Translate this page into:
Chemical characterization of Passiflora edulis extracts and their in vitro antioxidant, anti-inflammatory, anti-lipid activities, and ex-vivo vasodilation effect
⁎Corresponding author at: Expert Centre of Innovative Health Food, Thailand Institute of Scientific and Technological Research (TISTR), Pathumthani 12120, Thailand. pennapa@tistr.or.th (Pennapa Chonpathompikunlert)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Passion fruit is a dietary plant with the protective proterties against many diseases such as cardiovascular diseases. The aim of this study was to explore the biological properties (anti-oxidant, anti-inflammatory, anti-lipid, and vascular relaxation) of the P. edulis extracts obtained from seed (PSEE) and fruit (PFWE). Thin-layer chromatography (TLC) and HPLC were used to analyze the phytochemical constituents in the extracts. Antioxidant and enzyme inhibition properties were examined. Anti-inflammatory activities of PFWE and PSEE were evaluated in lipopolysaccharide (LPS)-induced macrophages and ex-vivo vascular relaxation were also determined. A high contents of piceatannol and polyphenolic stilbene were found in PSEE extracts. On the other hand, beta-carotene and gamma-tocopherol were predominant in PFWE. PFWE exhibited stronger anti-oxidant activity than PSEE. Both PSEE and PFWE showed significant inhibition of lipase and cholesterol esterase activities. However, only PFWE showed minimal inhibitory effect against HMG-CoA reductase. PSEE and PFWE caused a significant reduction of nitric oxide level in LPS-induced inflammation in RAW264.7 cells. Both extracts also showed ex-vivo vasorelaxation of rat aortic rings. It has been demonstrated that PFWE and PSEE possesses anti-oxidant activity, anti-inflammatory activity, pancreatic lipase and cholesterol esterase, and ex-vivo vasorelaxation of rat aortic rings, however, the activity of HMG-CoA reductase was inhibited by only PFWE. These findings suggest the beneficial effect of PSEE and PFWE against hyperlipidemia thus preventing the development of hypertension. However, further investigations in animal are needed to elucidate.
Keywords
Passion fruit
Nitric oxide
HMG-CoA reductase
Lipase
Cholesterol esterase
1 Introduction
One of the valuable causes of morbidity and mortality worldwide is cardiovascular disease (CVD). Major risk factors are associated with lipid factors and nonlipid factors such as hypertension, diabetes, cigarette smoking and menopause (Virani et al., 2020).
A meta-analysis and systematic review found that high content of polyphenolic compound administration have been demonstrated to improve the inflammatory markers and blood pressure (George et al., 2018). A plant-based natural compounds contained at least one aromatic ring and a hydroxyl group, known as polyphenols, are potent natural antioxidants. Several studies have investigated the association between cardiovascular diseases and plant polyphenols (Sharifi-Rad et al., 2020). Conspicuously, the therapeutic potential of hebal medicine offered the synergistic activity of multi-constituents (Zhou et al., 2016). Passiflora edulis f. flavicarpa, also known as passion fruit, belongs to the Passifloraceae family and is found in many areas of the world (tropical and subtropical) (He et al., 2020). Polyphenols, triterpenes, glycosides, carotenoids, polysaccharides, amino acids, essential oils, and elements are the most important constituents found in P. edulis (He et al., 2020; Yuan et al., 2017). Several in vitro and in vivo researchs have been noted the biological potentials of P. edulis on anti-oxidant, anti-microbial, hepatoprotective, anti-diabetic, and anxiolytic-like actions (Ishihata et al., 2016; Jagessar et al., 2017; He et al., 2020). However, pharmacological activities of the P. edulis associated with CVD have not been clarified yet. In this present study, several possible aspects of biological properties of the extracts obtained from seed and fruit of P. edulis, including anti-oxidant, anti-inflammatory, anti-lipid, and vascular relaxation were explored.
2 Materials and methods
2.1 Preparation of P. edulis f. flavicarpa Deg. extracts
Fruits of P. edulis were obtained from Chiang Mai, Thailand. A voucher specimen (BK No 082283) was authenticated by a taxonomist staff of plant varieties protection office and located at the Forest Herbarium, Royal Forest Department, Ministry of Agriculture and Cooperatives, Bangkok, Thailand. The seed of P. edulis was dried for 24 h in hot air oven (45 °C) before powdered. The powder of dried seed (1.2 kg) was extracted with 10 times (w/v) of 70% ethanol by maceration and shaken (150 rpm) at room temperature (RT) (30 ± 2 °C) in a orbital shaker (Ratek, Boronia, VIC, Australia) for 72 h and then filtered (Whatman®, No 1). The solvent was heated to dryness by evaporator (Buchi, Switzerland) under reduced pressure at 50 °C and preserved in a freezer. The yield of the seed P. edulis ethanolic extract (PSEE) was 4.55% (w/w) of the dry seed. P. edulis fruit juice and pulp (4.5 kg) was blent, filtered 3 times with cotton and then freeze dried. The percentage yield of the P. edulis fruit juice and pulp extract (PFWE) was 12.12% (w/w) of the fresh juice and pulp.
2.2 Thin-layer chromatography (TLC) fingerprint of PFWE and PSEE
The tested samples, PFWE and PSEE (10 mg/mL in methanol, 5 µL) were spotted to Aluminum TLC plates, silica gel coated with fluorescent indicator F254 (4 × 6 cm, Merck, Germany). There were 3 ratios (90:10, 95:5 and 98:2) of dichloromethane and methanol used in this study as developing solvents represented a polar, moderate polar and non-polar conditions, respectively. The spots appeared in TLC plate after developing in each mobile phase were observed under 4 conditions. There were 1) normal visual 2) observed under UV TLC cabinet (CAMAG, Switzerland) at the short wavelength (254 nm) 3) at the long wavelength (365 nm) and 4) after spaying with 10% sulfuric acid solution in ethanol then heat at 110 °C. The retention factor (Rf) values of each spot were calculated by dividing the ratio between the distance of the spot and the distance of solvent front.
2.3 Determination of piceatannol in PFWE and PSEE by high performance liquid chromatography (HPLC)
Reverse-phase HPLC with diode array detector was used to determine the amount of piceatannol in PFWE and PSEE. The extract solution was injected into a Hypersil ODS column (250 × 4.0 mm i.d.; 5 µm particle size). Deionized water with 0.1% formic acid was used as a mobile phase A, and acetonitrile with 0.1% formic acid was used as mobile phase B. Flow rate for all analysis was 0.75 mL/min. A wavelength of 320 nm was used to detect an optical absorbance. The amount of piceatannol was determined by comparing the peak area of the extract solution with the peak area from the standard curve.
2.4 Determination of beta-carotene and gamma-tocopherol in PFWE by HPLC
Beta-carotene and gamma-tocopherol in PFWE extract was examined by using HPLC with UV detector at 450 nm. A Hypersil ODS (250 × 4.0 mm i.d.; 5 µm particle size) and mobile phase system consisted of acetonitrile : dichloromethane : methanol (70:20:10) in isocratic condition were used for the analysis. Mobile phase system for gamma-tocopherol in an isocratic condition composed of methanol : acetonitrile (60:40) and the detection was set at 208 nm.
2.5 Determination of total phenolic, flavonoid, and carotenoid contents
Folin-Ciocalteu assay was utilized to evaluate the content of total phenolic with slight modifications at 760 nm (Spanos and Wrolstad, 1990). The amount of total phenolic was presented as mg gallic acid equals per gram extract (GAE). Aluminium chloride colorimetric method was utilized to detect the amount of total flavonoid content in PFWE and PSEE at 430 nm (Kamtekar et al., 2014) and presented as mg quercetin equivalents per gram extract (QE). The content of total carotenoids was detected at 450 nm and represented as mg β-carotene equivalents per gram of extract (mg β-carotene /g extract).
2.6 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
Free radical scavenging properties of each extracts was detected by DPPH assay as illustrated by Teixeira et al. (2013) at 517 nm. The activity of DPPH radical scavenging (%) was determined by the following formula:
2.7 Determination of HMG-CoA reductase inhibition activity
The inhibitory activity of HMG-CoA reductase in PFWE and PSEE were evaluated by HMG-CoA reductase assay kit (Sigma-Aldrich Co., St Louis, MO, USA) under conditions recommended by the manufacturer at 340 nm. Pravastatin was utilized as a positive drug. The inhibition of HMG-CoA reductase was determined using the following formula:
2.8 The pancreatic lipase inhibition assay
The inhibitory activity of pancreatic lipase was carried out following a previously described method with a slight modification (Od-Ek et al., 2020). Orlistat was used as a positive drug. All samples were carried out in triplicate, and determined at 405 nm with a microplate reader (Biotek, Vermont, USA). The percentage inhibition of pancreatic lipase was examined using the following formula:
2.9 The pancreatic cholesterol esterase inhibition assay
The inhibitory activity of pancreatic cholesterol esterase was determined at 405 nm according to Makynen et al. (2013) with some modification. In this study, we used simvastatin (Sigma-Aldrich, MO, USA) as a positive control. All samples were performed in triplicate. The inhibition of pancreatic cholesterol esterase were measured using the following formula:
2.10 In vitro model
2.10.1 Determination of the cytotoxicity of PFWE and PSEE in RAW264.7 macrophages
RAW264.7 macrophages (American Type Culture Collection, Manassas, VA, USA) were maintained with complete DMEM medium in a 96-well plate at 1 × 104 cells/well. The PFWE and PSEE (0, 1, 5, 10, 25, 50, 100, 250, 500 and 1000 µg/mL) was exposed to the cells for 24, 48 and 72 h. The viability of RAW264.7 macrophages were evaluated by colorimetric MTT assay with a microplate reader (Biotek, Vermont, USA) at 570 nm.
2.10.2 Determination of the effect of PFWE and PSEE on nitric oxide (NO) level in the medium of RAW264.7 macrophages
After 2 h of PFWE and PSEE pre-treatment, different concentrations of PFWE and PSEE (25, 50, and 100 µg/mL) were exposed with LPS (1 µg/mL) or without LPS to RAW264.7 macrophages for 24 h. Then, an equal volume of RAW 264.7 macrophage medium and Griess reagent were then mixed and left at RT for 10 min. The concentration of nitrite-containing samples were identified at 540 nm. A standard curve was made by using sodium nitrite to calculate the concentration of nitrate in the medium.
2.11 Ex-vivo vascular relaxation model
A 8 weeks male Sprague-Dawley rats (180–200 g) were supported from Nomura Siam International (Bangkok, Thailand). All animals were kept under a controlled condition (22 ± 1.0 °C with a 12-hour light/dark cycle) in the center for animal research at Naresuan University. The study protocol was permitted by the Institutional Animal Care and Committee of Naresuan University, Thailand (approval number: 63-01-003). Intraperitoneal injection of sodium thiopental (100 mg/kg BW) was used to euthanize the rats. Then, the descending thoracic aorta were removed and saturated in ice-cold Krebs-bicarbonate solution. The thoracic aorta was then cut into ring segments in length of 2–3 mm and suspended between two stainless steel hooks. One hook was connected to an isometric force transducer (AD Instruments, Australia), which was linked to a Bridge Amplifier (Powerlab, AD instrument Ltd, Australia) for isometric tension recording. The resting tension of each aortic ring was controlled at 1 gm, and authorized to equilibrate for 60 min. The resting tension of each aortic ring was stabilized. The integrity of the endothelium was established by more than 80% relaxation to acetylcholine (10 μM) after pre-constriction with phenylephrine (PE, 0.1 μM). To measured the relaxant effect of PFWE and PSEE, aortic rings were declined with submaximal concentration of PE. After the contraction reached a plateau phase, PFWE, PSEE, or vehicle were added cumulatively to the endothelium-intact aortic rings. The relaxation effect was evaluated as a percentage of the contraction with PE.
2.12 Statistical analysis
Mean ± standard error of the mean was utilized to display the values of experiments. The differences of the mean values were examined by independent t-test and ANOVA, followed by LSD post hoc test (in vitro experiment) and Newman-Keuls post hoc test (ex-vivo experiment) to compare between groups. P values less than 0.05 was established for statistically significant. The data was analyzed by R program.
3 Results
3.1 Chemical analysis of PFWE and PSEE by TLC and HPLC
The physical appearance and the percent yields of two tested extracts, PFWE and PSEE, were yellowish coarse powders (12.12%) and light brown slurry (4.55%), respectively. The TLC fingerprint in polar, moderate, and non-polar conditions were presented in Fig. 1. The TLC analysis showed normal visual of TLC plates in every conditions of both extract, and no spot were observed (Fig. 1A). However, the spots were observed under short and long wavelength, and also after spraying with 10% sulfuric acid solution and heating as demonstrated in Fig. 1B, 1C and 1D, respectively. The results indicated that PSEE appeared to have more spots than the PFWE. The Rf values of spots observed in TLC were calculated and shown in Table 1. These results suggested that PSEE which was extracted with 70% ethanol exhibited more chemical components than that of PFWE which was in aqueous solution. By the HPLC method, piceatannol was detected with retention time of 31.33 min. HPLC chromatogram from the standard solution (Fig. S1), and the extract solutions were demonstrated in Fig. 2A. Piceatannol content was detected only in PSEE (4.742 mg/g extract). Beta-carotene and gamma-tocopherol contents were found in PFWE (0.1917 mg/g extract and 20.03 μg/g extract, respectively) using HPLC as shown in Fig. 2B and 2C, respectively. HPLC chromatograms of the standard references of beta-carotene and gamma-tocopherol were snowed in Fig. S2 and Fig. S3.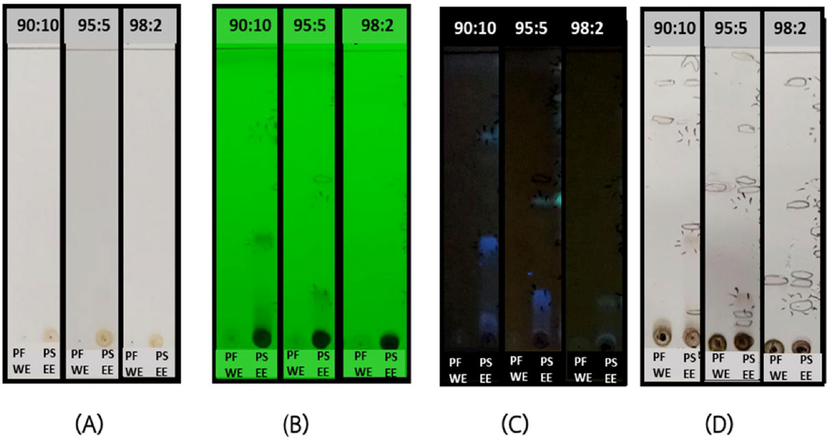
TLC-fingerprints of the PFWE and PSEE in three mobile phases; dichloromethane : methanol = 90:10, 95:5 and 98:2 which (A) normal visual, (B) under UV light λ 254 nm, (C) under UV light λ 365 nm and (D) after spraying with 10% Sulfuric acid solution in ethanol then heated at 110 °C.
Sample
Rf values
Dichloromethane: methanol 90:10
Dichloromethane: methanol 95:5
Dichloromethane: methanol 98:2
PFWE
0.88, 0.74
0.54
0.24
PSEE
0.98, 0.88, 0.74, 0.70, 0.40, 0.34, 0.08
0.98, 0.72, 0.56, 0.54, 0.48, 0.16, 0.10, 0.08
0.90, 0.50, 0.26, 0.24, 0.14
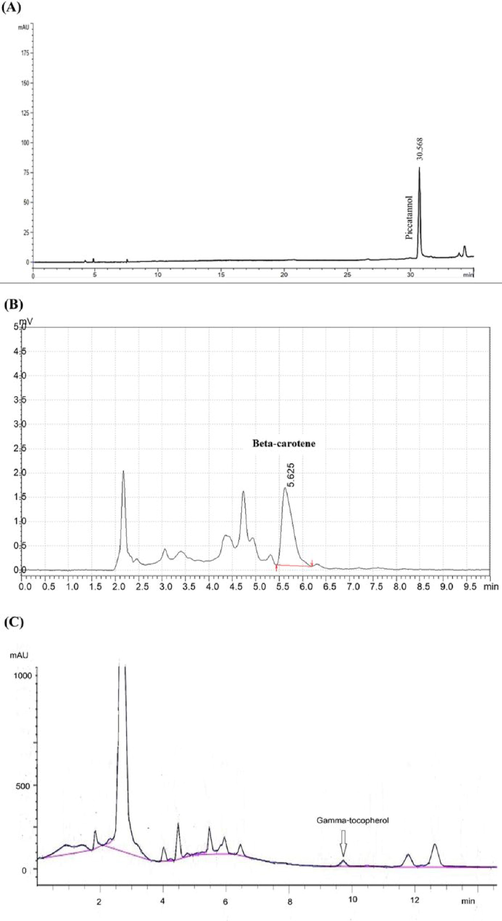
HPLC chromatograms of piceatannol, beta-carotene and gamma-tocopherol in the extract. (A) piceatannol in PSEE, (B) beta-carotene in PFWE and (C) gamma-tocopherol in PFWE.
3.2 The level of total phenolic, flavonoid and carotenoid constituents in PFWE and PSEE
Table 2 illustrated the contents of total phenolic, total flavonoid and total carotenoid level which were presented in terms of gallic acid equivalent, quercetin equivalent and β-carotene equivalents per gram of extract, respectively. Our result demonstrated that the levels of total phenolics, flavonoids, and carotenoids in PSEE were higher than PFWE (Table 2). * P < 0.05 compared with PFWE.
Extract
Total phenolic content
(mg gallic acid equivalent/g extract)Total flavonoid content
(mg quercetin equivalent/g extract)Total carotenoids content
(mg β-carotene/g extract)
PFWE
19.90 ± 5.35
4.96 ± 0.32
1.23 ± 0.24
PSEE
94.01 ± 7.59*
20.00 ± 0.99*
10.20 ± 0.55*
3.3 Percentage of radical scavenging activity of PFWE and PSEE
Fig. 3 demonstrated the radical scavenging effects of PFWE, PSEE and vitamin C. The antioxidant activities of the extracts were compared with a reference antioxidant vitamin C. The PFWE and PSEE had radical scavenging effects with values 56.32 ± 2.03 and 47.47 ± 0.26%, repectively. Vitamin C exhibited the most potent radical scavenging effect (90.94 ± 0.33%) compared with the extracts (Fig. 3).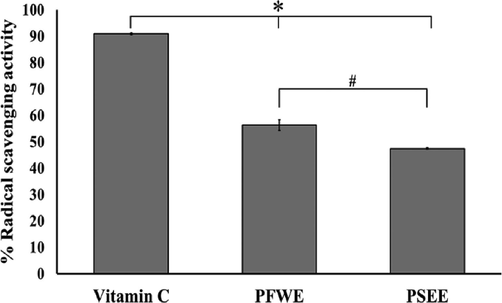
The effect of PFWE and PSEE on percentage of radical scavenging activity. Data shown are mean ± SEM of three replicates. * P < 0.05 compared with PFWE and PSEE, # P < 0.05 compared with PSEE.
3.4 The inhibitory effects of PFWE and PSEE on HMG-CoA reductase, lipase, and cholesterol esterase activity
HMG-CoA reductase inhibitory activity of PFWE and PSEE was presented in Fig. 4A, compared to the pravastatin (a standard drug). PFWE displayed minimal HMG-CoA reductase inhibition, whereas PSEE showed no effect (Fig. 4A). Both PFWE and PSEE exhibited anti-lipase activity (IC50 > 10 and 1.58 mg/mL, respectively) in a dose-dependent manner (Fig. 4B). PSEE were significantly higher inhibitory activity than PFWE. As shown in Fig. 4C, both PFWE and PSEE possess a significant anti-cholesterol esterase activity in a dose-dependent manner. The IC50 values of PFWE and PSEE were 1.36 and 0.34 mg/mL, respectively.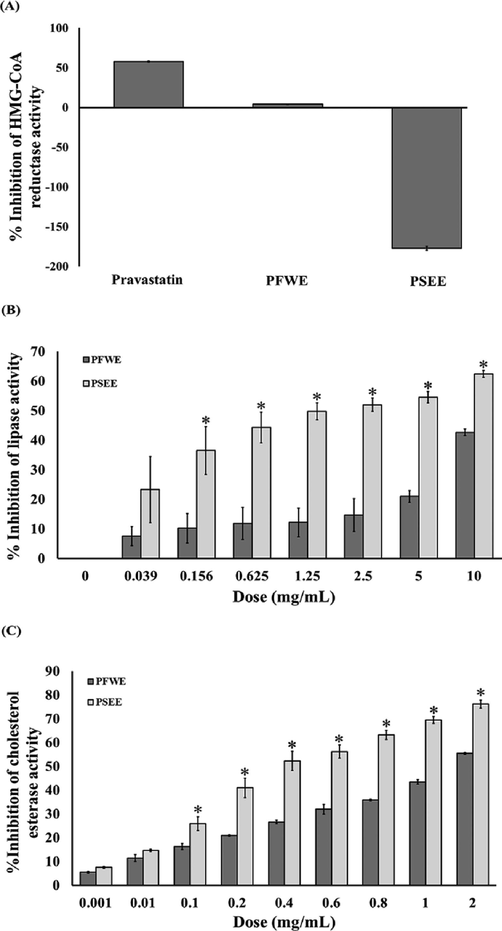
The inhibitory effects of PFWE and PSEE on HMG-CoA reductase, pancreatic lipase and cholesterol esterase activity. (A) HMG-CoA reductase, (B) pancreatic lipase and (C) cholesterol esterase. Data shown are mean ± SEM of three replicates. * P < 0.05 compared with PFWE.
3.5 Effect of PFWE and PSEE on RAW264.7 macrophage viability
The viability of RAW264.7 macrophages after receive to PFWE and PSEE for 24, 48, and 72 h were measured by the MTT assay. PFWE at 1–1000 µg/mL did not alter RAW264.7 macrophages viability after 24, 48 and 72 h of treatment (Fig. 5A and 5B). However, the exposure with PSEE (100–1000 µg/mL) for 48 and 72 h significantly suppressed RAW264.7 macrophages viability. These results indicated that PFWE and PSEE at 1–100 µg/mL are safe to the RAW264.7 macrophages at 24 h of treatment. Therefore, we selected the concentration of PFWE and PSEE at 1–100 µg/mL for further experiment.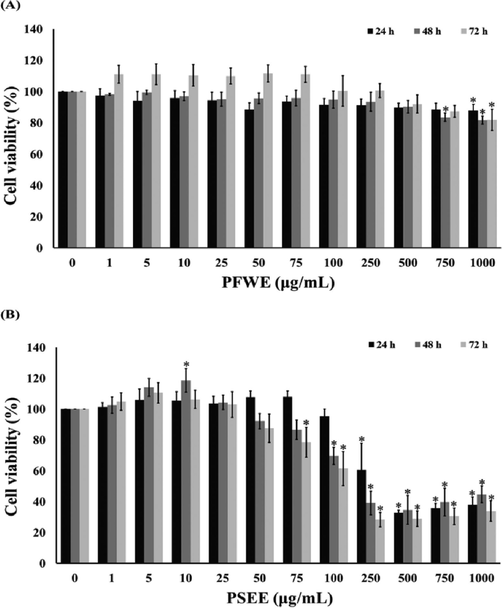
Cytotoxicity of PFWE (A) and PSEE (B) on RAW264.7 macrophages. RAW264.7 macrophages were treated with 1–1000 μg/mL of PFWE and PSEE for 24–72 h. The viability RAW264.7 cell was detected by MTT assay. Data shown are mean ± SEM of four independent experiments. *P < 0.05 compared to the control.
3.6 Effects of PFWE and PSEE on LPS-induced NO level in RAW264.7 macrophages
To explore the anti-inflammatory of PFWE and PSEE, LPS was utilized to activate the NO secretion into the medium of RAW264.7 macrophages. The exposure of LPS significantly enhanced NO secretion into the medium of RAW264.7 cells after 24 h. Pretreatment the cells with various doses of PFWE and PSEE prior to LPS administration demonstrated a markedly attenuated the augmentation of NO released into RAW264.7 medium. This indicated that PFWE and PSEE possess a potent anti-inflammatory activity against LPS-induced NO production (Fig. 6).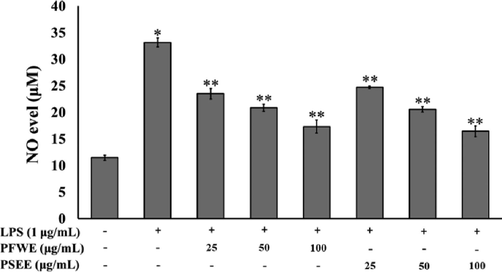
Effects of PFWE and PSEE on LPS-induced the secretion of nitric oxide in RAW264.7 macrophages. The cells were pre-treated with 25–100 μg/mL of PFWE and PSEE for 2 h and then treated with or without of LPS for 24 h. Data shown are mean ± SEM of four independent experiments. *P < 0.05 compared to the control. **P < 0.05 compared to the LPS alone group.
3.7 Effects of PFWE and PSEE on vascular function of rat thoracic aorta
As shown in Table 3, PFWE and PSEE (10, 100, and 200 μg/mL) caused vasorelaxation in endothelium-intact aortic rings precontracted with PE in a dose-dependent manner. In addition, the maximum relaxation stimulated by the highest concentration of PFWE and PSEE (200 μg/mL) was 31 ± 5 and 86 ± 6%, respectively, indicating the vasorelaxant effect produced by PSEE was greater than those of PFWE. * P < 0.05 compared with vehicle control groups, # P < 0.05 compared with PFWE. In the vehicle control, an equivalent volume of vehicle was used instead of the extracts.
Extract
Vasorelaxant activity (%)
10 μg /mL
100 μg/mL
200 μg/mL
Vehicle
0.6 ± 0.6
2.6 ± 1.4
5 ± 2.8
PFWE
20 ± 5.0*
23 ± 5.0*
31 ± 5.0*
PSEE
70 ± 5.0*,#
84 ± 5.0*,#
86 ± 6.0*,#
4 Discussion
Phenolic, flavonoid, and carotenoid compounds are commonly found in dietary plants and have gained a considerable examination due to their anti-cancer, anti-inflammation and anti-oxidant properties associated with human health (Pandey and Rizvi, 2009; Panche et al., 2016). In this study, high level of phenolic compound, flavonoid and carotenoids were observed in PSEE. The variation in phenolic, flavonoid and carotenoid content values might be related to the plant origins of the extractable compounds and the capability of the solvents used to identify the polyphenol, flavonoid and carotenoid from the plant substances (Altemimi et al., 2017). Moreover, the variation in contents of active compounds in different part might affect the biological activities. Therefore, TLC fingerprint and HPLC analysis were conducted to determined the active compounds, and might be used for quality control of the extract for the preparation of the new product from this plant. The TLC results indicated that PSEE exhibited more chemical components than that of PFWE. According to HPLC results, PSEE contained higher content of active compound, and piceatannol than that of PFWE. These might possibly due to the extract obtained from 70% ethanol extraction exhibited a wide range of secondary metabolites. Consistent with these results, piceatannol was found in high concentration from ethanolic extract fraction (Dos Santos et al., 2021; Krambeck et al., 2020). In addition, we also found that PFWE contained a high content of β-carotene and γ-tocopherol which are known as an anti-oxidant found in diet and herbal plant, and was consistent to its high potency in augment the percentage of scarvenging activity of PFWE. Previous evidences revealed that fruits of P. edulis possesses a high concentration of vitamin C (Septembre-Malaterre et al., 2016) supporting its anti-oxidant properties than seed extract.
P. edulis has been reported on the prevention of hyperlipidemia in traditional medicine. Firstly, we investigated an in vitro antihyperlipidemic potential mediated through the lipid synthesis, digestion, and absorption impairment by determining the inhibitory properties of HMG-CoA reductase, lipase, and cholesterol esterase activities of PFWE and PSEE. The enzyme HMG-CoA reductase is a rate-limiting enzyme in the synthesis of cholesterol and other isoprenoids that activate the changes of HMG-CoA to mevalonate (Friesen and Rodwell, 2004). The suppression of HMG-CoA reductase dramatically lowered cholesterol level. Consequently, the powerful of HMG-CoA reductase enzyme inhibitor can play a major role in the control of dyslipidemia worldwide (Friesen and Rodwell, 2004). Our present study displayed that only PFWE slightly suppressed the activity of HMG-CoA reductase enzyme. Current research findings informed that the digestion and absorption of fats as a potential preventive and medicinal therapy for dyslipidemia and obesity carried out by gastrointestinal (GI) mechanisms (Tucci et al., 2010). In the intestine, the pancreatic lipase which is a major enzyme digests the fats into glycerol and free fatty acids (Tucci et al., 2010). To date, the inhibition of pancreatic lipase mechanism have drawn a considerable attention in the anti-hyperlipidemic and anti-obesity efficacy of natural products. (Rajan et al., 2020). In this study, both PFWE and PSEE exhibited moderate anti-lipase activity, compared to the standard drug orlistat, which is a natural derivative from Streptomyces toxytricini (Heck et al., 2000). However, orlistat are likely to cause the unwanted GI adverse effects, for example, fecal incontinence, liquid stools, oily spotting, and abdominal cramping. As a result, new inhibitors separated from natural products without any adverse side effects are need to explore (Heck et al., 2000). Several studies reported that flavones, flavonols, tannins, and procyanidins, a polyphenolic compound, were observed in the inhibitory activity of lipase (Tucci et al., 2010). This study found that PSEE extracts had the high phenolic and flavonoid contents, which was consistent with the higher potency in suppression of lipase and cholesterol esterase activities than PFWE. Therefore, the effect of PSEE on lipase and cholesterol esterase suppression might be due to a synergistic effect of various components within the extract, including, polyphenolic, flavonoids rather than by a single compound. Free and esterified cholesterols in dietary cholesterol are hydrolyzed through pancreatic cholesterol esterase (Heidrich et al., 2004). Subsequently, the restriction of cholesterol esterase activity have a pivotal role in limiting dietary cholesterol absorption. In the present study, the in vitro inhibition of choresterol esterase activity was observed in PSEE and was higher compared to PFWE. Many studies reported that inhibitory properties of phenolic on cholesterol esterase. Consequently, it can be postulated that this activity might be due to the high phenolic content found in PSEE in the greater amount than PFWE.
In the body, macrophages are generally distributed immune cells and represented an important role in the innate and adaptive immune responses, including inflammation (Li et al., 2017). Acute macrophage stimulation contributes a protective and beneficial outcome. In macrophages, the chronic inflammatory responses though endotoxin and inflammatory negotiators develop massive proinflammatory mediators production involved in the pathogenesis of inflammatory diseases, for examples, cancer, atherosclerosis, rheumatoid arthritis, neurological diseases (Abarikwu, 2014; Ahn et al., 2016). LPS, a gram-negative bacteria component, induced the excretion of pro-inflammatory intermediary by binding toll-like receptor 4, and trigger various intracellular signaling pathways (Sukketsiri et al., 2019). Accordingly, LPS-activated macrophages model has been used to examine the anti-inflammatory potentials of natural products. In this study, we used the LPS-stimulated RAW264.7 macrophages to measure the anti-inflammatory activities of PFWE and PSEE. Nitric oxide (NO) is synthesized from different isoforms of the NO synthase (NOS) enzyme that is one of the crucial inflammatory mediators (Förstermann and Sessa, 2012). In the current study, pre-treatment with PFWE and PSEE inhibited the LPS-stimulated the generation of NO in RAW264.7 cells. Previous studies suggested that piceatannol decrease the expression of iducible NOS contributing to reduce the generation of NO in both in vitro and in vivo model (Peng et al., 2020; Ulanova et al., 2006), which might support the anti-inflammatory effects of PSEE. In addition, β-carotene and γ-tocopherol have been found to reduce the inflammation in both in vitro and in vivo (Gopal et al., 2013; Imamura et al., 2006; Novoselova et al., 2009), which may provide the support for PFWE against inflammation. These findings suggest that PFWE and PSEE has anti-inflammatory potential against LPS-induced inflammatory responses.
Vascular resistance play a crucial role in the regulation of arterial blood pressure (Oparil et al., 2018). Therefore, the reduction of vascular resistance caused by a direct action on vasodilation is thought to be one of the mechanisms to lower blood pressure in the antihypertensive medications (Loh et al., 2020; Oparil et al., 2018). The present finding showed that PSEE displayed higher potency of rat aortic rings relaxation than PFWE in a concentration-dependent manner, indicating the direct vasorelaxant effects of PFWE and PSEE. This effect of PSEE might be from the high content of piceatannol. Previous studes reported that piceatannol increased the endothelial NOS expression resulting in the enhancement of NO production in endothelial cells (Jeong et al., 2015; Sano et al., 2011) corresponding to the relaxation of vascular smooth mucle. These relaxant effects of PSEE may contribute to alleviate the development of hypertension. These present finding suggest a possibility to develop PSEE for the treatment of hypertension. However, further studies are required to explain the mechanism of PFWE and PSEE-induced vasorelaxation.
5 Conclusions
PSEE contained high levels of piceatannol, polyphenolic stilbene. On the other hand, PFWE contained high levels of beta-carotene and gamma-tocopherol contents. The results obtained in this study demonstrated that PFWE and PSEE have anti-oxidant activity, anti-inflammatory activity, and inhibition of pancreatic lipase, and pancreatic cholesterol esterase in vitro, and ex-vivo vasorelaxation of rat aortic rings, indicating their beneficial role in the hyperlipidemia and hypertension. Further investigations are in progress to elucidate the mechanism of PFWE and PSEE for the in vivo anti-hyperlipidemic or anti-hypertensive activities.
Acknowledgements
All authors gratefully acknowledge the financial support by Thailand Institute of Scientific and Technological Research (TISTR) and facilities provided by Faculty of Science-Prince of Songkla University, Faculty of Pharmaceutical Sciences-Khon Kaen University and Naresuan University and Faculty of Medical Sciences-Naresuan University, Thailand.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim. Biophys. Acta. 2014;1840:2373-2381.
- [Google Scholar]
- Gallic acid-g-chitosan modulates inflammatory responses in LPS-stimulated RAW264.7 cells via NF-κB, AP-1, and MAPK pathways. Inflammation. 2016;39:366-374.
- [Google Scholar]
- Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel). 2017;6:42.
- [Google Scholar]
- Selective extraction of piceatannol from Passiflora edulis by-products: application of HSPs strategy and inhibition of neurodegenerative enzymes. Int. J. Mol. Sci.. 2021;22:6248.
- [Google Scholar]
- The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol.. 2004;5:248.
- [Google Scholar]
- The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr.. 2018;30:1-24.
- [Google Scholar]
- Beta-carotene attenuates angiotensin II-induced aortic aneurysm by alleviating macrophage recruitment in Apoe(-/-) mice. PLoS ONE. 2013;8:e67098.
- [Google Scholar]
- Passiflora edulis: an insight into current researches on phytochemistry and pharmacology. Front. Pharmacol.. 2020;11:617.
- [Google Scholar]
- Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270-279.
- [Google Scholar]
- Inhibition of pancreatic cholesterol esterase reduces cholesterol absorption in the hamster. BMC Pharmacol.. 2004;4:5.
- [Google Scholar]
- Beta-carotene modulates the immunological function of RAW264, a murine macrophage cell line, by enhancing the level of intracellular glutathione. Biosci. Biotechnol. Biochem.. 2006;70:2112-2120.
- [Google Scholar]
- Vascular- and hepato-protective effects of passion fruit seed extract containing piceatannol in chronic high-fat diet-fed rats. Food Funct.. 2016;7:4075-4081.
- [Google Scholar]
- Antimicrobial activity of the ethanolic and aqueous extract of passion fruit (Passiflora edulis Sims), in the absence and presence of Zn(OAc)2.2H2O. World J. Pharm. Res.. 2017;6:230-246.
- [Google Scholar]
- Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol. Med. Rep.. 2015;12:937-944.
- [Google Scholar]
- Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. App. Pharm. Sci.. 2014;4:61-65.
- [Google Scholar]
- Identification and quantification of stilbenes (piceatannol and resveratrol) in Passiflora edulis by-products. Pharmaceuticals (Basel). 2020;13:73.
- [Google Scholar]
- The anti-inflammatory activities of two major withanolides from Physalis minima via acting on NF-κB, STAT3, and HO-1 in LPS-stimulated RAW264.7 cells. Inflammation. 2017;40:401-413.
- [Google Scholar]
- Creation of novel antihypertensive agent via structure-activity relationship study on phytochemicals towards vasorelaxant activity. J. Pharmacopuncture. 2020;23:88-89.
- [Google Scholar]
- Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem.. 2013;139:735-743.
- [Google Scholar]
- Naturally occurring antioxidant nutrients reduce inflammatory response in mice. Eur. J. Pharmacol.. 2009;615:234-240.
- [Google Scholar]
- Anti-obesity effect of Carica papaya in high-fat diet fed rats. Biomed. Rep.. 2020;13:30.
- [Google Scholar]
- Panche, A.N., Diwan, A.D., Chandra S.R., 2016. Flavonoids: an overview. J. Nutr. Sci. 5, e47.
- Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev.. 2009;2:270-278.
- [Google Scholar]
- Protective effect of piceatannol against acute lung injury through protecting the integrity of air-blood barrier and modulating the TLR4/NF-kappaB signaling pathway activation. Front. Pharmacol.. 2020;10:1613.
- [Google Scholar]
- Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res.. 2020;155:104681
- [Google Scholar]
- Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem.. 2011;59:6209-6213.
- [Google Scholar]
- Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem.. 2016;212:225-233.
- [Google Scholar]
- Diet, lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health. 2020;17:2326.
- [Google Scholar]
- Influence of processing and storage on the phenolic composition of Thomson seedless grape juice. J. Agric. Food Chem.. 1990;38:1565-1571.
- [Google Scholar]
- ECa 233 suppresses LPS-induced proinflammatory responses in macrophages via suppressing ERK1/2, p38 MAPK and Akt pathways. Biol. Pharm. Bull.. 2019;42:1358-1365.
- [Google Scholar]
- Hydroxycinnamic acid antioxidants: an electrochemical overview. Biomed. Res. Int.. 2013;2013:1-12.
- [Google Scholar]
- The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab. Syndr. Obes.. 2010;3:125-143.
- [Google Scholar]
- Involvement of Syk kinase in TNF-induced nitric oxide production by airway epithelial cells. Biochem. Biophys. Res. Commun.. 2006;351:431-437.
- [Google Scholar]
- Virani, S.S., Alonso, A., Benjamin, E.J., Bittencourt, M.S., Callaway, C.W., Carson, A.P., et al., 2020. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 141, e139–e596.
- Chemical constituents of leaves of Passiflora edulis. Chem. Nat. Compd.. 2017;53:1165-1166.
- [Google Scholar]
- Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front. Pharmacol.. 2016;7:201.
- [Google Scholar]
Further reading
- Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI J.. 2014;13:897-921.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102431.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







