Translate this page into:
Production of Anti-Camel IgY for diagnosis of infectious diseases affecting camels located in Kingdom of Saudi Arabia
⁎Corresponding author at: Department of Botany and Microbiology, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia. imoussa1@ksu.edu.sa (Ihab Moussa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Chicken can be used for the production of hyper-immune serum for many type of antigens specially, the antigens of mammals and other species. Therefore, the current study aimed to use the chickens for the productions of anti-camel IgY- antibodies using ammonium sulphate and ammonium sulphate - caprylic acid methods for detection of any infectious disease affecting camels.
One ml of purified camel IgG of mixed with complete freund's adjuvant was injected intramuscularly and S/C to four groups of laying hens (Hisex strain, 140 days old). The first booster dose was administrated two week after first immunization with one ml of purified camel IgG mixed with incomplete freund's adjuvant and the hens were repeatedly boostered (three times) at two weeks intervals. During the immunization periods we collect the eggs every day and the anti-camel immunoglobulins Y was separated by using two extraction methods; the ammonium sulphate precipitation method and the ammonium sulphate caprylic acid method, the sensitivity and the specificity of the recovered antibody against the camel IgG were evaluated using SDS-PAGE and ELISA.
Two weeks after the initial dose of immunizing the hens the anti-camels IgY-antibodies were detected then the mean antibody titer increased significantly (P < 0.05) till became the highest level at 10 weeks post immunization. The antibody titer remain at high level without significant increase (P > 0.05) up to 12 weeks after the first dose of immunization then started to decrease.
Therefore, there are several benefits of using immunized chicken to produce IgY antibodies rather than using other species such as mammals in order to prepare anti-camels antibodies conjugates for diagnosis of infectious disease affecting camels.
Keywords
Chickens IgY
Anti-camel IgY
Infectious diseases
Camel IgG
Ammonium sulphate extraction method
1 Introduction
Immunization of chickens intramuscularly with specific antigens for different time points and produces specific IgY- antibodies. These antibodies are transferred to progeny through the latent phase of egg. The transmission of serum immunolglobulins IgG antibodies in chicken to the egg yolk is similar to IgG cross placenta in mammals. It was revealed that IgY transmission is dependent receptor process (Morrison et al., 2002). After IgY antibodies are accumulated in the egg yolk then transportation to embryonic bloodstream were occurred especially in the last days of embryonic phase (Kowalczyk et al., 1985). It was reported that production of effective antibodies could be achieved by immunizing domestic chicken with antigen and adjuvant and therefore generation of long lasting titers of antibodies (Losch et al., 1986). These antibodies start to appear and accumulate in serum 7 days post immunization then passively transmitted to the yolk with a range between 3 and 35 mg/ml (Moussa et al., 2016). This avian IgG was dependent on receptors that are expressed on follicular epithelium of the ovary (Vera et al., 2015).
Kritratanasak et al., (2004), have reported that detection of anti-mouse specific IgG antibodies in chicken blood samples could be achieved two weeks later post immunization and hit the peak in week 10. These antibodies passively transferred to egg yolk and started to appear 2 weeks from the initial dose on immunization and reach to maximal level 11 weeks after, then remains until week 20 (Kritratanasak et al., 2004). The IgY –antibodies prepared against infectious bursal disease (IBD) were evaluated by Moussa et al., (2016). It was revealed that IgY are protective and specific and it could reduce the level of morbidity and mortality to around 15 % and 10 %, respectively (Moussa et al., 2016). Nikbakht and his colleague revealed that anti-camel IgY is capable to attach to the heavy chain of different animal species such as horse, sheep, cattle and camel. However, it can couple to the light chain of just only camel. This antibody has the specificity against camel IgG isotypes (IgG1, IgG2 and IgG3) (Nikbakht Brujeni, 2009). So, using camel components such as IgG antibody to raise specific production of IgY in chicken eggs is promising. Cai et al., and Rangel et al., were both demonstrated promising results in regards to development of methods for immunological diagnosis using IgY, for detection of Schistosoma japonicum antigens and Pythium insidiosum proteins, respectively (Cai et al., 2012, Rangel et al., 2010).
2 Materials and methods
2.1 Purification of camel IgG antibodies
Blood samples of 10 camels were collected by jugular venepuncture in anti-coagulant free universal tubes. Followed by separation of serum by centrifugation, and then kept in freezer at −20 °C. Purification of camel immunoglobulins was carried out by using ammonium sulphate precipitation. Pooled serum samples were precipitated with 50 % saturated ammonium sulphate solution and dialysed against phosphate buffer saline, until removal of all ammonium sulphate (de Almeida et al., 2008). SDS-PAGE had been carried out to confirm the purity of purified Camel IgG antibodies.
2.2 Animal handling and the ethical approval
The Ethics of Animal Rights was approved as recommended by the committee of King Saud University (Ethics Reference Number: KSU-SE-1978). Twenty white laying Leghorn hens (5–6 months, 1.1–1.4 kg body weight) had been used for serum collections and for preparation of anti-camel IgY antibodies, all the chickens were kept under comp;ete hygienic conditios with abundant food and and expert workers.
2.2.1 Schedule of laying hens immunization
Groups of 20 chickens were inoculated at three regions in the breast muscles with 50 µg camel IgG antibody mixed with complete Freund's adjuvant, as described in (Gross and Speck, 1996) Briefly, 100 mg of camel antibodies will be re-suspended in 500 ml of PBS. Antigen - adjuvant mixture will be injected intramuscularly at days zero, and after two weeks from the first dose of immunizations, the camel IgG mixed with incomplete Freund's adjuvants were inoculated inti the breast region by the same method. Every-two weeks after that booster doses from camel IgG only were inoculated by the same method explained before. From zero days and during the immunization period of the laying hens the eggs were collected every days for extraction of Immunoglobulin’s IgY by separation of the yolk from the albumins and kept at − 20 °C until separated by precipitation methods While blood samples were collected and the serum samples were separated and kept in the refrigerator to test the antibody titer.
2.2.2 Separation of anti-camel IgY antibodies by precipitation methods using ammonium sulphate caprylic acid method explained by (Moussa et al., 2015)
Ammonium sulphate caprylic acid precipitation method explained by (Moussa et al., 2015) was used for extraction of anti-camel IgY antibodies from the previously separated yolk that kept at − 20 °C. After separation of egg yolk from egg white, the egg yolk was diluted 5 times PBS pH 7.5. Adjustments of pH to 4.5 occur by using mixture of (6 % caprylic acid and acetic acid (volum/volum) for precipitation of non-immunoglobulins proteins. The extracted IgY antibodies were reconstituted in 5 ml PBS. The total protein content of the extracted immunoglobulins were measured using Biuret method. The extracted immunoglobulins were filtered using 0.45 um filter and kept as a aliquotes in ependorff tubes and stored in freezer.
2.3 Characteristics of IgY by SDS–PAGE and Western blotting analyses
The extracted IgY anti camel IgY antibodies were analysed by SDS-PAGE methods as described by (Moussa et al., 2015). Serum collected from camel and chicken were analysed as well by using molecular mass markers.
2.4 Measurement of antibody titer of camel IgY by indirect ELISA technique
Coating of 96 wells ELISA plates were occur using 100 µl of 5 µg camels IgG using carbonat bicarbont coating buffer and incubated at 4 °C/12 h. Tween 20 PBS were be used for washing and 10 % Skimmed milk were used for blocking. The camels IgY antibodies were diluted serially (1:10 to 1:320) in blocking and kept at 37° C for one hour. The plate washed three times using PBS/Tween. Rabbit anti-chicken IgY-HRP, diluted (1:1000) in PBS/Tween to each well. Then, incubating the plates for in the incubator at 37 °C for one hour. The plates were washed Three times with washing buffer followed by addition of 50 µl of OPD substrate solution and kept for 15 min at room temperature. Stopping of the reaction occur by using stopping solution of 2 N H2SO4. The absorbances were measured at 490 nm by using ELISA plate reader.
2.5 Statistical analysis
GraphPad Prism statistical software was used for statistical analysis. Two-way ANOVA were used to evaluate the differences between the groups. The p values in tables were calculated using the Mann-Whitney U. A p value < 0.05 was considered statistically significant.
3 Results
3.1 Total amount of protein present in the yolk of immunized hen with camel IgG antibodies at different period of immunization
Significant increase in the total protein of the extracted IgY were observed after four weeks from the zero day of immunization (p < 0.001and p < 0.01) as shown in Table 1 However, no significant increase was observed after two weeks from the zero day of immunization. The increase of the value of total protein remain until two weeks following the last booster dose where it reached to 1.46
g/ dl in case of IgY- antibodies extracted by ammonium sulphate precipitation method and 1.37
g/ dl for IgYantibodies extracted by caprylic acid precipitation method followed by decrease in the level of protein contents as shown in Table 1 and Fig. 1.
: Mod. Sign.(p < 0.01).
Highly Sign. (p < 0.001).
: Non significant. SDn; stander deviation.
Period
(Weeks)
Immunization
Ammonium sulphate method
Ammonium sulphate caprylic acid method
Mean value of TPC gm/dl
X
SDn
Mean value of TPC gm/dl
X
SDn
0
Zero day
0.42
0.02
0.32
0.020
2
2 weeks (First booster dose)
0.52
0.02
0.42
0.031
4
4 weeks (Second booster dose)
0.75
0.05
0.62
0.020
6
6 weeks (Third booster dose)
1.10
0.05
0.95
0.017
8
8 weeks (Fourth booster dose)
1.38
0.030
1.22
0.025
10
!0 weeks
1.44
0.02
1.31
0.036
12
12 weeks
1.46
0.02
1.37
0.017
14
14 weeks
1.35
0.05
1.23
0.0131
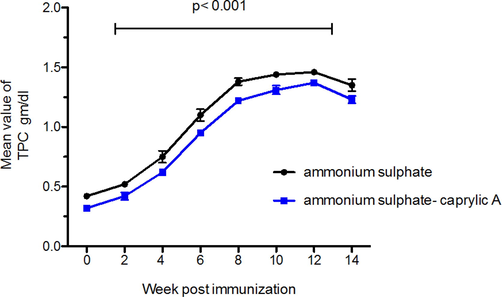
The level of total protein of the extracted immunoglobulins IgY from the egg yolk of immunized laying chickens with camel IgG antibodies. The blue curve represents the extraction using caprylic acid methods, while the black one presents ammonium sulphate methods the mean of IgY-antibodies response following immunization at different immunization period with different methods. Analysis was performed to compare between two different methods, p < 0.001 is considered significant by usingTwo- way ANOVA.
The total protein level significantly increased in the extracted immunoglobulines by Boostering. However, the caprylic acid method showed lower value than total protein value extracted by ammonium sulphate as it could remove some of the non– immunoglobulin proteins.
3.2 Result of electrophoretic analysis of anti- camels IgY- antibodies extracted after each immunization time intervals using SDS-PAGE
polyacrylamide slab gel (12.5 %) were used for analyzing the extracted anti-camels IgY antibodies either by extracted by ammonium sulphate or ammonium sulphate caprylic acid methods. The recovered protein bands were visualized directly by staining the gel with coomassie blue stain. Clear and distinct bands were observed with the anti-camels IgY antibodies extracted with caprylic acid ammonium sulphate methods more than that observed with ammonium sulphate only. Moreover, The addition of caprylic acid help in the remove of some of the non-specific proteins (non – immunoglobulin protein) that have low molecular weight that had been observed with the anti-camels IgY antibodies extracted by ammonium sulphate only as shown in Figs. 2,3.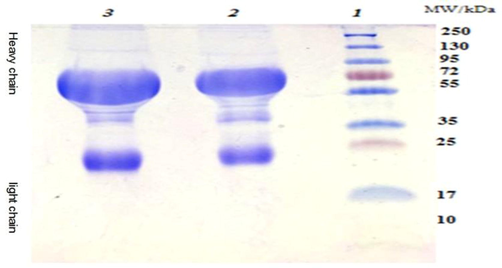
Analysis of camels IgG extracted by ammonium sulphate using SDS-PAGE.Lane 1: molecular weight marker (10–250 kDa), Lane 2 & 3: IgG extracted by ammonium sulfate.

Anti-camel IgY analysis by SDS-PAGE. Lane 1 represents the high range molecular weight marker. Lane 2–9 represent a repeated samples showing both the heavy and light chains.
Analysis of anti-camels-IgY antibodies fractionation with ammonium sulfate using SDS-PAGE showed two main distinct bands at 68 and 27 kDa representing the heavy and light chains of IgY, many other major and minor bands between 28 and 55 kDa are also found (high protein impurities) Figs. 2,3.
While, SDS-PAGE analysis of anti-camels-IgY antibodies fractionation with the additions of caprylic acid to ammonium sulphate showed two main distinct bands representing the heavy chains and light chains of anti-camel IgY. Non – immunoglobulin protein whivh have low molecular weight had been removed by the effect of caprylic acid as shown in Figs. 2,3.
3.3 Antibody titers of the extracted anti-camel IgY antibodies and from the collected serum samples from the zero day until the end of immunization using ELISA
The antibody titer against camels IgG appear after two weeks of the first dose of immunization, then significantly incrase (P < 0.05) after each booster dose of immunization and reach to the highest level at 10 weeks post immunization (2 weeks following 4th booster dose) and remained high with no significant difference (P > 0.05) up to 12 weeks. The titer after that became to decrease as shown in Table 2 and Fig. 4.Fig. 5Table 3.
Interval
Group
Geometrical mean serum antibodies after zero day of immunization
2 weeks
4 weeks
6 weeks
8 weeks
10 weeks
12 weeks
14 weeks
Test
0.975 ± 0.027
1.952 ± 0.041
2.631 ± 0.025
3.791 ± 0.035
4.114 ± 0.021
3.761 ± 0.035
3.211 ± 0.021
Control
0.293 ± 0.040
0.293 ± 0.040
0.293 ± 0.040
0.293 ± 0.040
0.293 ± 0.040
0.293 ± 0.040
0.293 ± 0.040
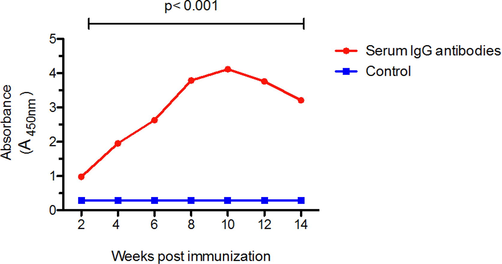
Geometrical mean IgG antibodies post immunization using ELISA test. The mean of IgG antibodies response following immunization at different time points compared to control (pre-immunization). Two- way ANOVA analysis was performed to compare between two different groups, p < 0.001 is considered significant.
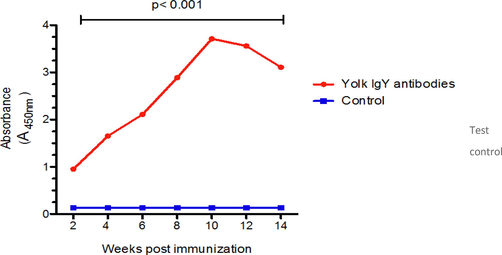
Geometrical mean IgY antibodies post immunization using ELISA test.The mean of IgY antibodies response following immunization at different time points compared to control (pre-immunzation). Two- way ANOVA analysis was performed to compare between two different groups, p < 0.001 is considered significant.
Interval
Group
Geometrical mean IgY antibodies post immunization
2 weeks
4 weeks
6 weeks
8 weeks
10 weeks
12 weeks
14 weeks
Test
0.951 ± 0.022
1.652 ± 0.021
2.110 ± 0.015
2.891 ± 0.040
3.714 ± 0.031
3.561 ± 0.025
3.110 ± 0.011
Control
0.133 ± 0.040
0.133 ± 0.040
0.133 ± 0.040
0.133 ± 0.040
0.133 ± 0.040
0.133 ± 0.040
0.133 ± 0.040
4 Discussion
Diagnosis of infectious disease affecting camels occur by using protein A conjugate due to the absence of anti-camel conjugate, however, protein A conjugate have many non-specific reactions and false positive results (Abdel-Rahman et al., 2017, kandil et al., 2020). Many authors suggested rabbit for the production of anti-camel conjugates but the use of rabbit has many problem among of which is the animal suffering and the sever side effect beside the high cost required for keeping and handling of such laboratory animals (Zhang et al., 2017). Chickens used by many authors for the productions of hyper- immune serums for a variety of antigens, many advantage had been obtained among of which is its cheap and the chickens produce high titer of antibodies specially when using antigens form mammals (Almeida et al., 2008, Wilmar Dias da Silva and Denise Tambourgi 2010). Our investigations aimed to use the chickens as a source for the productions of anti-camel conjugates in order to prepare a diagnostic kits for diagnosis of infectious diseases affecting camels to avoid the non-specific reactions that obtained by using protein A conjugate as anti-species.
Groups of 20 chickens were inoculated with 50 µg camel IgG antibody mixed with complete Freund's adjuvant, at days zero, and after two weeks from the first dose of immunizations, the camel IgG wereinjected with incomplete Freund's adjuvants and every-two weeks after that booster doses from camel IgG only were inoculated by the same method explained before.
Significant increase in the total protein of the extracted IgY were observed after four weeks from the zero day of immunization (p < 0.001and p < 0.01). The increase of the value of total protein remain until two weeks following the last booster dose. The increase in the total protein value in the extracted IgY is attributed to the increase in the antibody titer and other proteins in response to the immunization with camels IgG (Abdel-Rahman et al., 2017, kandil et al., 2020).
The protein content in the extracted IgY by ammonium sulphate only is higher than that extracted by additions of caprylic acid 6 % as the caprylic acid could some of the low molecular weight proteins (non-immunoglobulins) and help in the purification and concentration of anti-camel IgY and it has been clear when the anti-camel IgY extracted by addition of caprylic acid in SDS- PAGE (Bernardo et al., 2019; Redwan et al., 2021).
The antibody titer against camels IgG appear after two weeks of the first dose of immunization, then significantly incrase (P < 0.05) after each booster dose of immunization and reach to the highest level at 10 weeks post immunization (2 weeks following 4th booster dose) and remained high with no significant difference (P > 0.05) up to 12 weeks. The titer after that became to decrease (Bernardo et al., 2019, Moussa et al., 2015; Redwan et al., 2021).
5 Conclusion
We can conclude that anti-camels antibodies prepared by immunization of chickens and extraction of IgY from the yolk of such laying hens could be used for the production of anti-camels conjugates and any other species conjugate to prevent animal suffering and costly better than mammals.
Acknowledgment
This project had been funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-04-001-0007)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation of goat and rabbit anti-camel immunoglobulin G whole molecule labeled with horseradish peroxidase. Vet World.. 2017;10(1):92-100.
- [Google Scholar]
- Development of process to produce polyvalent IgY antibodies anti-African snake venom. Toxicon.. 2008;52(2):293-301.
- [Google Scholar]
- Extraction of phospholipid-rich fractions from egg yolk and development of liposomes entrapping a dietary polyphenol with neuroactive potential. Food Chem. Toxicol.. 2019;133(2019):110749
- [Google Scholar]
- Chicken egg yolk antibodies (IgY) for detecting circulating antigens of Schistosoma Japonicum. Parasitol Int.. 2012;61:385-390.
- [Google Scholar]
- IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Veterinary Immunol. Immunopathol.. 2010;135(3-4):173-180.
- [Google Scholar]
- Avian yolk antibodies in diagnosis and research. Dtsch Tierarztl Wochenschr. 1996;103:417-422.
- [Google Scholar]
- Quantitation of maternal-fetal IgY transport in the chicken. Immunology. 1985;54:755-762.
- [Google Scholar]
- Production of IgY anti- mouse IgG antibodies from chicken eggs. Asian Pac J. Allergy Immunol.. 2004;22:61-68.
- [Google Scholar]
- Sequences in antibody molecules important for receptor-mediated transport into the chicken egg yolk. Mol. Immunol.. 2002;38:619-625.
- [Google Scholar]
- Immunological properties of anti Naja Haje Arabica (the arabian cobra) snake venom antibodies prepared in chicken. Int. J. Pharmacol.. 2015;11:956-959.
- [Google Scholar]
- Production and evaluation of the immuno-protective efficacy of the immunoglobulins IgY- antibodies prepared against infectious bursal disease. Int. J. Pharmacol.. 2016;12:749-753.
- [Google Scholar]
- Development of IgY antibodies in chickens and IgG in rabbits immunized against proteins of pythium insidiosum isolated from horses in the State of Rio de Janeiro. Pesquisa Veterinaria Brasileira. 2010;30:87-93.
- [Google Scholar]
- Simple and efficient protocol for immunoglobulin Y purification from chicken egg yolk. Poult. Sci.. 2021;100(3):100956.
- [Google Scholar]
- Molecular characterization of infectious bursal disease virus (IBDV) isolated in Argentina indicates a regional lineage. Arch. Virol.. 2015;160:1909-1921.
- [Google Scholar]
- IgY: a key isotype in antibody evolution. Biol. Rev. Camb. Philos. Soc.. 2017;92(4):2144-2156.
- [Google Scholar]







