Translate this page into:
Morphological and molecular approaches of the nematode parasite Desportesius invaginatus (Acuariidae) infecting the cattle egret Bubulcus ibis (Ardeidae)
⁎Corresponding author. rabdelgaber.c@ksu.edu.sa (Rewaida Abdel-Gaber)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Investigations on the parasites that infect migrating birds worldwide are ongoing. To identify the parasite species that infect ardeids, morphological and morphometric characteristics were used. A total of 20 Bubulcus ibis (Ardeidae) specimens were collected from the agricultural lands belonging to the Faculty of Agricultural at Cairo University, which were then examined for nematode parasites. Only one acuariid species, belonging to the Acuariidae family, has been identified, with a prevalence rate of 50 % (10/20) among infected egrets. The Desportesius species isolated from the gizzard of the egret host is morphologically and morphometrically compatible with Desportesius invaginatus, which was previously identified from several Ciconiiformes hosts of Bubulcus ibis (Egypt, India, and Taiwan), Egretta garzetta (France), and Egretta rufescens (USA). Additionally, utilizing the partial small subunit ribosomal RNA (18S rRNA) gene sequence, maximum parsimony based on the Tamura-Nei model was used to infer the phylogeny of the recovered Desportesius species. The query sequences revealed 99.37 % identity for the 18S (MW358651.1) of the previously mentioned D. invaginatus. In addition to clarifying several morphological features of D. invaginatus, this study also provided new DNA data for this species.
Keywords
Host-specificity
Ardeidae
Acuariidae
Morphology
Phylogeny
1 Introduction
Small nematodes known as acuarioids are frequently found inside the esophagus, proventriculus, and gizzard of birds, notable waders from the Scolopacidae and Charadriidae families. The cordons, ptilina, or serrated shields seen on the cephalic structures of members of the superfamily are distinctive features (Chabaud, 1975). Species with cordons are members of the Acuariinae subfamily (there is only the single-family Acuariidae in the superfamily).
Chabaud and Campana (1949) designated the parasitic helminths that are mostly found in fish-eating birds of the order Ciconiiformes as belonging to the genus Desportesius (Nematoda, Acuariidae). Although many academics now acknowledge it as a distinct genus, it was once thought to be a subgenus of Synhimantus (Chabaud and Petter, 1959; Skryabin et al., 1965; Chabaud, 1975; Wong and Anderson, 1986; Anderson et al., 1996). Desportesius is a genus that has about eight species, however, because of how similar they are, it can be difficult to classify them (Chabaud and Campana, 1949; Wong and Anderson, 1986; Abou Shafeey, 2019).
The most common acuariid parasitic nematode found in Egypt’s egret gizzards is Desportesius invaginatus Skrjabin, Sobolev et Ivaschkin, 1965 which inhabits the gizzard of egrets in Egypt. The morphology of Desportesius invaginatus’ morphology has been researched by several authors (Skrjabin, 1917; Skryabin et al., 1965; Baruš et al., 1978; Wong and Anderson, 1986; Smogorzhevskaya, 1990; Varjabedian, 2006; Wheeb et al., 2015). The previous redescription only provided information on a few characters and considered the metrical data and its variance (Mutafchiev and Georgiev, 2009).
The primary method for identifying species has been morphological observation, which required extensive training and was a time-consuming process (Dkhil et al., 2022). As a defense mechanism, molecular methods of nematode identification offer precise and different diagnostic techniques (Nega, 2014). The primary taxonomic identifier for species identification has been the DNA sequence of the target regions (Al-Hoshani et al., 2021). Analysis of the mitochondrial cytochrome c oxidase subunit I (COI) gene and the nuclear 18S and 28S ribosomal RNA genes served as the basis for the genetic identification of acuariid parasites (Choi et al., 2014; Ebner et al., 2017; Kim et al., 2018; Ivanova et al., 2018; Mutafchiev et al., 2020). The nuclear ribosomal DNA 18S rRNA gene is very repetitive, comprises variable regions flanked by more conserved parts, and has only recently been utilized to research inter- and intraspecific connections, not to identify Desportesius specimens (Lee et al., 2021). Even though Desportesius specimens’ genetic divergence implies that they are conspecific with Synhimantus species (Mutafchiev and Georgiev, 2009).
To learn more about the morphology and phylogeny of the acuariid parasites infecting the cattle egret in Egypt, this study was designed.
2 Materials and methods
2.1 Experimental animals
Twenty cattle egrets, Bubulcus ibis (Family Ardeidae), were randomly selected from the agricultural lands owned by the Faculty of Agricultural at Cairo University and transported using special boxes to the Parasitology Laboratory in the Department of Zoology, Faculty of Science, Cairo University, Egypt; for further examination. According to Goodman et al. (1989), collected samples were taxonomically recognized.
3 Parasitological examination
3.1 Light microscopic (LM) examination
Within 8 to 24 hrs of collection, each chosen bird was sacrificed by receiving an intraperitoneal injection of a dilute solution of Nembutal (sodium pentobarbital). Each specimen’s alimentary canal was dissected, separated into segments, and inspected using a stereo-dissecting microscope (Nikon SMZ18, NIS ELEMENTS software) to check for intestinal parasites. Using a brush or tiny pipettes, the collected intestinal parasites were transferred to a clean saline solution and repeatedly washed to remove any mucus or debris that was typically adhering to their body surface. For further investigation and identification, the helminth parasites were fixed in 70 % ethanol. For each egret, the sites and the numbers of each parasite species were noted. According to Bush et al. (1997), the parasitological term of the prevalence was estimated.
3.2 Morphology and morphometry
To prepare the worms for whole mounts (n = 5), they were first fixed, stained with Semichon's acetocarmine, dried using a graduated ethanol series, cleared in clove oil, and mounted with Canada balsam in permanent preparations. The parasitic nematode specimens (n = 5) were preserved in pure glycerine as semi-permanent mounts for examination (Ryss, 2003). Using a Leica DM 2500 microscope (NIS ELEMENTS software, version 3.8), the mounted specimens and the pertinent structural features were studied, documented, and photographed at different magnifications. Using ImageJ 1.53e software (Wayne Rasband and contributors, National Institute of Health, USA), measurements were collected from digitalized drawings and represented in millimeters (mm).
3.3 Scanning electron microscopic (SEM) examination
The specimens (n = 10) were fixed at 3 % glutaraldehyde, rinsed in sodium cacodylate buffer, dehydrated in a graded sequence of ethanol, and infiltrated with amyl acetate (Al-Hoshani et al., 2021). They were processed in a critical point dryer “LEICA, EM CPD300“ with Freon 13 after passing through an ascending series of Genesolv D, then coated with gold–palladium using an auto fine coater (JEOL, JEC-3000FC), before the examination using an Etec Autoscan at 10-kV JEOL-SEM (JSM-6060LV) in the Electron Microscopy Unit at the Faculty of Science, Ain Sham University.
3.4 Type material
Permanent slides and the preserved nematode samples were placed in the collection of parasitology of the Department of Zoology at College of Science, King Saud University, Riyadh, Saudi Arabia.
4 Molecular analysis
4.1 Extraction of the genomic DNA
Using a DNeasy tissue kit© (Qiagen, Hilden, Germany), genomic DNA was extracted from ethanol-preserved samples (n = 10) by following the manufacturer's instructions. Genomic DNA was utilized for the polymerase chain reaction (PCR) and its concentration and purity were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fischer Scientific, Inc., Wilmington, DE, USA) (Al-Hoshani et al., 2021).
4.2 Amplification via polymerase chain reaction (PCR)
Through the PCR, a partial nuclear small subunit ribosomal RNA (18S rRNA) gene was targeted and amplified in this study. A total volume of 50 µl was used for all PCR reactions, which includes 5 μl of 10 × buffer, 5 μl of each dNTP (10 mM), 10 μl of each primer (1 pmol/ μl), 0.3 μl of Taq polymerase (5 U/ml), 2.5 μl MgCl2 (50 mM), and 2 μl of total genomic DNA. Primers used for the targeted gene were 5′-CGT ACC GGC GAC GTA TCT AT-3′ (forward) and 5′-AGG TGA GTT TTC CCG TGT TG-3′ (reverse) as mentioned by Lee et al. (2021). The PCR thermocycling profile included initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 1 min. The post-PCR extension was then completed for 10 min at 72 °C. On 1.5 % w/v agarose gel in 1 × Tris-acetate–EDTA (TAE) stained with SYBR green, the amplicons were examined, and the results were seen with a UV trans-illuminator. Following the manufacturer’s instructions, PCR products of the anticipated size were gel-extracted and purified using the GeneJETTM PCR Purification Kit [Thermo (Fermentas)] (Al-Quraishy et al., 2020).
4.3 Sequencing and phylogenetic analysis
After sequencing procedures using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA), PCR amplicons were submitted to automated sequencing utilizing an automated DNA Sequencer (ABI-PRISM 310). Using ABI Editview (Perkin-Elmer), each sequence was manually modified for accuracy. Using BioEdit 7.0.1 (Hall, 1999), a partial 18S rRNA gene region was aligned with those in the National Center for Biotechnology Information (NCBI) database. Phylogenetic trees were inferred using Molecular Evolutionary Genetics Analysis (MEGA 7.0) software (Kumar et al., 2016) and Maximum Likelihood (ML) analysis. The bootstrap approach with 1000 replicates was used to evaluate the ML tree. With branch lengths in the same units as the evolutionary distances used to estimate the phylogenetic trees that were rendered to scale.
5 Results
The investigated cattle egret, Bubulcus ibis, has nematode parasites that naturally present in ten out of twenty (50 %) specimens, primarily in the gizzard. This parasitic species was identified as Desportesius invaginatus. Each parasitized egret has an infection intensity that is between 5 and 10 (mean of 7).
5.1 Morphological studies
The worm has a cylindrical, white body (Fig. 1A). Two conical pseudolabia containing two amphids and a pair of cephalic papillae encircled the mouth opening (Figs. 1A-D, and 2A-C). It had a thin, striated cuticle (Fig. 1G, H). The buccal capsule has the appearance of a long, thin funnel (Fig. 1A-C). Before becoming anastomosed close to the nerve ring, cordons that began at the dorso-ventral sides of the mouth opening extended posteriorly and repeatedly (Figs. 1A-E, and 2A-E). Small spines were given for the cordons. The cordons were laterally followed by a pair of tricuspid deirids (Figs. 1F, and 3A, B). There were lateral alae that were present and extended to the tail’s tip behind the deirids (Fig. 1A, I, L, and 3C, D). The cylindrical esophagus has both a muscular and a glandular part (Fig. 1A, B).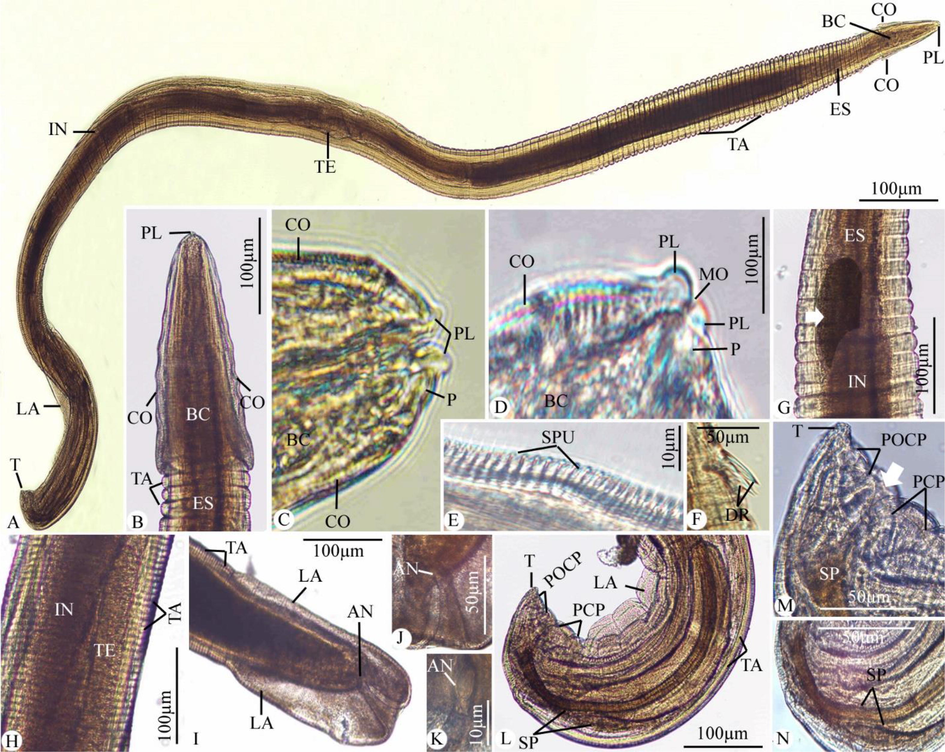
Light photomicrograph of Desportesius invaginatus showing different body parts. (A) Whole mount preparation. (B-N) High magnifications for different body parts: (B-E) Anterior extremity of the body. (F) Deirids. (G) The esophagus is followed by the intestinal diverticula (arrow) and intestine. (H) The middle part of the body shows testes. (I-K) The posterior extremity of the female worm. (L-N) The posterior extremity of the male worm with the cloacal opening (arrow). Note: PL, pesudolabia; BC, buccal capsule; CO, cordons; TA, transverse annulations; DR, deirids; SPU, spinules; ES, esophagus; TE, testes; IN, intestine; LA, lateral alae; T, tail; P, papillae; MO, mouth opening; PCP, precloacal papillae; POCP, postcloacal papillae; AN, anal opening; SP, spicules.
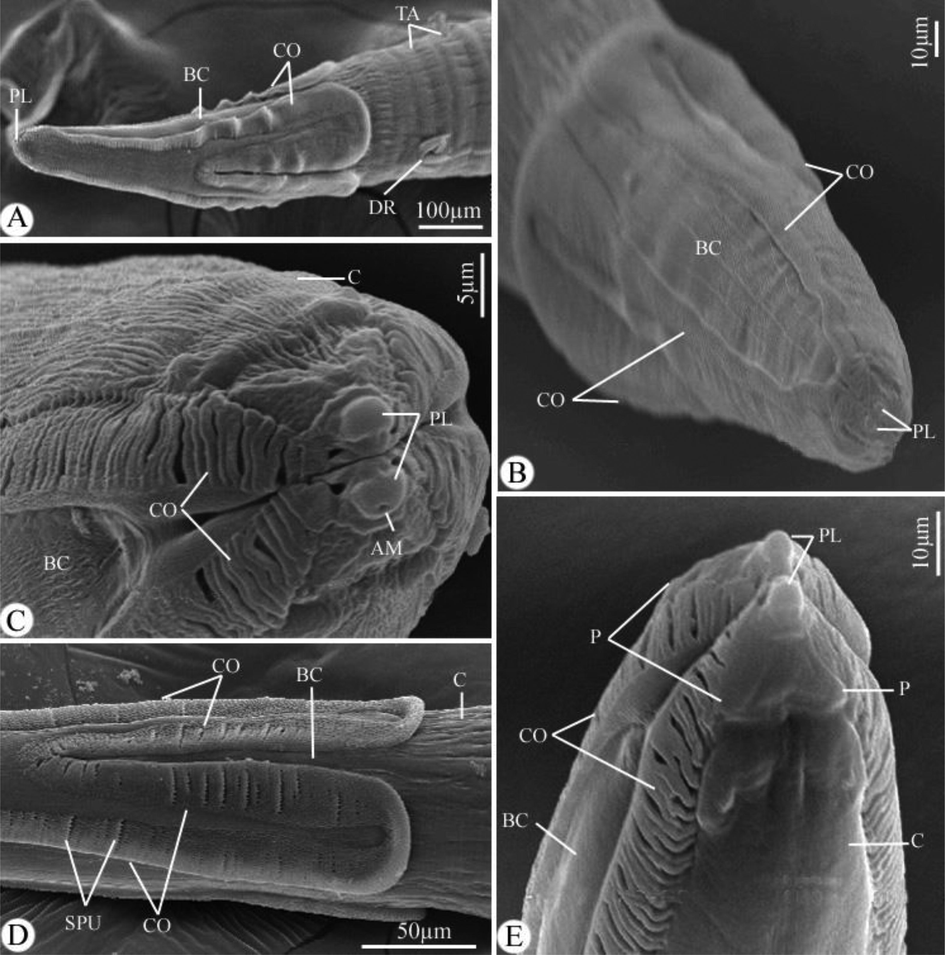
Scanning electron micrographs of Desportesius invaginatus showing different parts in the anterior extremity of the body. Note: AM, amphid; PL, pseudolabia; BC, buccal capsule; CO, cordons; TA, transverse annulations; DR, deirids; C, cuticle; SPU, spinules.
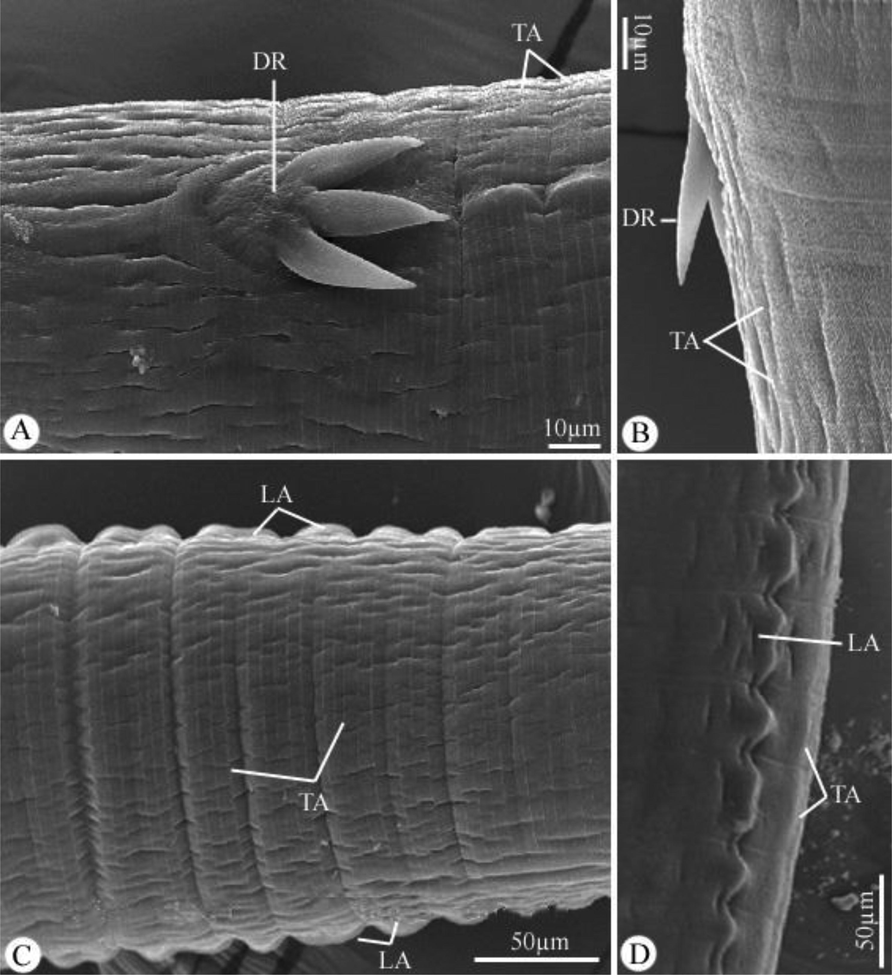
Scanning electron micrographs of Desportesius invaginatus showing different parts in the middle region of the body. Note: DR, deirids; TA, transverse annulations; LA, lateral alae.
5.2 Description of the female worm
The body’s dimensions were 9.89–12.88 (11.87) long and 0.30–0.39 (0.37) wide. Cordons were 0.278–0.377 (0.342) long. The length of the buccal cavity was 0.221–0.278 (0.245). The muscular portion of the esophagus measured 0.60–1.28 (1.09) long, while the glandular portion was 2.36–3.01 (2.98) long. The nerve ring and excretory pore were situated at 0.167–0.198 (0.170) and 0.204–0.238 (0.228) from the anterior end, respectively. The tricuspid deirids, which were extremely large, were located 0.432–0.711 (0.667) from the body’s anterior end and were 0.047–0.060 (0.056) long. The distance between the vulva and the posterior extremity was 0.051–0.069 (0.061). The tail was conical and had a structure in the form of a rounded knob (Figs. 1I-K, and 4C).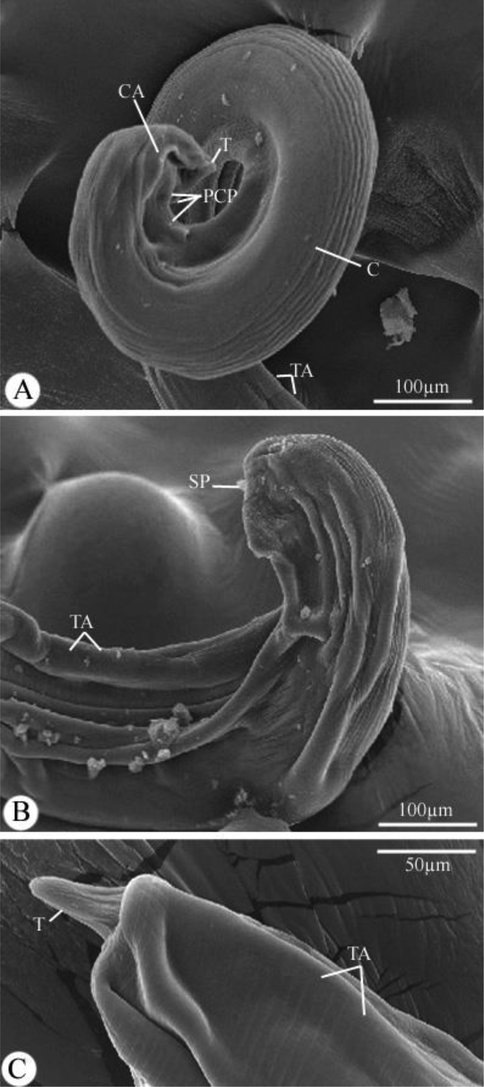
Scanning electron micrographs of Desportesius invaginatus showing different parts in the posterior extremity of the male (A,B) and female (C) body. Note: CA, cervical alae; T, tail; C, cuticle; TA, transverse annulations; SP, spicule.
5.3 Description of the male worm
The body’s dimensions were 6.98–8.23 (7.60) long and 0.189–0.201 (0.199) wide. Cordons were 0.234–0.302 (0.298) long. The buccal capsule had a length of 0.199–0.243 (0.220). The muscular portion of the esophagus measured 0.211–0.821 (0.786) long, while the glandular portion was 1.29–2.89 (2.10) long. The nerve ring and excretory pore were situated at 0.147–0.169 (0.158) and 0.201–0.288 (0.225) from the body’s anterior end, respectively. Large tricuspid deirids were situated at 0.290–0.632 (0.576) from the body’s anterior end and were 0.029–0.039 (0.035) long. The caudal portion was spirally coiled, and the caudal alae were inflated and vesicular in appearance (Figs. 1L-N, and 4A). Nine pairs of caudal papillae were observed on the posterior extremity, including four pairs of precloacal and five pairs of postcloacal papillae (Fig. 1L, M). Two unequal spicules were present (Figs. 1L, N, and 4B), the right spicule measured 0.690–0.803 (0.729) long, and the left spicule measured 0.401–0.520 (0.492) long.
5.4 Molecular phylogenetic analysis
The partial 18S rRNA sequence of the studied nematode species was 630 bp and included 50.6 % GC content [A(24.6 % 155) | C(21.75 % 137) | G(28.89 % 182) | T(24.76 % 156)] and given the accession number ON564731.1 in GenBank. Nucleotide sequencing data from 26 taxa were aligned across 621 positions using the ML method to construct a phylogenetic dendrogram that represented one class of Chromadorea (Table 1). The average distance between all specimen sequences was 0.030 overall. The identity of the genus Desportesius was verified by pairwise comparison with the GenBank 18S rRNA gene data collection (Table 1, and Fig. 5).
Superfamily
Family
Species
Accession No.
% identity
Acuarioidea
Acuariidae
Desportesius invaginatus
MW358651.1
99.37
Acuariidae
Synhimantus hamatus
EU004819.1
98.57
Acuariidae
Synhimantus laticeps
EU004818.1
98.41
Acuariidae
Synhimantus cf. laticeps
KP861914.1
98.25
Acuariidae
Echinuria borealis
EF180064.1
95.58
Acuariidae
Stegophorus macronectes
HE793715.1
97.74
Habronematoidea
Tetrameridae
Crassicauda magna
MT496895.1
98.10
Habronematidae
Cyrnea leptoptera
EU004815.1
96.35
Physaopteroidea
Physalopteridae
Proleptus sp.
JF934733.1
97.78
Physalopteridae
Heliconema longissimum
JF803949.1
97.46
Physalopteridae
Physaloptera apivori
EU004817.1
95.40
Physalopteridae
Turgida torresi
EF180069.1
95.40
Spiruroidea
Cystidicolidae
Cystidicola farionis
MG594291.1
97.62
Cystidicolidae
Comephoronema werestschagini
MN294781.1
97.78
Cystidicolidae
Ascarophis arctica
DQ094172.1
97.78
Cystidicolidae
Neoascarophis longispicula
JF803921.1
97.62
Cystidicolidae
Capillospirura ovotrichuria
MN294783.1
96.83
Cystidicolidae
Cystidicoloides vaucheri
KY558630.1
96.67
Cystidicolidae
Metabronema magnum
JF803918.1
96.67
Cystidicolidae
Salmonema ephemeridarum
JF803927.1
96.19
Rhabdochonidae
Rhabdochona mazeedi
JF803936.1
96.51
Rhabdochonidae
Spinitectus gracilis
MG594297.1
95.29
Spirocercidae
Mastophorus muris
MG818763.2
96.19
Filarioidea
Setariidae
Setaria digitata
DQ094175.1
95.08
Onchocercidae
Onchocerca cervicalis
DQ094174.1
95.08
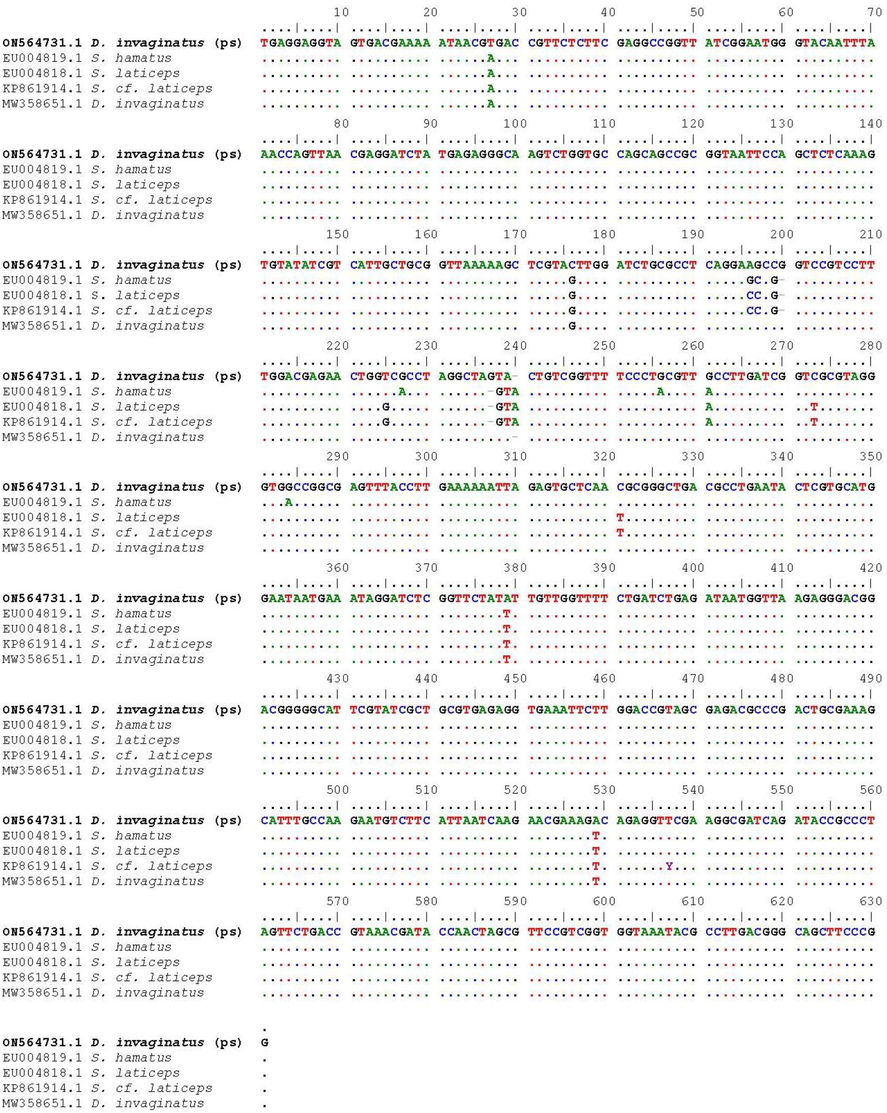
Partial 18S rRNA gene sequence alignment of Desportesius invaginatus with the most closely related species (Only variable sites are given). Bases that are similar to those in the initial sequences are shown by dots, whereas gaps are indicated by dashes. (PS mentioned specimen in the present study).
Taxa from the following five superfamilies were included in the phylogenetic analysis: Acuarioidea (represented by the family Acuariidae), Habronematoidea (represented by two families Tetrameridae and Habronematidae), Physalopteroidea (represented by family Physalopteridae), Spiruroidea (represented by three families Cystidicolidae, Rhabdochonidae, and Spirocercidae), and Filarioidea (represented by family Setariidae). Different ranges of identities exist for species within the Acuarioidea clade that range from 99.37 to 95.58 %, 98.10–96.35 % for Habronematoidea, 97.78–95.40 % for Physalopteroidea, 97.78–95.29 % for Spiruroidea, and 95.05 % for Filarioidea (Table 1).
A well-resolved separate clade containing acuariids and the recovered nematode species, especially those from the Acuariidae family, was visible on the phylogenetic dendrogram (Fig. 6). The recovered species exhibit high sequence identities for genera within the Acuariidae, with taxa of the Desportesius having a 99.37 % identity, Synhimantus having a 98.57–98.25 % identity, Echinuria having a 95.58 % identity, and Stegophorus having a 97.74 % identity. The sequence of the present species clustered with the Desportesius invaginatus (MW358651.1) sequences from earlier species in the same clade with a strong support value (99).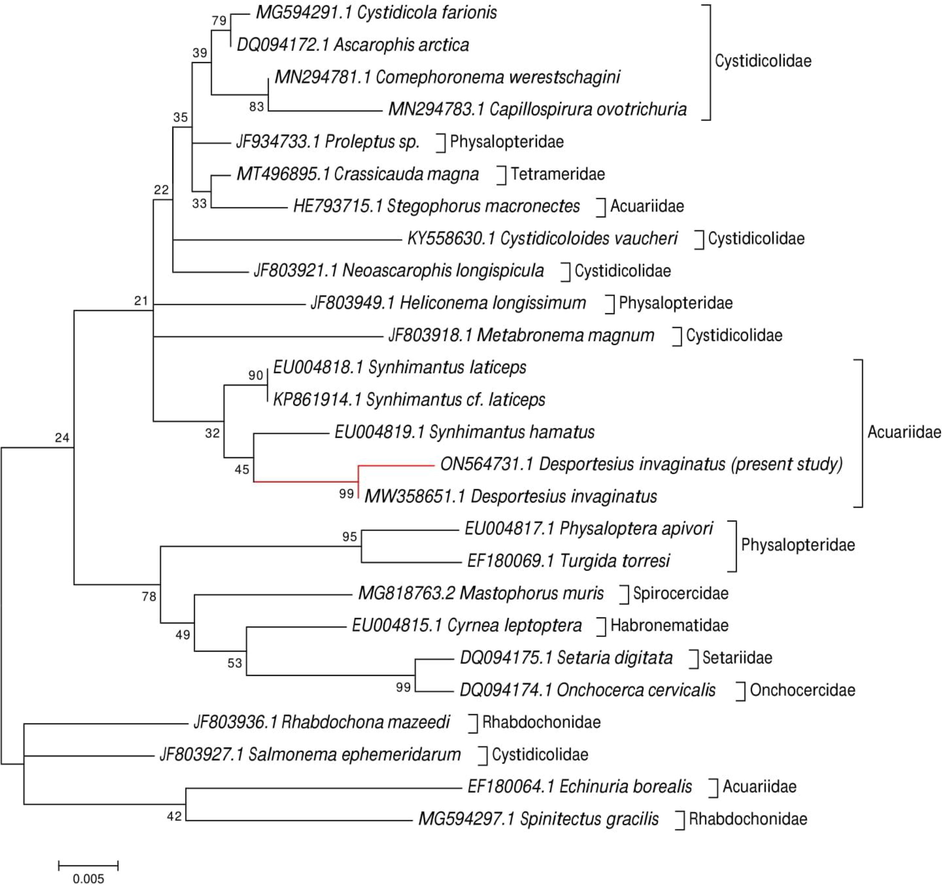
Molecular phylogenetic analysis was done by the ML method for the 18S rRNA gene region based on the Tamura-Nei model. The tree with the highest log likelihood (-2122.15) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach and then selecting the topology with a superior log-likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site.
6 Discussion
Chabaud (1954) categorized the members of the family Acuariidae according to the structure of cordons, and the subfamily Acuariinae consists of 25 genera. These taxa may be identified by the location of the vulva, the monodelphy, the posteriorly enlarged cordons, and the males’ inflated, vesicular caudal alae (Chabaud, 1975). The genus Synhimantus was divided into three subgenera by Chabaud and Petter (1959), namely Desportesius, which has cordons that are anastomosed and posteriorly enlarged, and the vulva is located close to the posterior end; Synhimantus, which has cordons that are anastomosed and the vulva is located in the middle of the body; and Dispharynx, which has cordons that non-anastomosed and the vulva lied not at the posterior end. The recovered nematode species in this study has the key characteristics of the former subgenus, making it possible to identify it as one Desportesius species.
Various ciconiform birds (ardeid birds, primarily herons, bitterns, and egrets) harbor nematodes of the genus Desportesius in their gizzard as common and generalist parasites (Chabaud and Campana, 1949; Mawson, 1982; Wong and Anderson, 1986; Anderson et al., 1996), but little is known about their pathogenic effects on the hosts (Tomás et al., 2016). In the current investigation, this Desportesius species were found in the gizzard of 50 % of the Bubulcus ibis that was examined. This infection rate was lower than that reported by Abou-Shafey (2012), who found D. invaginatus in Ardeola ibis ibis at an infection rate of 83.33 %, and Wheeb et al. (2015), who found the same parasite species in the cattle egret at an infection rate of 71.42 %.
Desportesius is made up of eight species that are widely known: D. invaginatus, D. brevicaudatus, D. bubulcusi, D. equispiculatus, D. longevaginatus, D. orientalis, D. sagittatus, and D. triaenucha. The recovered Desportesius species was in good agreement with the description and characterization of the previously observed D. invaginatus based on both morphological and morphometric features, including the body length, the margins of the tips of the two recurrent cordons are anastomosed on both ventral and dorsal surfaces with the other cordon, length of the muscular and glandular esophagus, the length of the spicules (right spicule is longer than the left one), and the arrangement of the caudal papillae (four pairs of preanal and five pairs of postanal papillae). Wong and Anderson (1986), Varjabedian (2006), Abou Shafeey et al. (2018), and Lee et al. (2021) all described D. invaginatus in very few reports. Additionally, D. invaginatus has been observed in several Ciconiiformes hosts, including Bubulcus ibis (Egypt, India, and Taiwan), Egretta garzetta (France), Egretta rufescens (USA) (Wong and Anderson, 1986).
The taxa within the genus Desportesius have insufficient descriptions, according to Wong and Anderson (1986). The precise identification of nematodes within the Acuariidae at a specific level has recently been made possible utilization of molecular approaches that employ the ribosomal gene regions as genetic markers (Lee et al., 2021). The nuclear ribosomal DNA 18S rRNA gene was used to study inter- and intraspecific relationships to identify Desportesius specimens, which agreed with Choi et al. (2014) and Kim et al., (2018) who stressed that this genetic marker is highly repeated and contains variable regions that are flanked by more conserved regions. The 18S rRNA sequences of specimens were separated from other Synhimantus species, which agreed with Chabaud and Petter (1959) and Lee et al. (2021) who regarded this as the morphology of the cordon and the position of the vulva opening. Genetic divergence of Desportesius specimens suggests their conspecific relationship with S. hamatus (EU004819.1) and S. laticeps (EU004818.1 and KP861914.1), respectively. Additionally, there was a strong relationship to the D. invaginatus specimens that had already been submitted on GenBank (MW358651.1).
7 Conclusion
Morphological and molecular phylogenetic data confirm the existence and placement of Desportesius invaginatus within the Acuariidae. It has been determined that this is the second genetic record for Desportesius species been discovered. Future research should employ more samples and genetic markers to further understand this group of acuariid parasites.
Conflict of interest
The author(s) declare that they have no conflict of interest regarding the content of this article.
Acknowledgments
This study was supported by the Researchers Supporting Project (RSP-2021/25), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Scanning Electron Microscopy of Microtetrameres spiralis (Nematoda, family Tetrameridae) Parasitising Cattle Egret “Ardeola ibis ibis” in Egypt. Egypt. J. Zool.. 2019;72:57-67.
- [Google Scholar]
- Assessment of sulfur, calcium and phosphorus in the cuticle covering the cordons of Desportesius invaginatus (Nematoda, Acuariidae) using energy dispersive X-ray microanalysis (EDXMA) PUJ. 2018;11(2):99-111.
- [Google Scholar]
- Desportesius invaginatus (Linstow, 1901) Chabaud and Campana, 1949 (Nematoda, Acuariidae) from Ardeola ibis ibis with reference to the fine structure of the cordons. PUJ. 2012;5:49-57.
- [Google Scholar]
- Cucullanus bulbosus (Lane, 1916) Barreto, 1918 (Nematoda, Cucullanidae) from the common ponyfish Leiognathus equulus (Leiognathidae): Morphology and molecular study. Microb. Pathog.. 2021;154:104821.
- [Google Scholar]
- Morphological and molecular characteristics of the gastro-intestinal nematode parasite Ascaridia colunbae infecting the domestic pigeon Columba livia domestica in Saudi Arabia. Acta Parasitol.. 2020;65:208-224.
- [Google Scholar]
- The acuarioid and habronematoid nematodes (Acuarioidea, Habronematoidea) of the upper digestive tract of waders a review of observations on their host and geographic distributions and transmission in marine environments. Parasite. 1996;4:303-312.
- [Google Scholar]
- Helminths of fish-eating birds of the Palaearctic Region. Moscow-Prague: I. Academia; 1978. p. :319.
- Shostak, A.W.,Parasitology meets ecology on its own terms: margolis,revisited. J. Parasitol.. 1997;83:575-583.
- [Google Scholar]
- Sur le cycle evolutif des Spirurides et de Nematodes ayant une biologie comparable. Valeur systématique des caractères biologiques. Ann Parasitol Hum Comp. 1954;39:42-88.
- [Google Scholar]
- Keys to the genera of the order Spirurida. Part 2. Spiruroidea, Habronematoidea and Acuarioidea. In: Anderson R.C., Chabaud A.G., Willmott S., eds. CIH keys to the nematode parasites of vertebrates. Vol No. 3. Farnham Royal: Commonwealth Agricultural Bureaux; 1975. p. :29-58.
- [Google Scholar]
- A propos d’une variété nouvelle de Synhimantus equispiculatus Wu & Liu, 1943. Création d’un nouveau sous-genre (Desportesius) n. subgen. Ann. Parasitol. Hum. Comp.. 1949;24:77-92.
- [Google Scholar]
- Essai de classification des Nématodes Acuariidæ. Ann Parasitol Hum. Comp.. 1959;34(3):331-349.
- [Google Scholar]
- Next-Generation in Situ Hybridization Chain Reaction: Higher Gain, Lower Cost. Greater Durability. ACS Nano. 2014;8(5):4284-4294.
- [Google Scholar]
- Morphological and phylogenetic studies of a copepod species, Irodes parupenei Ho and Lin (2007), infecting Parupeneus rubescens in Saudi Arabia. J. Ocean Univ. China. 2022;21(2):457-464.
- [Google Scholar]
- Excreted Cytoplasmic Proteins Contribute to Pathogenicity in Staphylococcus aureus. Infect. Immun.. 2017;84(6):1672-1681.
- [Google Scholar]
- The birds of Egyptian western desert. Mus: Mich; 1989.
- BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT. Nucleic Acids Symp. Ser.. 1999;41:95-98.
- [Google Scholar]
- Identification of Natural Compounds against Neurodegenerative Diseases Using In Silico Techniques. Molecules. 2018;23:1847.
- [Google Scholar]
- Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PloS ONE. 2018;13(11):e0207801.
- [Google Scholar]
- MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol.. 2016;33:1870-1874.
- [Google Scholar]
- Morphological and molecular characterization of Desportesius invaginatus (Nematoda: Acuariidae) from Egretta garzetta and Bubulcus ibis in Korea. J. Vet. Clin.. 2021;38(2):75-81.
- [Google Scholar]
- Some Acuariinae (Nematoda) from Australian birds. T. Roy. Soc. South. Aust.. 1982;106:19-30.
- [Google Scholar]
- Redescription of Desportesius brevicaudatus (Spirurida, Acuariidae) based on nematodes from Ixobrychus minutus (Aves, Ciconiiformes) from Bulgaria. Helminthologia. 2009;46(2):90-96.
- [Google Scholar]
- A 28S rDNA-based phylogeny of the nematode family Acuariidae (Spirurida) parasitic in vertebrates. Zool. Scr.. 2020;49(5):641-657.
- [Google Scholar]
- Review on concepts in biological control of plant pathogens. J. Biol. Agric. Healthcare. 2014;4(27):33-54.
- [Google Scholar]
- Express technique to prepare collection slides of nematodes. Zoosystematica Ross.. 2003;11:257-260.
- [Google Scholar]
- Skrjabin, K.I., Sobolev, A.A., Ivaschkin, V.M., 1965. Ž . Osnovi nematodologii. Vol. 14. Spirurata, Izdatel’stvo ‘Nauka’, Moscow, pp. 572.
- Nematodes. Part 3. Acuarioidea. In: Sharpilo V.P., ed. Fauna Ukrainy. Kiev: Naukova Dumka; 1990. In Russian
- [Google Scholar]
- Occurrence of helminth parasites in the gastrointestinal tract of wild birds from wildlife rehabilitation and investigation centre of Ria Formosa in southern Portugal. Vet. Parasitol. Reg. Stud. Reports. 2016;8:13-20.
- [Google Scholar]
- An ultrastructural study on Desportesius invaginatus (Nematoda, Acuariidae) from Ardeola ibis ibis in Egypt. J. Egypt. Soc. Parasitol.. 2006;36:149-157.
- [Google Scholar]
- Some helminthes parasites infecting wild birds at Edko, Behira province, Egypt. AJVS. 2015;47:65-70.
- [Google Scholar]
- Revision of the genus Desportesius Chabaud and Campana, 1949 (Nematoda: Acuarioidea) mainly from the gizzard of ciconiiform birds. Can. J. Zool.. 1986;64:2520-2530.
- [Google Scholar]







