rajeshkumar and Li, 2018; Rahman and Singh, 2019 Balali-Mood et al., 2021). In addition, a recent study suggested that consumption of contaminated vegetables also induce potential health hazards due to the atmospheric concentration of metals originated from industrialization as well as transport or marketing techniques (Alengebawy et al., 2021).
Furthermore, habits such as smoking and use of smokeless tobacco are the sources of contamination of toxic elements such as cadmium (Cd), mercury (Hg), arsenic (As), lead (Pb) and nickel (Ni) (Alhazmi et al, 2018) which together with the harmful chemicals are the causative factors for cancer, cardiovascular diseases, and lung diseases (Bao et al., 2022). Heavy metals are highly carcinogenic that can induce oral cancer, liver diseases and even death in extreme cases (Alrobaian and Arida, 2019; Yao et al., 2021). The heavy metal ions such as cadmium (Cd2+), mercury (Hg2+), and copper (Cu2+) are environmentally and occupationally widespread pollutants which have mutagenic, carcinogenic, and teratogenic effects (Yang and Wang, 2022). The exposure to heavy metals during day-to-day life can impair both adaptive innate and humoral immune responses. This condition could lead to an augmented susceptibility to infections and might also be influencing the diagnostic errors. Thus, although metals and metal ions play a vital role in sustaining life, their overabundance and misallocation might result in unprecedented problems in human life.
Hepatitis is an inflammatory disease of the liver caused due to infection by the hepatitis B virus, or due to non-viral causes such as trace element poisoning, aflatoxins, bacterial infections, and autoimmune diseases (Christen and Hintermann, 2019; Mekuria et al., 2020). It is primarily diagnosed by measuring serological markers using ELISA technique. The present study was carried out to understand the interaction between metal ions and recombinant HBsAg protein that could lead to interference in the antigen–antibody interaction during ELISA. As some of the metal ions are known to bind to biological macromolecules and even interfering with their functions, the present study was important to understand their effects on the diagnosis of these proteins. The amino acids such as tryptophan, cysteine, and histidine are exposed at the surface of the HBsAg and are accessible to the metal ion ligands. These amino acid residues are known to participate in binding to the metal ions and the binding interaction depends upon the type of metal ions and their planarity. The metal ions upon binding to the proteins disrupt their structure by disrupting the disulfide bonds leading to partial unfolding of their secondary structure. This would also change their conformation and therefore the antibody binding site will be less accessible for the antigen–antibody interaction. Various electrochemical properties were also measured for antigen solutions containing different metal salts to assess the protein-metal interaction. The results of the present investigation will highlight how various metallic salts interfere during disease diagnosis by interacting with the target proteins.
2 Materials and methods
2.1 Chemicals and reagents
All chemicals used were of analytical reagent grade. Metallic salts ZnCl2 (≥97 %), CaCl2 (≥97 %), NiCl2 (98 %), FeCl3 (97 %), MgCl2 (≥98 %), AlCl3 (>99 %), LiCl (>99 %), and BaCl2 (>99 %) were purchased from Sigma-Aldrich, USA. Tris powder (>99 %) was also purchased from Sigma Aldrich, Germany. The extra pure deionized double distilled water was used throughout the study and was produced in our lab. ELISA test kit HBsAg one Version ULTRA was purchased from Dia Pro Diagnostic Bioprobes Srl, Italy. All the items were locally supplied by Bayouni Trading Co. Ltd., Saudi Arabia.
2.2 Preparation of test sample in tris buffer
The tris buffer (20 mmol/L) was prepared by dissolving 2.42 g of tris powder in 200 mL double-distilled water, adjusting the pH to 7.4 using dilute HCl and making up the volume to 1L using double-distilled water. Solutions for each metal salt (MSS) were prepared in a concentration of 20 µM using the prepared tris-HCl buffer.
2.3 ELISA protocol
A simple step sandwich ELISA was performed as per the manufacturer’s instructions to determine the behavior of recombinant HBsAg in the presence and absence of metal salts. Briefly, the required number of test strips was placed in a plastic holder, and wells in a microtiter plate were designated as blank, positive control and samples (positive control + metal salts). A positive control recombinant HBsAg sample in the kit was used as the control as well as for the test samples. The recombinant HBsAg (500 ng/mL) stock was diluted in a standard diluent at 1:2 ratio to get the working concentration of 250 ng/mL, and 100 µL of this was added to each well. Subsequently, MSS was added to individual wells, followed by incubation for 60 min at 37 °C. The plate was thoroughly washed 5 times with the washing buffer provided in the kit. After washing, 100 μL of horseradish peroxidase (HRP)-conjugated antibody was added and incubated for 60 min at 37 °C. The plate was washed 5 times using washing buffer to remove unbound proteins and HRP-conjugates. Subsequently, 200 μL of substrate (0.03 % tetra-methyl-benzidine and 0.02 % hydrogen peroxidase) was added and incubated for 30 min at room temperature in the dark. Finally, 100 μL of sulfuric acid was added and the optical density of the reaction mixture was measured using an ELx 800 ELISA reader (BioTek, USA). The experiment was performed in triplicate and the reaction was evaluated by the intensity of color development measured at 450 nm, with a reference wavelength at 650 nm. The effect of metal ions on the recombinant HBsAg protein was quantified by comparing the optical density (OD) value of the standard with the samples. The concentration of HBsAg in samples with metal ions was calculated and the % difference in the concentration was measured.
Furthermore, the results were calculated by determining the cut-off value on the mean OD 450 nm of the negative control (NC). The cut-off value can be determined by employing the formula, Cut-off (Co) = NC + 0.050. The results were interpreted according to the protocol described in the manual by calculating the sample (S) OD at 450 nm divided by the cut-off value (S/Co). The value of S/Co less than 0.9 reported as negative, whereas, S/Co > 1.1 was considered as positive.
2.4 Measurement of zeta potential, electrical conductivity and redox potential
The zeta potential and electrical conductivity of the reaction mixture were measured by Zetasizer Nano ZS (Malvern Panalytical, UK), and were expressed in millivolts (mV) and milli Siemens per centimeter (mS/cm), respectively. The redox potential of the reaction mixture was determined using Oakton pH 700 instruments (Thermo Fischer Scientific, Singapore).
3 Results and discussion
Diagnostic laboratory tests are crucial for detecting the occurrence and/or severity of numerous diseases. Heavy metals are highly invasive since they accumulate in tissues as well as organs and able to generate reactive oxygen species (ROS) inducing damage to the cell (Liu et al., 2018; Aalami et al., 2022). Presence of metal ions in diagnostic samples may interfere with the tests and result in erroneous readings. The present study provided a comparative assessment of how various metal ions might influence the results of diagnostic testing of recombinant HBsAg levels using ELISA technique. Table 1 summarizes the effects of various metal ions on the absorption of recombinant HBsAg. As evident from the table, all the metal salts changed the absorption of HBsAg protein and the calculated concentrations of free recombinant protein in the samples decreased from the initial value (25 ng/100 µL) to various extents depending upon the metal ion. The metal and protein interaction also affected the S/Co values to determine the outcome of the test as positive or negative.
Samples
Concentration of free Recombinant HBsAg protein (ng/ 100 µL)
% decrease in the free HBsAg protein concentration
S/CO
Test Result
Recombinant HBsAg protein in tris buffer
25.0
0.0
9.1
Positive
Zinc (II) chloride* + HBsAg in tris buffer
24.3
3.1
8.3
Positive
Calcium (II) chloride + HBsAg in tris buffer
23
7.96
8.57
Positive
Nickel (II) chloride + HBsAg in tris buffer
2.1
91.62
0.8
Negative
Iron (III) chloride + HBsAg in tris buffer
13
47.92
4.6
Positive
Magnesium (II) chloride + HBsAg in tris buffer
13.1
47.48
4.5
Positive
Aluminium (III) chloride + HBsAg in tris buffer
1.63
93.5
0.78
Negative
Lithium (I) chloride + HBsAg in tris buffer
1.12
95.5
0.6
Negative
Barium (I) chloride + HBsAg in tris buffer
1.48
94.1
0.75
Negative
The results of this study demonstrated that the metal salts nickel (II) chloride, aluminium (III) chloride, lithium (I) chloride, and barium (I) chloride changed the test results to negative as the protein concentrations detected in these samples decreased considerably (Table 1), since strong meal-protein interactions were observed these cases. The treatment of recombinant HBsAg protein with iron (III) chloride and magnesium (II) chloride salts also showed interaction with the protein and decreased the free protein concentration to 47–48 % of the original value; however the S/Co values remained>1.1 showing the test results positive. Other metal salts, zinc (II) chloride and calcium (II) chloride showed less than 8 % decrease in the protein concentration upon interaction with S/Co values >8 showing positive results in the ELISA.
The interaction between the metal ions and HBsAg protein was further studied by determining the physicochemical parameters of the recombinant protein with and without metal ions. Zeta potential, redox potential, conductivity, mobility and polydispersity index (PDI) were calculated for the protein in presence and absence of metal ions and the results obtained are summarized in Table 2. Zeta potential is an indicator of electrostatic interaction between charged surfaces and manifests in the presence of an aqueous medium when functional groups dissociate or ions are adsorbed on the surface of a molecule. The changes in zeta potential of the protein changed considerably upon addition of metal ions to the solution (Table 2). The zeta potential of HBsAg protein without metal ions was −19.2 mV which increased (less negative) to −0.278, −0.164 and −0.219 upon addition of Al3+, Li+ and Ba+ ions, respectively showing strong interaction of the protein with these metal ions. In all other cases, zeta potential increased considerably and turned out to be positive and more than unity, except for Ni2+ ions, where the zeta potential was positive but was less than one (0.422). In this study, the percentage decrease in the concentration of free recombinant HBsAg was observed to be 95.5 % due to Li+ interaction, and 94.1 % due to Ba2+ interaction. Al3+ and Ni2+ interaction resulted in 93.5 % and 91.6 % decrease in the free protein concentration, respectively. This decrease in the concentration of free protein is attributed to the strong interaction of these metal ions to the protein showing most of the binding sites of the protein occupied by these metal ions.
Samples
Zeta potential
(mV)Redox potential
(mV)Conductivity
(mS/cm)Mobility
(µm.cm/Vs)PDI
% Mass r.dnm
Recombinant HBsAg protein in tris buffer
−19.2
31
4.56
−1.505
0.421
50
Zinc (II) chloride + HBsAg in
tris buffer1.42
233
12.8
0.1160
0.4
35.57
Calcium (II) chloride + HBsAg in
tris buffer1.48
242
13.8
0.1157
0.723
49.93
Nickel (II) chloride + HBsAg in tris buffer
0.422
201
10.9
0.3339
0.48
34.6
Iron (III) chloride + HBsAg in
tris buffer1.58
236
11.2
0.07979
0.4
42.6
Magnesium (II) chloride + HBsAg in tris buffer
1.48
220
13.8
0.1164
0.3
45.6
Aluminum (III) chloride + HBsAg in tris buffer
−0.278
193.1
9.82
−0.0384
0.868
38.5
Lithium (I) chloride + HBsAg in
tris buffer−0.164
182.4
9.24
−0.01284
0.788
40.2
Barium (I) chloride + HBsAg in
tris buffer−0.219
191
9.78
−0.0768
0.658
43.5
Electrophoretic mobility is another important factor to assess the stability of proteins. Zeta potential measurements were used to derive the electrophoretic mobility of suspended proteins in the colloidal reaction mixture and to determine the surface charge. The present study demonstrated that the electrophoretic mobility changed with the change in zeta potential (Table 2). The electrophoretic mobility is the direct function of the charge present on the analyte and therefore, samples with negative zeta potential showed negative electrophoretic mobilities, whereas samples with positive zeta potential showed positive electrophoretic mobility values. Out of all metal ions, Li+ ions showed strongest interaction with the recombinant HBsAg protein as the percent change in the free protein concentration (95.5 %) was highest in this case. It was followed by Ba+, Al3+ and Ni2+, respectively. The interaction with these metal ions was so strong that the free protein concentration in presence of these metal ions decreased markedly, resulting in the S/Co values less than 0.9 and changing the outcome of the result as negative.
Metal ions are integral part of our life as they play crucial role in the maintenance of normal physiological functions in our body. Additionally, metal-based drugs are being widely utilized nowadays in the treatment and diagnosis of a number of diseases. These metal ions are known to bind to the macromolecules present in our body including proteins, enzymes, and nucleic acids. Binding of these metal ions to the proteins changes the conformation of the protein molecule which might lead to unfolding and even aggregation in some cases depending upon the concentration of metal ions (Alhazmi et al., 2021). Previously, a number of studies have shown interactions of various monovalent and divalent metal ions with many proteins and the type and extent of interactions have been reported. The specificity of binding interactions between proteins and metal ions as ligands depends upon the planarity of ligands and it was reported that these metal ions bind to the tryptophan residue present on the outer surface of protein and is the most accessible one (Hu et al., 2021).
Few metal ions bind covalently to the proteins which might lead to disruption of their structure and inhibition of their function. Partial unfolding of proteins due to binding of these metal ions might disrupt the disulfide bonds and perturbation of their secondary structure. The α-helix component in the secondary structure of the protein is partially lost due to the protein unfolding which changes the polarity around the tryptophan (Trp) and tyrosine (Tyr) residues (Ehteshami et al., 2013). Due to the rearrangement of structure of protein, energy transfer and collision quenching processes change in the protein, which changes the microenvironment and polarity of amino acids present at the binding site (Jalali et al., 2014; Grigoryan et al., 2022). The cationic metal ions act as electron deficient moieties which bind to the negatively charged (electron-rich) amino acid residues present at the binding site of protein. The extent of this interaction depends upon the valency, charge-accepting capacity and atomic radius of the metal ions and stronger interactions influence the structural stability of the protein.
Previous studies reported that the zeta potential of biomolecules decreased proportionately with an increase in salt concentration due to decreasing thickness of the electrical double layer (Midekessa et al., 2020). The zeta potential of unbound recombinant HBsAg protein was −19.9 mV in the control samples, which considerably changed upon interaction with metal salts due to redox reactions. The behaviour of a protein in an environment is attributed to both reduction and oxidation potentials; where the reduction potential tends to gain electrons and oxidation potential tends to lose electrons. Redox reactions indicate the transfer of electrons between atoms, and the availability of electrons is directly proportional to the oxidation and reduction potential of proteins. The reduction potential of metals is the tendency to lose electrons, and thus, reduce other atoms or molecules. Metalloproteins and oxidoreductases act as significant catalysts in numerous biological processes that involve electron transfer, such as respiration, metabolism, photosynthesis, and molecular signaling (Andberg et al., 2007).
Metal complexes significantly increase the possibility of partial unfolding of the protein at redox sites or portions of the molecule prone to oxidation and/or reduction reactions. The untreated recombinant HBsAg exhibited 31 mV redox potential value in 20 mM Tris buffer, whereas interactions with metal ions increased the potential to 191–242 mV, indicating interaction with the electron deficient species. A mechanistic depiction of how metal salts affect the interaction between recombinant HBsAg and antibodies in the diagnostic kit is provided in Fig. 1. The figure shows that the metal ions from their respective salts can interact with the HBsAg protein leading to change in their secondary structure and partial unfolding. The change in the polarity and microenvironment around the amino acids present in the binding site of the protein does not allow the antigen binding leading to negative ELISA test results.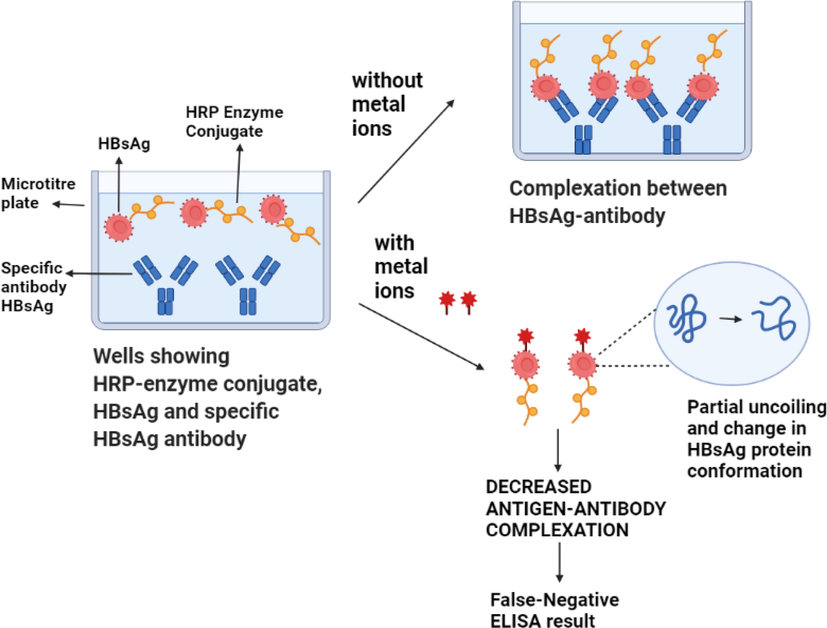
Figure showing interaction of metal ions with the HBsAg protein causing partial unfolding and conformational changes leading to negative ELISA test results.
The mode by which a metal ion binds to the protein is an important consideration while studying the complexation of metals and proteins. These metal ions can bind to the protein in two specific ways: intermolecular and intramolecular interactions. In case of intermolecular interaction, there is formation of intermolecular bridges between the protein molecules and the metal ions leading to aggregation of proteins. The intramolecular interaction takes place when the atoms which participate in the coordination with metal ions are present in the same protein molecule and this changes the secondary structure of the protein, reduction in α-helical structure and partial unfolding of protein. The polarity and microenvironments around the important amino acids present at the binding site is changed which ultimately changes the binding capacity of protein. These metal ions generally bind to the regions of high hydrophobicity in proteins as the electronic distribution in metal ions being highly symmetric attracts the Lewis bases present in the proteins. These Lewis bases are the electron-pair donors such as oxygen, nitrogen and sulphur atoms present in the amino acid residues. The transition metal ions favour binding with nitrogen and sulphur of histidine and cysteine amino acid residues, while the group IIa metals prefer oxygen atoms present in the glutamate and aspartate side chains. Therefore, proteins containing these amino acids are expected to have good binding affinity with the metal ions.
4 Conclusion
This study concluded the incidence of strong interaction of HBsAg protein with several metal ions which resulted in remarkable effects on the diagnosis of hepatitis B. Interestingly, all the tested metal ions influenced the test results as the concentration of free protein decreased in all the cases to various extents. However, metal ions including Ni (II), Al (III), Li (I) and Ba (I) showed remarkable decrease in the free antigen concentration in the samples leading to erroneous test results. The presence of metal ions did not allow the immune complex to form and the antigens were not detected in the test. Therefore, presence or contamination of metal ions should always be considered as an important factor in the diagnosis of diseases involving detection of proteins and proper measures should be taken to improve the accuracy and reliability of these tests. This study also emphasizes on the toxicity of several metal ions on biological systems and shows the extent to which these metal ions can bind to the body proteins and disrupt their structure and functions. Measuring the effects of more metal ions on the diagnosis of different diseases involving antibody detection along with their proper mechanistic investigations is warranted nevertheless.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project with number: ISP22-12.
Funding
This work was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through the project with number: ISP22-12.
Author contributions
All authors contributed to the work presented in the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carcinogenic effects of heavy metals by inducing dysregulation of microRNAs: A review. Mol. Biol. Rep.. 2022;49(12):12227-12238.
- [Google Scholar]
- Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics.. 2021;9(3):42.
- [CrossRef] [Google Scholar]
- FT-IR spectroscopy for the identification of binding sites and measurements of the binding interactions of important metal ions with bovine serum albumin. Sci. Pharm... 2019;87:5.
- [CrossRef] [Google Scholar]
- A comprehensive platform to investigate protein–metal ion interactions by affinity capillary electrophoresis. 2015. J. Pharm. Biomed. Anal.. 2015;107:311-317.
- [Google Scholar]
- Elemental profiling of smokeless tobacco samples using inductively coupled plasma-mass spectrometry, their chemometric analysis and assessment of health hazards. Phcog. Mag.. 2018;14:587-596.
- [CrossRef] [Google Scholar]
- Spectroscopic characterization of the interactions of bovine serum albumin with medicinally important metal ions: platinum (IV), iridium (III) and iron (II) Acta Biochim. Pol.. 2021;68(1):99-107.
- [CrossRef] [Google Scholar]
- Assessment of heavy and toxic metals in the blood and hair of Saudi Arabia smokers using modern analytical techniques. Int. J. Anal. Chem.. 2019;2019:7125210.
- [CrossRef] [Google Scholar]
- Cleavage of recombinant proteins at poly-His sequences by Co(II) and Cu(II) Protein Sci.. 2007;16(8):1751-1761.
- [CrossRef] [Google Scholar]
- Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol.. 2021;12:643972
- [CrossRef] [Google Scholar]
- Environmental toxic metal contaminants and risk of stroke: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int.. 2022;29(22):32545-32565.
- [CrossRef] [Google Scholar]
- Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon.. 2020;6(9):e04691.
- [Google Scholar]
- Targeting Metalloenzymes for Therapeutic Intervention. Chem. Rev.. 2019;119(2):1323-1455.
- [Google Scholar]
- Pathogens and autoimmune hepatitis. Clin. Exp. Immunol.. 2019;195(1):35-51.
- [CrossRef] [Google Scholar]
- Characterization of 6-mercaptopurine binding to bovine serum albumin and its displacement from the binding sites by quercetin and rutin. J. Lumin.. 2013;135:164-169.
- [CrossRef] [Google Scholar]
- Spectroscopic analysis of 2-(5-mercapto-1,3,4-oxadiazol-2-yl)-6-methylquinolin-4-ol binding to blood plasma albumin. Monatsh. Chem.. 2022;153(5–6):507-515.
- [CrossRef] [Google Scholar]
- Chemical modifications of tryptophan residues in peptides and proteins. J. Pept. Sci.. 2021;27(1):e3286.
- [Google Scholar]
- Binding of the neuroleptic drug, gabapentin, to bovine serum albumin: Insights from experimental and computational studies. J. Lumin.. 2014;148:347-352.
- [CrossRef] [Google Scholar]
- Role of ROS and nutritional antioxidants in human diseases. Front. Physiol.. 2018;9:477.
- [CrossRef] [Google Scholar]
- Aflatoxins as a risk factor for liver cirrhosis: a systematic review and meta-analysis. BMC Pharmacol. Toxicol.. 2020;21(1):39.
- [CrossRef] [Google Scholar]
- Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega. 2020;5(27):16701-16710.
- [CrossRef] [Google Scholar]
- Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal Methods. Chem. Rev.. 2019;119(6):3510-3673.
- [CrossRef] [Google Scholar]
- The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ. Monit. Assess.. 2019;191(7):419.
- [CrossRef] [Google Scholar]
- Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake. China. Toxicol Rep.. 2018;5:288-295. Erratum. In: Toxicol Rep. 2020. 8, 28-29
- [CrossRef] [Google Scholar]
- Aluminum, arsenic, beryllium, cadmium, chromium, cobalt, copper, iron, lead, mercury, molybdenum, nickel, platinum, thallium, titanium, vanadium, and zinc: Molecular aspects in experimental liver injury. Int. J. Mol. Sci.. 2022;23(20):12213.
- [CrossRef] [Google Scholar]
- The epitranscriptomic mechanism of metal toxicity and carcinogenesis. Int. J. Mol. Sci.. 2022;23(19):11830.
- [CrossRef] [Google Scholar]
- Stratification of population in NHANES 2009–2014 based on exposure pattern of lead, cadmium, mercury, and arsenic and their association with cardiovascular, renal and respiratory outcomes. Environ. Int.. 2021;149:106410.
- [Google Scholar]







