Translate this page into:
Detection of heavy metals from Harpadon nehereus, Channa punctata and Pampus chinensis dried fishes and toxicity study of Channa punctata on embryonic zebrafish
⁎Corresponding author. Faruk_geb@ru.ac.bd (Md. Faruk Hasan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Globally, heavy metal pollution is one of the major serious environmental issues. The present investigations were carried out to determine the heavy metals concentrations from three dried fish along with the toxicity effects of Channa punctata dried fish on embryonic development of zebrafish.
Methods
The concentrations of heavy metals, Nickel (Ni), Chromium (Cr), Cadmium (Cd), Arsenic (As), and Lead (Pb) were detected from Harpadon nehereus, C. punctata and Pampus chinensis dried fish’s samples by Atomic Absorption Spectrophotometry (AAS) method.
Results
The highest concentrations of As and Pb were 3.65 ± 0.08 and 3.29 ± 0.07 ppm, found in C. punctata followed by 3.11 ± 0.05 and 3.26 ± 0.07 ppm, in H. nehereus, respectively. Meanwhile, no significant levels of Ni, Cr and Cd were found in all the three tested dried fishes, compared with standard references. The treatments of 50 µg/ml and 100 µg/ml C. punctata dried fish solution on zebrafish embryos showed significant toxicity and abnormalities of unequal blastomeric curve, yolk sac edema, pericardial edema, spinal curvature and tail deformation at 24, 33, 48, 72 and 96 h post fertilization (hpf).
Conclusions
The present investigation revealed that the tested dried fishes contain heavy metals and C. punctata is highly toxic for zebrafish embryos.
Keywords
Dried fishes
Heavy metals
Toxicity
Danio rario
1 Introduction
Fishes are one of the richest source of protein in the human diet. A number of studies have been reported on heavy metal addition in different fishes (Türkmen et al., 2005). Bangladesh has a vast fisheries resources comprising of freshwater, brackish and marine water inhabited. Among their 260 species are fresh water and brackish, 475 species of marine fish, 12 species of exotic fish and 24 species of prawn (Hasan et al., 2022; Shamsuzzaman et al., 2016). Fish and fishery products are important food components for a large part of the world’s population, with an average consumption level of 20.1 kg per capita (Mohiuddin et al., 2022; FAO, 2016). In developing countries, fish is a relatively cheap and accessible protein source, suitable for complementing high carbohydrate-based diets of West African and Asian population (Adeyeyeet al., 2015; Belton et al., 2022). Many people in our country depend on dried fish industry. Dried fish and dried fish products are stay a heart element of production, trade, diets and cuisines crossways the globe, mainly in the world of South Asia (Belton et al., 2022; Mohiuddin et al., 2022). It also used in producing fish and poultry meal which have a significant role in Bangladesh economy (Hossain et al., 2015),
In the coastal area, pollutants like heavy metals are possibly accumulated in marine fishes and subsequently transferred to human and animals (Giordano et al., 1991; Zhu et al., 2020). Metals are found in two forms in nature essential and non-essential both are playing an important role in our biological systems. Heavy metals are poisonous when presence in extra amount in the nature (Soylak et al., 2005). Heavy metals constitute are the core group of aquatic pollutants for their toxicity, long persistence, bio accumulative and non-biodegradable properties in our food chain. Heavy metals level in aquatic ecosystems increased by mining, industrial and agricultural activities that makes potential human health hazards (Ikem and Egiebor, 2005, Zhu et al., 2020). It is well known that fish muscle is not an active tissue in accumulating heavy metals. But it was informed that heavy metal levels in muscle and skin of some fish in polluted regions presented acceptable levels. For this reason, determination of heavy metal levels of fish is extremely important for human health. Dried fishes traditionally consumed by humans in many parts of Bangladesh. But day by day pollutants affects this product which is harmful to us. In spite of having both economic and human health importance, the current research focused to determine specific heavy metals (Ni, Cr, Cd As and Pb) from different types of dried fish and identify the cytotoxic effects on embryonic and larval development of Danio rario.
There are several reports on detecting the status of heavy metals contamination in sea fishes collected from costal area of Bangladesh (Mohiuddin et al., 2022; Hossain et al., 2019; Ahmed et al., 2015). But, as far our knowledge goes, there is no suitable report on the contamination of heavy metals in dried fishes collected from Cox’s Bazar Sea beach market, Bangladesh. Hence, the current research was designed to identify the levels of heavy metals in dried fishes as well as their cytotoxic capability on developmental stages of zebrafish embryos.
2 Materials and methods
2.1 Ethical statement**
Selected dried fishes were collected from the dried fish market of Cox’s Bazar Sea beach area. An ethical clearance certificate was taken from I.B.Sc., University of Rajshahi, Bangladesh (IAMEBBC, Memo No.: 255(14)/320/IAMEBBC/IBSc).
2.2 Samples collection
The dried fish’s samples of Harpadon nehereus (Loitta), Channa punctata (Taki) and Pampus chinensis (Rupchada) were collected from the biggest dried fish market of Cox’s Bazar Sea beach area, Bangladesh. The samples were collected in sterile air tight plastic bag separately to prevent extraneous contamination. Collected dried fish samples were stored at 4 °C in the laboratory for next investigations. Collected samples are presented in Fig. 1.
Samples of dried fishes collected from Cox’s Bazar Sea beach area in Bangladesh; (a) Harpadon nehereus, (b) Channa punctata and (c) Pampus chinensis.
2.3 Analysis of heavy metals concentration
2.3.1 Preparation of reagents
All reagents used for the analysis were analytical grade and consisted of nitric acid (65 % v/v) and hydrogen peroxide (30 % v/v) (Merk, Darmstandt, Germany). Standard stock solutions of Ni, Cd, Cr, Pd, and As with concentration of 1000 mg/L were prepared by diluting 1 ml of each single element stock in the combination list to 100 ml with deionized distilled water that contains 1 % (v/v) nitric acid.
2.3.2 Atomic adsorption spectroscopy
Atomic absorption spectrophotometer (AAS) technique was used to assess the concentrations of heavy metals (Tsade, 2016) methods with slide modification. Fish powder was converted into atomic vapor and feed into atomizer continuously at a constant rate giving a spectral signal. The spectral signal will rises to top pic and absorbs radiations of features. Wavelength from an external source, the atoms of lead, chromium, copper, Cadmium, calcium, magnesium absorb radiations of wavelengths of 217.0 nm, 357.9 nm, 228.8 nm and 324.0 nm respectively (Kilic et al., 2014). This technique has been extensively engaged in soils, water, nuts, wine and wine products (Narin et al., 2000).
2.3.3 Sample analysis
The samples were performed with flame AAS (Model Shimadzu AA-7000, Japan) method (Kilic et al., 2014). The absorption wavelengths and detection limits for the heavy metals were 211.8 nm and 0.001 ppm for As, 217.0 nm and 0.001 ppm for Pb, 228.8 nm and 0.002 for Cd, 324.7 nm and 0.02 ppm for Ni and 232.0 nm and 0.01 ppm for Chromium.
2.3.4 Calibration of instrument
The standards and unknown treatment absorption to be instrumentation. They will showed a signal from interpreted in the graph of real concentration of each metals. It was calculated using the following formula:
Where:
(mg/kg) R = AAS reading of digest.
Dilution Factor = Volume of digest.
Dilution factor = volume of the digest used/ weight of digested sample.
2.3.5. Toxicity study of Channa punctata dried fish
2.4 Preparation of dried fish solutions
Collected Channa punctata dried fish sample was rinsed in disinfected water to remove dust and other elements. Later, the sample was dried at 60 °C for 12 h. Dry fish will be griended into powdered. Total 1 mg of dried fish powder was added in 1 ml of autoclave distilled water. Fish powder was mixed well. Different concentrations (50–500 µg/ml) were tested on zebrafish embryos developmental stages.
2.5 Zebrafish embryo collection
For all the experiments, zebrafish embryos from the same spawn of eggs were used, supplied by Department of Pharmacy, Faculty of Sciences, University of Rajshahi, Bangladesh. Zebrafish eggs were collected and chosen under light microscope at 1 hpf. These fertilized eggs were cleaned using distilled water and incubated at 28 °C in E3 medium (Fazry et al., 2018; Khan et al., 2021). All fishes were maintained, breed and eggs were collected according to standard procedure of Dulay et al. (2012) with some modifications.
2.6 Toxicity of dried fish
Toxicity of Channa punctata dried fish was assessed on zebra fish embryos with the test times after of 24, 33, 48, 72 and 24––96 hpf according to the procedures of previous studies (Wibowo et al., 2018; Ponrasu et al., 2012). From the different concentrations 500 µg/ml dried fish solution was selected as the final exposure volume. Three replicate treatment groups (3 × 30 embryos) were assayed in 96 well microplates incubated at 27 °C ± 1 °C. Evaluation was conducted by using parameters of lethality, i.e., coagulation, non-detachment of the tail, somite disruption, and lack of heart-beat. Morphological abnormalities viz., delayed growth, limited movement, abnormal head-trunk angle, scoliosis/flexure, and yolk sac edema of zebrafish embryos were studied by Nagel (2002) methods. Images were taken under a digital biological microscope (Labomed, CA, USA).
2.7 Statistical analysis
Findings were analyzed using the statistical package, SPSS 20.0, (USA) with the means and standard errors (m ± SE). Figures were prepared using GraphPad Prism 8. Other calculations were performed by Microsoft Excel 2016.
3 Results
3.1 Detection of heavy metals
The levels of Ni, Cd, Cr, Pd, and As in Harpadon nehereus, Channa punctata and Pampus chinensis dried fishes with standard references were presented in Fig. 2. Highest levels of As and Pb concentrations were found with 3.65 ± 0.08 and 3.29 ± 0.07 ppm, respectively in Taki fish followed by 3.11 ± 0.05 and 3.26 ± 0.07 ppm, respectively in Loitta fish. Lowest levels of As and Pb was found with 2.61 ± 0.15 and 3.09 ± 0.04 ppm, respectively in Rupchada fish. On the other hands, no significant levels of Ni, Cr and Cd were found in Rupchada, Loitta and Taki fishes, compared with standard references.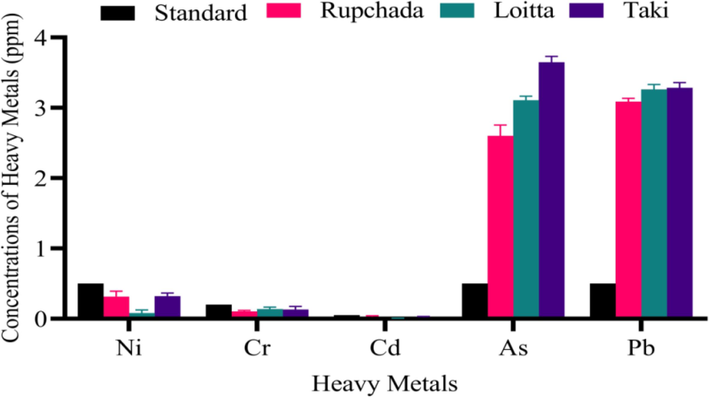
Concentrations (in ppm) of heavy metals in dried Rupchada, Loitta and Taki fishes.
3.2 Effects of dried fish treatments on embryonic development of Danio rario
3.2.1 Hatching rate
The hatching rate of Danio rario embryos were evaluated at 24, 48, 72 and 96 hpf. These hatching rates of embryo had been observed in four different hours for both control and treated groups and given in Table 1.
Treatment
Mean Hatching rate at several hpf
Treatments (Mean ± SE)
24 h
48 h
72 h
96 h
Control
78.33 ± 2.02a
87.66 ± 1.45a
85.33 ± 1.45a
92.00 ± 1.15a
85.83 ± 1.52a
50 µg/ml (Low dose)
71.66 ± 2.02b
61.33 ± 1.76b
40.66 ± 1.76b
24.66 ± 1.45b
49.58 ± 1.75b
100 µg/ml (High dose)
63.66 ± 1.20c
53.66 ± 2.02c
30.33 ± 1.45c
11.66 ± 2.02c
39.83 ± 1.67c
3.2.2 Mortality rate
The mortality rate of Danio rario embryos were evaluated at 24, 48, 72 and 96 hpf. These mortality rates of embryos were observed in four different hours for both control and treated groups are presented in Table 2.
Treatment
Mean mortality rate at several hpf
Treatments (Mean ± SE)
24 h
48 h
72 h
96 h
Control
4.00 ± 1.73c
6.00 ± 1.73c
12.33 ± 2.02c
12.00 ± 1.15c
8.58 ± 1.66c
50 µg/ml (Low dose)
23.66 ± 1.76b
33.33 ± 3.28b
45.00 ± 2.88b
57.66 ± 3.17b
39.91 ± 2.77b
100 µg/ml (High dose)
38.00 ± 3.46a
52.33 ± 4.3a
68.66 ± 4.09a
81.00 ± 3.78a
60.00 ± 3.92a
3.2.3 Heartbeat rate
In current study, the heart rates of Danio rario embryos were evaluated at 24, 48, 72 and 96 hpf. These heart rates of embryo were observed in four different hours for both control and treated groups are described in Table 3.
Treatment
Mean heart rate at several hpf
Treatments (Mean ± SE)
24 h
48 h
72 h
96 h
Control
96.66 ± 0.88a
94.33 ± 1.45a
89.66 ± 2.61a
81.33 ± 1.85a
90.51 ± 1.69a
50 µg/ml (Low dose)
84.67 ± 1.45b
77.33 ± 1.43b
82.33 ± 1.45a
65.01 ± 2.88b
77.35 ± 1.81b
100 µg/ml (High dose)
66.67 ± 2.27c
55.33 ± 2.96c
44.33 ± 2.33b
39.67 ± 2.63c
51.50 ± 2.46c
3.3 Cytotoxic effects of dried fish treatments on embryonic development of Danio rario
3.3.1 24-hpf
In case of 24 hpf control group have shown cleavage stage there after normal inverted blastomeric curve was formed then gastrulation period started with blastomeric epiboly. After treatments with 50 µg/ml and 100 µg/ml dried fish on the zebrafish embryo didn’t show any normal developmental stage like; cleavage, blastomare and gastrulation period (Fig. 3). They are functionally abnormal and growth was retarded.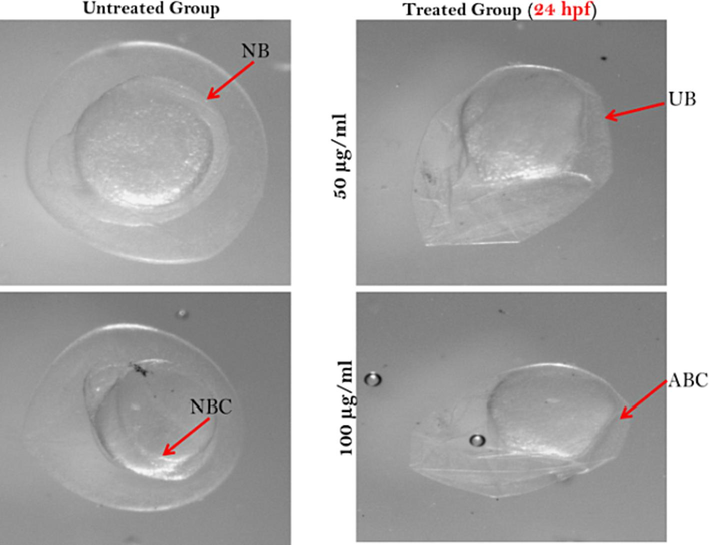
Zebrafish embryos after 24 hpf exposed to dried fish treatments. Here, NB- normal blastomere; UB- unequal blastomere; NBC- normal blastomeric curve and ABC- abnormal blastomeric curve.
3.3.2 33-hpf
At 33 hpf the normally grown embryo well known as organogenesis stage. The normal developing embryo without applied any dry fish supplement for this reason the tail was totally detached from the yolk, eyes were clearly be seen both heart rate and blood circulations were observed. In this developmental stage the spine and skin pigments also observed. When heavy metals supplementations were done to the zebra fish embryo deformities were observed like; skin pigment, spine and eyes were not seen clearly. Heart rate and blood circulation become slower or almost stopped in contrast to normally grown embryo and disrupted normal yolk shape (Fig. 4).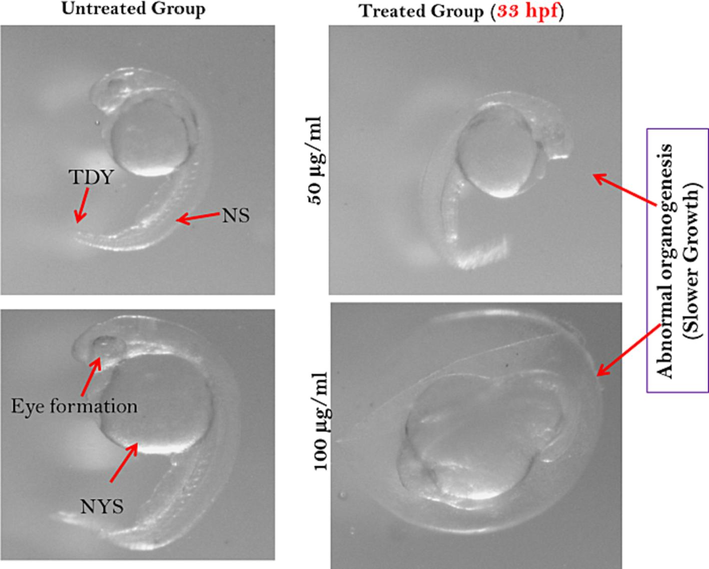
Zebrafish embryos after 33 hpf exposed to dries fish treatments. Here, TDY- tail detached from yolk sac; NS- normal spine and NYS- normal yolk sac.
3.3.3 48-hpf
At 48 hpf the normally hatched embryo was subjected to its normal length, eyes and skin pigments were well visible as well as size and shape of the yolk sac were normal and gradually it becomes longer. Heavy metal can deformities the zebrafish embryo, when possess of adequate dry fish in embryo the yolk sac deformities were observed, yolk sac edema was found. It was shorter than control. Skin pigments and eyes were not clearly visible (Fig. 5).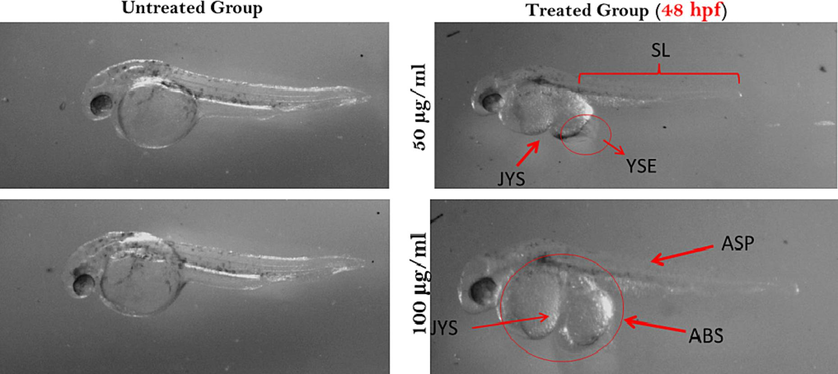
Zebrafish embryos after 48 hpf exposed to dried fish treatments. Here, SL- shorter length; JYS- joint yolk sac; YSE- yolk sac edema; ASP- abnormal skin pigmentation and ABS- abnormal body shape.
3.3.4 72- and 96-hpf
At 72 and 96 hpf normally grown embryo have shown its normal skin pigments, shape and size, clearly visible eyes, normal yolk sac and elongated spine. Exposure of dry fish treatment in zebra fish embryo which was effectively forms spinal curvature, tail bending, yolk sac edema, pericardial edema and lost its normal pigmentation with tail deformities (Fig. 6).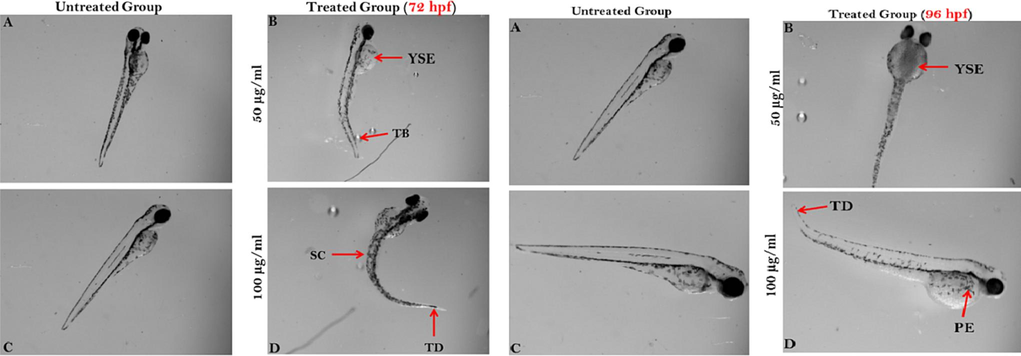
Zebrafish embryos after 72 and 96 hpf exposed to dried fish treatments. Here, YSE- yolk sac edema; TB- tail bending; SC- spinal curvature; TD- tail deformation and PE- pericardial edema.
4 Discussion
The investigations were carried out to determine the Nickel, Chromium, Cadmium, arsenic, lead concentrations and revealed out the effects of dried fishes on embryonic development of zebrafish embryos. Here, we measured the levels of Ni, Cd, Cr, As and Pb in Channa punctata, Harpadon nehereus and Pampus chinensis. In the present investigation, the highest concentrations of As and Pb were 3.65 ± 0.08 and 3.29 ± 0.07 ppm, respectively, found in C. punctata followed by 3.11 ± 0.05 and 3.26 ± 0.07 ppm, respectively in H. nehereus. Ali et al. (2022) evaluated the concentrations of As, Cr, Cd, and Pb in 12 different commercially important fish species. Kalay et al.(1999) studied the heavy metal concentrations in different fish species from Iskenderun Bay in 1996. Ahmed et al. (2015) reported several heavy metals in Ailia coila, Gagata youssoufi, and Mastacembelus pancalus fishes collected from the Buriganga River, Bangladesh. In contrast, Kalay et al. (1999) showed higher levels, except Ni, Cr and Cd from our findings. Similar results were found by Canli and Atli (2003) in different fish species but As and Pb concentrations were similar to the present results.
In current study, dried fish solution was applied on some developmental stages of zebrafish in different time’s interval at the concentrations of 50–500 µg/ml. In the control group, the mean hatching rate was 85.83 % during the experiment. Meanwhile, in case of 50 µg/ml, this was decreased to 49.58 % whereas; due to the 100 µg/ml of the respective dry fish agent this value was tremendously reduced to 39.83 %. Therefore, it is statistically evident that, the values of the hatching rate were significantly diminished in terms of treated groups compared to the control group. Littleton and Hove (2013) stated that hatching rates and embryonic development of C. carpio fish was 75 % and 60 % after treated with tartrazine at low and high concentrations, respectively. In the contrarily, Manjunatha et al. (2014) observed that the hatching rates of zebrafish embryos were reduced tremendously when treated with higher concentrations of henna hair dye.
In the present study, the mean mortality rate of zebrafish larvae were 39.917 % and 60 % at the low dose and high dose treated groups, respectively, while the control group showed only 8.58 % mortality. Witeska et al. (2014) reported that azo dyes holding toxic chemicals induced (0.1 mg/ml) a significant increase in mortality rate of zebrafish larvae in different periods of times. Nebeker et al. (1985) indicated the same result while he was observing the developmental stages of rainbow trout. These reports are supporting our present findings. So, it is statistically proved that the larvae mortality rate was significantly increased due to the effects of dried fish in the treated groups than the control group. Moreover, D. rario larvae did not show notable abnormalities in terms of mortality rates in control group.
In our experiment, the value of larval heart rates of control sample was 90.5 %, whereas, after treatment of dried fish at the concentrations of 50–500 µg/ml, larva showed 77.33 % and 51.5 % of heart rates, respectively. Singh et al. (1987) stated that synthetic food colors cause heart failure or circulatory failure of zebra fish larvae when exposed to higher concentration of color and hence, affect heart functions. They also observed that after 96 h of hatching, heart rates of the treated groups immensely decreased to 40 %. Recently, Cho et al. (2023) reported abnormal cardiac development of zebrafish embryos in 3-PCA-treated at a concentration of 5 mg/L. So, the findings proved that the larval heart rates had been affected by the dried fish and these heart rates of the treated groups abated significantly compared to the control group.
Zebra fish embryo didn’t show any normal developmental stage in 24 hpf they were shown abnormal cleavage, blastomare and gastrulation period when treated with two different concentrations of dry fish. They are functionally abnormal and growth was retarded. Schiwy et al. (2015) reported exposures of Altrip sediments in the concentrations of 300 mg/ml the larvae were reached only the epiboly stage. Shaikh et al. (2019) also reported on the same fish model to applied aqueous extract of Ficus glomerata after 24hpf delayed the growth, limited movement and slightly detached tail in four different concentrations. Zebrafish embryo deformities were also observed in 24 hpf skin pigments, spine and eyes were not seen clearly. Heart rate and blood circulation become slower or almost stopped in contrast to normally grown embryo and disrupted normal yolk shape compare to untreated group.
After 48 hpf deformities were observed on zebra fish embryo, when possess of dried fish treatment of 500 µg/ml in embryo the yolk sac deformities were observed, joint yolk sac pericardial edema was found. Skin pigments and eyes were not clearly visible. Similar result was observed by Shaikh et al. (2019) at similar duration when exposed aqueous extract of Ficus glomerata. Schiwy et al. (2015) also reported no eye and skin pigments formation. Li et al. (2018) observed the cardiovascular toxicity by the exposure of cyproconazole at similar duration.
Exposure of dried fish treatment with the concentrations of 50 µg/ml and 100 µg/ml at 72 hpf and 96 hpf in zebrafish embryos showed effectively forms spinal curvature, tail bending, yolk sac edema and lost its normal pigmentation with tail deformities. Shaikh et al. (2019) observed yolk sac flexure and head-trunk angle changes after 72 hpf treated with 250 and 500 ppm, aqueous extract of Ficus glomerata. In 96-h-old zebrafsh embryos, re-sorption was reduced the volume of the yolk sac and the intestinal tract was fully developed (Hellfeld et al., 2020). Schiwy et al. (2015) reported edema in the heart and yolk sac after 72 hpf treated with 1.8 mg/ml verning canal sediment on fish larvae. Hermsen et al. (2011) observed at 72 hpf exposure of triadimefon 300 µM concentration pericardial edema and morphological abnormalities were found. Similar result was also observed by Aksakal and Ciltas (2018) in 96 hpf exposure of 1.6 mg/ml and 2.4 mg/ml penconazole in Danio rerio embryos.
5 Conclusions
The tested dried fishes contain heavy metals as follows: As > Pb > Ni > Cr > Cd. Obtained results revealed that As and Pb concentrations found in all the tested dried fishes were significantly higher than standard Food and Agriculture Organization (FAO) recommended values. Channa punctata and Harpadon nehereus contain high levels of As and Pd. The mean As and Pd concentrations in the current research confirmed the highest concentration level in Pampus chinensis, which slightly beat Bangladeshi rules and regulations on food safety and security. The treatments of C. punctata on zebrafish embryos showed significantly intoxication progresses in developmental abnormalities, embryonic and larval malformation and disability. As the levels of As and Pd found in C. punctata were higher than the standard limit and showing highly toxicity on zebrafish embryos development. The toxicity findings of the present work revealed that C. punctata dried fish available in dried fish market of Cox’s Bazar Sea beach area in Bangladesh contain some toxic substances which is not safe for health.
Funding
This work was financially supported by the Faculty of Biological Sciences (Project grant Number: 101/5/52/RU/life16/20–21), University of Rajshahi, Rajshahi 6205, Bangladesh.
Acknowledgements
The authors are grateful to the Faculty of Biological Sciences, University of Rajshahi, Bangladesh (Grant/Award Number: 101/5/52/RU/life16/20-21) for financial support during the parts of this investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Quality and safety assessment of traditional smoked fish from Lagos State. Nigeria. Int. J. Aqua.. 2015;5(15):1-9.
- [CrossRef] [Google Scholar]
- Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga. Bangladesh. Environ. Sci. Pollut. Res.. 2015;22(20):15880-15890.
- [CrossRef] [Google Scholar]
- Developmental toxicity of penconazole in Zebrfish (Danio rerio) embryos. Chemosphere. 2018;200:8-15.
- [Google Scholar]
- Seasonal behavior and accumulation of some toxic metals in commercial fishes from Kirtankhola tidal river of Bangladesh – A health risk taxation. Chemosphere 2022
- [CrossRef] [Google Scholar]
- Dried fish at the intersection of food science, economy, and culture: a global survey. Fish Fisher.. 2022;00:1-22.
- [CrossRef] [Google Scholar]
- The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut.. 2003;121(1):129-136.
- [CrossRef] [Google Scholar]
- Developmental toxicity of a pymetrozine photo-metabolite, 3-pyridinecarboxaldehyde, in zebrafish (Danio rerio) embryos: abnormal cardiac development and occurrence of heart dysfunction via differential expression of heart formation-related genes. Ecotoxicol. Environ. Saf.. 2023;253(2023):114654
- [CrossRef] [Google Scholar]
- Teratogenic and toxic effects of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (higher Basidiomycetes), on zebrafish embryo as model. Int. J. Med. Mushrooms. 2012;14(5):507-512.
- [CrossRef] [Google Scholar]
- Cytotoxicity and toxicity evaluation of xanthone crude extract on hypoxic human hepatocellular carcinoma and zebrafish (Danio rerio) embryos. Toxics. 2018;6(60):1-10.
- [CrossRef] [Google Scholar]
- Heavy metals in mussels and fish from Italian coastal waters. Mar. Pollut. Bullet.. 1991;22(1):10-14.
- [CrossRef] [Google Scholar]
- Presence of microplastics in two common dried marine fish species from Bangladesh. Mar. Pollut. Bullet.. 2022;176:113430
- [CrossRef] [Google Scholar]
- Adverse efects in the fsh embryo acute toxicity (FET) test: a catalogue of unspecifc morphological changes versus more specific effects in zebrafsh (Danio rerio) embryos. Environ. Sci. Eur.. 2020;32:122.
- [CrossRef] [Google Scholar]
- Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol. In Vitro. 2011;25(3):745-753.
- [CrossRef] [Google Scholar]
- Hossain, M.A.R., Belton, B., Thilsted, S.H. 2015. Dried Fish Value Chain in Bangladesh. World Fish, Bangladesh and South Asia Office, Dhaka, Bangladesh, p. 122.
- Baseline study of heavy metal contamination in the Sangu River estuary, Chattogram. Bangladesh. Mar. Pollut. Bull.. 2019;140:255-261.
- [CrossRef] [Google Scholar]
- Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America) J. Food Compos. Anal.. 2005;18(8):771-787.
- [CrossRef] [Google Scholar]
- Heavy metal concentrations in fish tissues from the Northeast Mediterranean Sea. Bull. Environ. Contamin. Toxicol.. 1999;63(5):673-681.
- [CrossRef] [Google Scholar]
- Carbofuran accelerates the cellular senescence and declines the life span of spns1 mutant zebrafish. J. Cell Mol. Med.. 2021;25:1048-1059.
- [CrossRef] [Google Scholar]
- Development and operation of gold and cobalt oxide nanoparticles containing polypropylene based enzymatic fuel cell for renewable fuels. Biosens. Biolelectron.. 2014;61:500-505.
- [CrossRef] [Google Scholar]
- Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere. 2018;207:781-790.
- [CrossRef] [Google Scholar]
- Zebrafish: a nontraditional model of traditional medicine. J. Ethnopharmacol.. 2013;145(3):677-685.
- [CrossRef] [Google Scholar]
- The effects of henna (hair dye) on the embryonic development of zebrafish (Danio rerio) Environ. Sci. Pollut. Res.. 2014;21(17):10361-10367.
- [CrossRef] [Google Scholar]
- Human health risk assessment for exposure to heavy metals in finfish and shellfish from a tropical estuary. J. King Saud Univ. – Sci.. 2022;34:102035
- [CrossRef] [Google Scholar]
- DarT: the embryo test with the zebrafish Danio rerio-a general model in ecotoxicology and toxicology. ALTEX. 2002;19(Suppl 1):38-48.
- [Google Scholar]
- Determination of trace metal ions by AAS in natural water samples after preconcentration of pyrocatechol violet complexes on an activated carbon column. Talanta. 2000;52(6):1041-1046.
- [CrossRef] [Google Scholar]
- Sensitivity of rainbow trout early life stages to nickel chloride. Env. Toxicol. Chem.. 1985;4(2):233-239.
- [Google Scholar]
- Developmental toxicity evaluation of ethanolic extract of Annona squamosa in zebrafish (Danio rerio) embryo. J. Pharm. Res.. 2012;5(1):277-279.
- [Google Scholar]
- A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res.. 2015;22(21):16305-16318.
- [CrossRef] [Google Scholar]
- Teratogenic effects of aqueous extract of Ficus glomerata leaf during embryonic development in zebrafish (Danio rerio) J. App. Pharma. Sci.. 2019;9(05):107-111.
- [CrossRef] [Google Scholar]
- Legal status of Bangladesh fisheries: issues and Responses. Indian J. Geo-Mar. Sci.. 2016;45(11):1474-1480.
- [Google Scholar]
- Acute and short-term toxicity studies on orange II. Vet. Human Toxicol.. 1987;29(4):300-304.
- [Google Scholar]
- Determination of trace metals in mushroom samples from Kayseri, Turkey. Food Chem.. 2005;92(4):649-652.
- [CrossRef] [Google Scholar]
- Atomic absorption spectroscopic determination of heavy metal concentrations in Kulufo River, Arbaminch, Gamo Gofa. Ethiopia. J. Env. Anal. Chem.. 2016;3(1):177.
- [CrossRef] [Google Scholar]
- Heavy metals in three commercially valuable fish species from Iskenderun Bay, Northern East Mediterranean Sea. Turkey. Food Chem.. 2005;91(1):167-172.
- [CrossRef] [Google Scholar]
- Ethanolic extract of pomegranate (Punica granatum L) peel: acute toxicity tests on zebrafish (Danio rerio) embryos and its toxicity prediction by in silico. J. App. Pharma. Sci.. 2018;8(06):082-086.
- [CrossRef] [Google Scholar]
- The effects of cadmium and copper on embryonic and larval development of ide Leuciscus idus L. Fish Physiol. Biochem... 2014;40:151-163.
- [CrossRef] [Google Scholar]
- Evaluation of ecosystem health and potential human health hazards in the Hangzhou Bay and Qiantang Estuary region through multiple assessment approaches. Environ. Pollut.. 2020;264:114791
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102963.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







