Translate this page into:
Label free detection of vitamin D by microcantilever-based aptasensor
⁎Corresponding author. Alzkhalid@ksu.edu.sa (Khalid E. Alzahrani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Vitamin D deficiency or insufficiency is a growing public health problem. In this work, a novel and sample approach based on microcantilever for measuring the level of 25-hydroxyvitamin D (25(OH)D) was proposed. The Bruno aptamer was utilized to functionalize the microcantilevers, serving as a sensing layer for detecting 25(OH)D. The working principle of the sensor is based on the changes of the microcantilever deflection and frequency as a result of the aptamer removal from the surface of the microcantilever resulting from its interaction with vitamin D. The results showed a linear dependence between vitamin D concentration and the microcantilever deflection and frequency shift in a range of concentrations from 1 to 300 nM. For this biosensor, the limit of detection (LOD) was calculated to be ∼ 0.3 nM. The biosensor selectivity was tested against vitamin C and the sensor showed high selectivity towards vitamin D.
Keywords
Microcantilever
Aptamer
Vitamin D
25-hydroxyvitamin
Aptasensor
1 Introduction
Vitamin D is an essential substance that our body needs for maintaining our overall health and preventing chronic illnesses (Nair and Maseeh 2012, Hossein-nezhad and Holick 2013). A lack of vitamin D can lead to muscle weakness, osteoporosis and rickets in children. Recently, many studies have linked a low level of vitamin D to a high risk of some chronic diseases including diabetes, metabolic syndrome, cancer and cardiovascular diseases (Latic and Erben 2020, Luo et al., 2021, Zittermann et al., 2021, Zhang et al., 2022). However, vitamin D deficiency or insufficiency is a growing public health problem. According to estimates, more than one billion individuals globally suffer from an inadequate level of vitamin D (Tsiaras and Weinstock 2011). Hydroxylation of vitamin D in the liver is a crucial step in the metabolism of the vitamin. When vitamin D (either from dietary sources or synthesized in the skin) enters the bloodstream, it is transported to the liver where it undergoes the first hydroxylation step, mediated by the enzyme 25-hydroxylasem converting vitamin D. into 25-hydroxyvitamin D (25(OH)D) (Chang and Lee 2019, Gupta et al., 2021). The level of vitamin D is usually determined by measuring the concentration of 25(OH)D in the serum(Holick 2007, Wagner et al., 2009), which helps identify if the measured level of vitamin D in the body is within a healthy range, 30–100 ng/ml (75–250 nM) (St-Arnaud et al., 1997, Holick 2007). There are currently several techniques used for detecting the level of (25(OH)D). These techniques include high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent assay (ELISA), chemiluminescent immunoassay (CLIA), radioimmunoassay (RIA) and liquid chromatography-mass spectrometry (HPLC-MS). They are highly sensitive techniques, but its widespread use is constrained by its technical complexity, tedious process and high cost (Van den Ouweland et al., 2010, El-Khoury et al., 2011, Madenci et al., 2017). Moreover, the serum 25(OH)D level obtained by different conventional methods varied significantly (Lips et al., 1999).
To overcome the limitations of the conventional techniques, several biosensors have been developed. Due to their ability to detect and measure very low concentrations of nutrients, toxins, chemical, and other molecules, biosensors have been considered as potential alternatives to the conventional techniques for the detection of vitamin D. The most used biosensors are those based on electrochemical and surface plasmon resonance (SPR) transduction. Chauhan et al. (Chauhan et al., 2018)used an electrochemical biosensor with an electrode modified with Fe3O4 nanoparticles incorporated in PAN nanofibers for vitamin D detection. The PAN nanofibers treated with Fe3O4 nanoparticles was used as a matrix to immobilize vitamin D antibody. The fabricated biosensor showed a low limit of detection (LOD) at approximately 0.12 ng/ml and the linearity from 10 to 100 ng /ml. Carlucci et al. (Carlucci et al., 2013) reported the detection of 25(OH)D using SRP and electrochemical biosensors. SRP was employed to directly estimate the vitamin D concentration using vitamin D antibody, obtaining LOD of 2 μg/ml which is insufficient for clinical analysis. However, the sensitivity of SRP was further improved in an indirect way by Vitamin D binding protein that leads to LOD of 45 ng/ml. They also constructed an electrochemical biosensor based on screen-printed gold electrode with 4-ferrocenylmethyl-1,2,4-triazoline-3,5-dione (FMTAD) modification. This senor showed linear detection of vitamin D in the range from 20 to 200 ng/ml with a LOD at about 10 ng/ml. In another study, an enzyme-modified electrode to detect 25(OH)D (Ozbakir, Sambade et al. 2016). The enzyme (CYP27B1) was immobilized on glassy carbon electrodes, and the fabricated sensor demonstrated the successful detection in the range of 5–200 ng/ml.
Microcantilevers are widely used in the field of sensing and detection due to their high sensitivity and versatility, enabling the detection of a wide range of analytes such as biological molecules, gases, and chemicals (Ji et al., 2000, Rogers et al., 2003, Lavrik et al., 2004; Lam et al., 2023; Wang et al., 2023). The microcantilever as a biosensor holds much promise for the future. Its small size, portability, and ease of use make it ideal for many applications in health care and medical diagnostics. The microcantilever can be fabricated with different surface coatings that are selective for specific biomolecules. By modifying the surface chemistry of the microcantilever, different interactions can be detected such as DNA hybridization, protein–protein interactions, and small molecule-protein interactions (Wang et al., 2023, Zhang et al., 2023a, Zhang et al., 2023b). By measuring the cantilever response to different stimuli, it can give quantitative data regarding the characteristics of a sample. The microcantilever-based biosensors are relatively affordable and offer unique advantages over traditional testing methods, including high sensitivity, low cost and rapid response.
In recent years, aptamers have been gaining increasing attention as promising alternatives to traditional antibodies in various applications, including biosensing, drug delivery, and therapeutics. Their high affinity and selectivity for specific targets, combined with their versatility and ease of synthesis, make them attractive candidates for a wide range of biomedical and environmental applications. Aptamers are a short, single-stranded DNA or RNA molecule that can bind to a specific target molecule, such as a protein or small molecule., with high affinity and specificity. The biosensors that use aptamers as the recognition element are called aptasensors. They are capable of rapidly recognizing and binding to the target molecules, causing a detectable signal that can be used for sensing in a variety fields, such as in biotechnology, medicine, and environmental science (Zhang et al. 2016, Xiao et al., 2019).
Aptasensors have been used for detecting vitamins. Lee et al. (Lee and Gu 2017) reported the development of gold nanoparticles- based colorimetric aptasensor for the detection of 25(OH)D using VDBA14 aptamer. The limit of detection was determined to be 1 µM. A graphene oxide-based aptasensor was developed for the detection of 25(OH)D (Gupta et al., 2021). The reaction of fluorescently tagged aptamers with the graphene oxide resulted in the quenching of the fluorescence which can be recovered by adding 25(OH)D. The limit of detection achieved by this aptasensor was estimated to be ∼ 150 ng/ml. Using a target-induced dissociation-based aptasensor, the level of 25(OH)D in buffer was estimated (Prante et al., 2019). Aptamers specific to 25(OH)D were immobilized on a glass slide and then hybridized with a complementary oligonucleotide sequence that was fluorescently tagged. A decrease in fluorescence was observed because of the complementary strand's dissociation in the presence of 25(OH)D. The limit of detection measured by the aptasensor was 5.4 nM. One study established a highly sensitive detection approach using a fluorescence-based assay modified with a highly specific aptamer directed against 25(OH)D (Alyamani et al., 2019). The best detection of limit that achieved using this method was 1 fM.
In this study, we employed an aptamer as a recognition element immobilized on the surface of microcantilever for the detection of vitamin D. The working principle of the biosensor is based on the dissociation of aptamer from the surface of microcantilever upon the interaction with the vitamin D molecules causing a change in the microcantilever deflection and resonance frequency that can be utilized for estimating the vitamin concentration. The limit of detection using this method was estimated to be ∼0.3 nM.
2 Material and methods
2.1 Materials
Silicon microcantilevers with a length of 300 µm, width of 80 µm and thickness of 1 µm were purchased from Micromotive Gmbh (model:Oto 500S, Germany). Phosphate Buffer Saline (PBS) and was provided by Fisher Scientific and used for washing. 25(OH)D was purchased from Carbosynth Limited (Berkshire, UK). The stock solution of vitamin (1 mM) was prepared in an aqueous solution of methanol in water (5 %). The desired concentrations were obtained by dilution the stock solution using water and methanol (5 %). The use of methanol with water due to the hydrophobic nature of 25(OH)D (Daiger et al., 1975). The aptamer targeting 25(OH)D, known as Bruno aptamer, was synthesized by Operation Technology Inc, and its sequence is kept as a trade secret of Operation Technology Inc. under the inventory code: OTC Biotech Catalog number 070, VDA. The aptamer was dissolved in in deionized water (Milli-Q) and kept at −20 °C before use.
2.2 Microcantilever functionalization
The microcantilevers were washed in a piranha solution for 3 min and then rinsed with deionized water to remove organics. The microcantilevers on one side were coated by a 40 nm layer of gold using thermal evaporation system (Oerlikon, leybold vacuum, Germany). Prior to gold deposition on the microcantilever, a ∼ 5 nm layer of chromium was vacuum evaporated to serve as adhesion layer between gold and silicon. Next, the gold-coated microcantilevers were washed several times using absolute ethanol. After washing, the microcantilevers were immersed in a solution containing the aptamer (1 mL, 1 -15 M) and incubated for 3 h at room temperature. The aptamer-modified microcantilever was taken out, thoroughly washed with PBS, and dried gently with nitrogen gas.
2.3 Measurements
The detection system used to monitor the change in the microcantilever’s bending is based on an optical technology integrated with the Picomeasure PM3 system (FOURIEN, CANDA). In this system, a laser beam focused on the backside of the microcantilever is reflected off onto a four-quadrant photodiode, and then the output signal is used to record the microcantilever’s deflection and resonance frequency. After the microcantilever was functionalized by Bruno aptamer, it was inserted into a measurement cell fabricated for this experiment. The desired concentration of the vitamin was then injected into the measurement cell to interact with the aptamers immobilized on the surface of the microcantilever, and then washed by injecting deionized water. The deflection/ resonance frequency of the microcantilever was recorded in-situ, and the differential deflection (or change in the resonance frequency) was obtained by subtracting it from that of the aptamer-modified microcantilever.
2.4 Surface characterization
The contact angle measuring system (Attension T330, Biolin Scientific) was used to measure the surface wettability/hydrophilicity. To perform this, droplets of approximately 3 μL of deionized water were dispensed onto the surface using a microliter syringe and allowed to equilibrate before the contact angle was measured at the point of intersection with the surface. To ensure the consistency of the measurements, the experiment was repeated at least five times in different locations on the surface. The measurements were conducted on three different surfaces: gold coated surface, gold coated surface modified with the aptamer, and gold coated surface modified with the aptamer and treated with 300 nM of 25(OH)D. The Fourier Transform Infrared (FTIR) spectrum of the aptamer-modified surface before and after adding 25(OH)D was acquired using FTIR (Nicolet 6700 FTIR spectrophotometer).
3 Results and discussion
The process of immobilizing aptamer onto the gold-coated microcantilever is influenced by both the specific properties of the aptamer and the surface chemistry of gold. Aptamers, typically short strands of single-stranded DNA or RNA. Since this aptamer lacks a thiol label, it could only interact with the gold surface through its nucleobases. These nucleobases possess the capacity to engage with gold atoms, most likely through relatively weak van der Waals forces and electrostatic interactions (Liu, 2012; Farrokhpour et al., 2019). These interactions are not as strong or stable as covalent bonds, but they can still facilitate stable binding between the aptamer and the gold surface. This interaction entails a bond between the metal atoms on the gold surface and the nitrogen or oxygen atoms within the nucleobases of the aptamer. The strength of this interaction dictates the stability of the immobilization process. If the bond between the aptamer and the target molecule proves to be stronger than the interaction between the aptamer and the gold surface, the likelihood of the aptamer dissociating from the surface increases. It can be easily displaced from the surface when it recognizes and binds with target molecules (vitamin D). As illustrated in Fig. 1, the detection mechanism is based on the removal of the aptamer from the microcantilever surface generated by the aptamer-vitamin interactions. The removal of the aptamer from the microcantilever surface leads to a decrease in the number of aptamers absorbed to the surface which in turn results in a shift in the microcantilever resonance frequency and generating a differential surface stress causing the microcantilever to bend or deflect. The deflection of the gold-coated microcantilever and its resonance frequency were set as a reference for the subsequent measurements.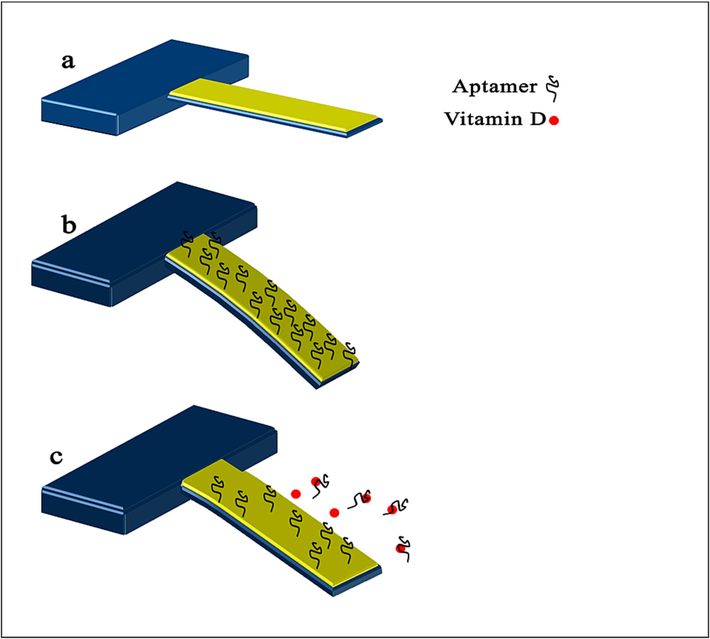
Schematic showing the experimental principle.
To confirm the successful immobilization/removal of the aptamers from the microcantilever surface, contact angle method is employed. The change in contact angle of a surface functionalized with aptamer before and after adding vitamin refers to the alteration in the wetting behavior of the surface due to the interaction between the aptamer and the vitamin. Contact angle is the angle formed at the interface between a liquid and a solid surface, measured through the liquid droplet's curvature at the point of contact with the surface. The contact angle is an indicator of the surface's hydrophobicity or hydrophilicity. A high contact angle indicates that the surface is hydrophobic, and the liquid droplet beads up on the surface, while a low contact angle indicates that the surface is hydrophilic, and the liquid droplet spreads out over the surface.
As shown in Fig. 2, the contact angle value measured for bare gold has been found to be approximately 88° (Fig. 2a), and for aptamer/Au, it is 56° (Fig. 2b). In the literature, it has been reported that gold-coated surfaces have a contact angle of approximately 90°(Davis et al., 2003; Zina et al., 2018). The aptamer-modified surface exhibited lower contact angle compared to that of the bare gold surface. Coating the surface with the aptamer is expected to result in a decrease in the contact angle value due to the hydrophilic nature of aptamers(Hasegawa et al., 2016, Witt et al., 2019). A decrease in contact angle values confirms a change in surface wettability and thus the presence of the aptamer on the surface, indicating that the aptamer was successfully immobilized on the gold coated microcantilever. The contact angle increased after the addition of 25(OH)D) (at a concentration of 300 nM), which can be attributed to the removal of the aptamer from the surface.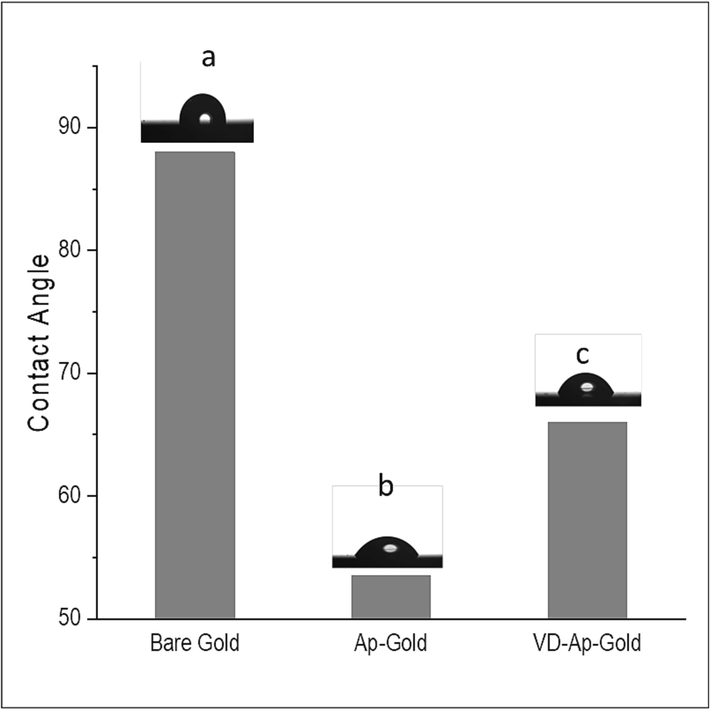
Contact angle of the different stages of process. (a) Bare gold, (b) Aptamer-functionalized gold surface, (c) Aptamer-functionalized gold surface following treatment of 25(OH)D.
For further confirmation, Fourier transform infrared (FT-IR) spectra were recorded before and after the addition of 25(OH)D, as shown in Fig. 3. In general, aptamers are short, single-stranded DNA or RNA molecules that can recognize and bind to specific targets, such as proteins or small molecules, with a high level of specificity and affinity Nucleotide bases such as adenine, cytosine, guanine, and uracil are typically present in aptamers, which can be detected by analyzing the Fourier-transform infrared (FTIR) spectra. The characteristic peaks in the 1200–1800 cm−1 range indicate the presence of these bases (Saito et al., 2012, Han et al. 2018).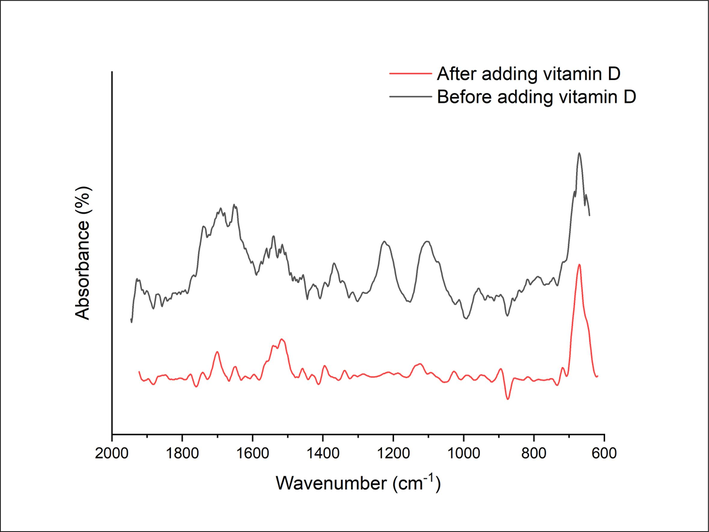
FTIR spectrum of a gold coated microcantilever modified with the aptamer before and after adding the vitamin D at a concentration of 300 nM.
Fig. 3 shows the FT-IR spectra before and after removing the aptamer from the surface of a gold-coated microcantilver in the region from 600 to 2200 cm−1. Before removing the aptamer, the FTIR spectrum has characteristic peaks corresponding to the functional groups present in the aptamer. The FT-IR spectrum of the aptamer immobilized on the gold-coated microcantilever surface illustrated the expected peaks of the aptamer and the overlapping peaks with slight shifts. The peaks at 1363 and 1531 cm−1 were assigned to carboxylate symmetric and asymmetric stretching bands, respectively, in aptamer molecules. The peaks between 1550 and 1750 cm−1 correspond to C⚌O band and heterocyclic moieties of nucleotide units in the aptamer. Furthermore, there were some peaks in the region from 800 to 1100 cm−1 attributed to the vibrations bands of C—O and C—H. The presence of these peaks in the spectrum would indicate that the aptamer is successfully immobilized on the surface of the microcantilever. After adding vitamin D, the FTIR spectrum would likely show a decrease or absence of the peaks corresponding to the functional groups present in the aptamer. The absence of peaks corresponding to the functional groups present in the aptamer would indicate that the aptamer has been successfully removed from the surface of the microcantilever. By comparing the FT-IR spectra before and after removal of the aptamer, one can confirm the success of the removal process.
Once the immobilization of the aptamer on the microcantilever was confirmed, the biosensor was exposed to different concentration of 25(OH)D ranging from 1 nM to 300 nM. The binding of 25(OH)D to the aptamer immobilized on the surface of the microcantilever resulted in the removal of the aptamer from the surface. The removal of aptamer causes a reduction in the surface stress of the microcantilever leading to a decrease of the bending or deflection of the microcantilever. The aptamer binding to or dissociating from the microcantilevers can alter the microcantilever’s surface stress, which is then immediately translated into a gradual change in the microcantilever deflection (known as static deflection). Initially, the immobilization of aptamers induced more deflection in the downward direction compared to those only gold-coated microcantilevers. By increasing the vitamin D concentrations, more aptamers were removed from the microcantilever surface, and more deflection in the upward direction can observed. Fig. 4 illustrates the relationship between the microcantilever deflection and the vitamin D concentration, 25(OH)D. The deflection was calculated by subtracting the deflection of aptamer-functionalized microcantilevers before and after exposure to 25(OH)D. As can be show in Fig. 4, the relationship is linear. The concentration of 25(OH)D as low as 1 nM can be easily detected by this biosensor. The deflection of the microcantilever produced by treating the microcantilever with 1 nM of 25(OH)D was estimated to be ∼65 nm, which is by far above the noise level induced by the microcantilever thermal fluctuation. The limit of detection (LOD) for this mechanical biosensor is calculated to be 0.26 nM. Table 1 illustrated the evaluation of microcantilever sensors in comparison to other techniques for detecting the level of vitamin D.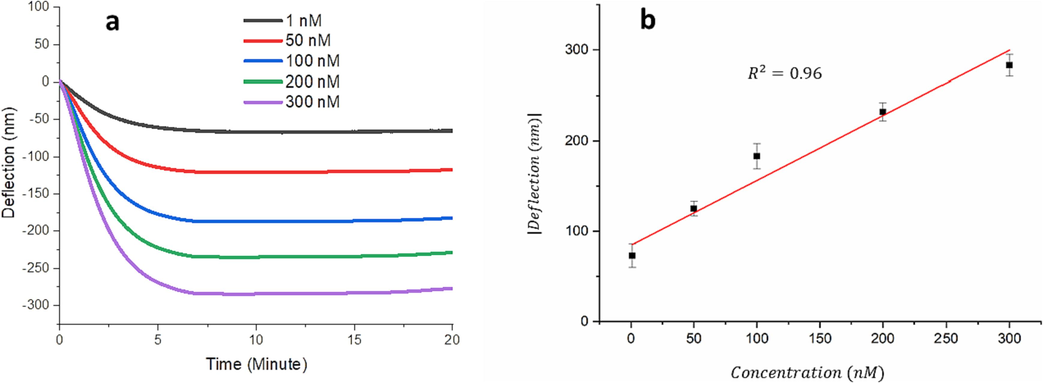
(a) Aptamer-functionalized deflection curves of microcantilevers treated with different concentrations of the vitamin D as a function of time. (b) Calibration curve of the differential deflection of the aptamer-functionalized microcantilver as a function of the vitamin D concentration. The error bars display the standard deviation of data (n = 3).
Analytical method
Recognition element
Concentration range
Limit of detection
References
Electrochemical
Antibody specific to vitamin D
0.01–106 pg/ml
0.23 pg/ml
(Kaur et al., 2023)
Electrochemical
Antibody specific to vitamin D
7.4–70 ng/ml
2.4 ng/ml
(Polli et al., 2023)
Electrochemical
Carbon dots-modified chiston and vitamin D antibody
10–50 ng/ml
1.35 ng/ml
(Sarkar et al., 2018)
Optical
Antibody specific to vitamin D
5–50 µg/ml
2 µg/ml
(Carlucci et al., 2013)
Electrochemical
Vitamin D binding protein
20–200 ng/ml
45 ng/ml
(Carlucci et al., 2013)
Electrochemical
The enzyme (cyp27b1)
5–200 ng/ml
10 ng/ml
(Ozbakir et al., 2016)
Optical
VDBA14 aptamer
0.4–20 µg/ml
0.4 µg/ml
(Lee and Gu 2017)
Mechanical
Bruno aptamer
0.4–120 ng/ml
0.12 ng/ml
This work
In addition to the deflection, the microcantilever showed a frequency response. Microcantilevers with similar mechanical properties to those utilized in the deflection measurements were used for the frequency measurements. Prior to performing the experiments, resonance frequency of the gold-coated microcantilever was recorded as shown in Fig. 5. The resonance frequency of the gold-coated microcantilever was found to be ∼5690.61 ± 1.201 Hz. However, after immobilizing the aptamer onto the microcantilever surface the resonance frequency of the microcantilever dropped by ∼5669.902 ± 1.239 Hz. This reduction in the resonance frequency ensures that the aptamer was effectively attached to the microcantilever. The aptamer-modified microcantilevers show an increase in resonance frequency with exposure to increasing concentrations of 25(OH)D. This behavior is in well agreement with that observed with the microcantilever deflection. The microcantilever response can be attributed to the change in the mass loading caused by the removal of aptamer. As the 25(OH)D concentration increases, a further decrease in the aptamer mass adsorbed on the microcantilever surface was obtained, leading to a higher frequency shift. By evaluating the shift in the microcantilever resonance frequency, we were able to approximate the concentration of 25(OH)D. Fig. 5b shows a plot of resonance frequency as a function of 25(OH)D concentration in the range of 1–300 nM. As illustrated in Fig. 5b, the frequency shift of the microcantilever to 300 nM 25(OH)D is approximately 15 Hz, while its response to 1 nM of 25(OH)D is approximately 5 Hz. The limit of detection and the sensor sensitivity were measured to be ∼0.3 nM.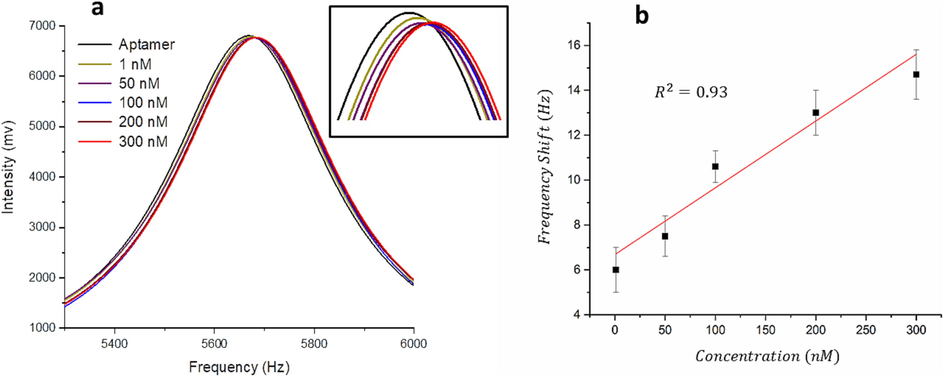
(a) Aptamer-functionalized resonance frequency curves of microcantilevers treated with different concentrations of the vitamin D as a function of time. (b) Calibration curve of the frequency shift of the aptamer-functionalized microcantilver as a function of the vitamin D concentration. The error bars display the standard deviation of data (n = 3).
To investigate the specificity of the biosensor towards 25(OH)D, three control experiments were carried out and the results are depicted in Fig. 6. The aptamer-modified microcantilevers were exposed to vitamin C (at a concentration of 300 nM), cholesterol at a concentration of 300 nM and the blank solution. The blank solution in which this experiment was performed is the solvent used to dilute the vitamin. The blank solution was prepared under the same conditions and using the same equipment as the sample solution to ensure consistency in the biosensor measurements. In contrast to 25(OH)D, no significant changes in the microcantilever deflection/frequency were observed, as shown in Fig. 6. It follows that the aptamer used in the study has minimal interaction with vitamin C and cholesterol, and any alterations observed in the microcantilever deflection or frequency are solely attributed to the presence of 25(OH)D. Using both cholesterol and vitamin C as control experiments helped to ensure the accuracy and reliability of the results obtained from the experiment. The high degree of structural similarity between cholesterol and 25(OH)D is noteworthy, as it indicates that the aptamer used in the study has a high specificity for 25(OH)D.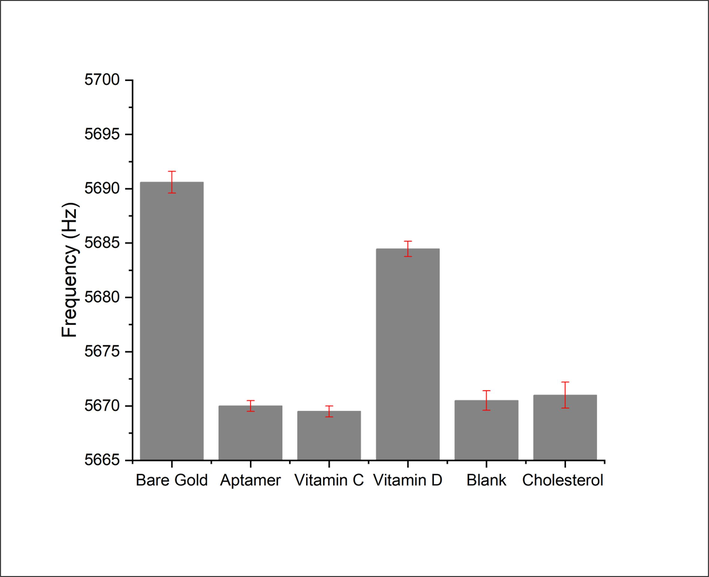
Experiments conducted to assess the aptamer-based assay's specificity.
4 Conclusion
The detection of vitamin D through biosensors remains to be challenging. In this study, a label-free microcantilever biosensor, incorporating the Brun aptamer for specifically identifying 25(OH)D, was designed, developed, and tested for the accurate detection of vitamin D. The change in the microcantilevers deflection and frequency were linearly dependent on 25(OH)D concentration over the range from 1 to 300 nM. Notably, the demonstrated high selectivity towards 25(OH)D over substances like vitamin C and with closely related chemical structures such as cholesterol highlights the biosensor’s exceptional specificity. With a limit of detection of approximately 0.3 nM, this biosensor exhibits promising capabilities for sensitive and accurate vitamin D quantification. Investigating the biosensor’s performance in complex biological matrices, such as serum or plasma, would be crucial to assess its feasibility in real-world applications. With its notable stability, ease of use, and low detection limit, the biosensor is a highly promising candidate for point-of-care diagnostics.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-277)”
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Label-free fluorescent aptasensor for small targets via displacement of groove bound curcumin molecules. Sensors. 2019;19(19):4181.
- [Google Scholar]
- Several approaches for vitamin D determination by surface plasmon resonance and electrochemical affinity biosensors. Biosens. Bioelectron.. 2013;40(1):350-355.
- [Google Scholar]
- Vitamin D and health-The missing vitamin in humans. Pediatr. Neonatol.. 2019;60(3):237-244.
- [Google Scholar]
- Electrochemical immunosensor based on magnetite nanoparticles incorporated electrospun polyacrylonitrile nanofibers for Vitamin-D3 detection. Mater. Sci. Eng. C. 2018;93:145-156.
- [Google Scholar]
- Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc. Natl. Acad. Sci.. 1975;72(6):2076-2080.
- [Google Scholar]
- Ionic strength effects on hexadecane contact angles on a gold-coated glass surface in ionic surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2003;221(1–3):69-80.
- [Google Scholar]
- Progress of liquid chromatography-mass spectrometry in measurement of vitamin D metabolites and analogues. Clin. Biochem.. 2011;44(1):66-76.
- [Google Scholar]
- Directional affinity of a spherical Gold nanoparticle for the adsorption of DNA bases. Colloids Surf. B: Biointerfaces. 2019;173:493-503.
- [Google Scholar]
- Graphene oxide and fluorescent aptamer based novel biosensor for detection of 25-hydroxyvitamin D 3. Sci. Rep.. 2021;11(1):23456.
- [Google Scholar]
- Key factors in FTIR spectroscopic analysis of DNA: the sampling technique, pretreatment temperature and sample concentration. Anal. Methods. 2018;10(21):2436-2443.
- [Google Scholar]
- A novel self-assembled monolayer (SAM) coated microcantilever for low level caesium detection. Chem. Commun.. 2000;6:457-458.
- [Google Scholar]
- MC-Au/MSS-Z8 porous network assisted advanced electrochemical immunosensing of 25-hydroxyvitamin D3. Talanta. 2023;257:124376
- [Google Scholar]
- Vitamin D and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int. J. Mol. Sci.. 2020;21(18):6483.
- [Google Scholar]
- Cantilever transducers as a platform for chemical and biological sensors. Rev. Sci. Instrum.. 2004;75(7):2229-2253.
- [Google Scholar]
- Highly sensitive detection of 25-HydroxyvitaminD3 by using a target-induced displacement of aptamer. Biosens. Bioelectron.. 2017;88:174-180.
- [Google Scholar]
- An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos. Int.. 1999;9:394-397.
- [Google Scholar]
- Adsorption of DNA onto gold nanoparticles and graphene oxide: surface science and applications. Phys. Chem. Chem. Phys.. 2012;14(30):10485-10496.
- [Google Scholar]
- Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people. J. Nutr.. 2021;151(1):98-103.
- [Google Scholar]
- Evaluation of new Beckman Coulter 25 (OH) Vitamin D assay and potential improvement of clinical interpretation. Biochemia Medica. 2017;27(2):332-341.
- [Google Scholar]
- Detection of 25-Hydroxyvitamin D3 with an Enzyme modified Electrode. J. Biosens. Bioelectron.. 2016;7(01)
- [Google Scholar]
- ASu@ MNPs-based electrochemical immunosensor for vitamin D3 serum samples analysis. Talanta. 2023;251:123755
- [Google Scholar]
- Characterization of an Aptamer directed against 25-hydroxyvitamin D for the development of a competitive aptamer-based Assay. Biosensors. 2019;9(4):134.
- [Google Scholar]
- Mercury vapor detection with a self-sensing, resonating piezoelectric cantilever. Rev. Sci. Instrum.. 2003;74(11):4899-4901.
- [Google Scholar]
- Study of DNA–emodin interaction by FTIR and UV–vis spectroscopy. J. Photochem. Photobiol. B Biol.. 2012;111:59-63.
- [Google Scholar]
- Carbon dots-modified chitosan based electrochemical biosensing platform for detection of vitamin D. Int. J. Biol. Macromol.. 2018;109:687-697.
- [Google Scholar]
- The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J. Bone Miner. Res.. 1997;12(10):1552-1559.
- [Google Scholar]
- Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J. Chromatogr. B. 2010;878(15–16):1163-1168.
- [Google Scholar]
- An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin. Biochem.. 2009;42(15):1549-1556.
- [Google Scholar]
- Production of polycaprolactone nanoparticles with hydrodynamic diameters below 100 nm. Eng. Life Sci.. 2019;19(10):658-665.
- [Google Scholar]
- Rationally engineered nucleic acid architectures for biosensing applications. Chem. Rev.. 2019;119(22):11631-11717.
- [Google Scholar]
- Association of serum 25-hydroxyvitamin D with cardiovascular outcomes and all-cause mortality in individuals with prediabetes and diabetes: results from the UK biobank prospective cohort study. Diabetes Care. 2022;45(5):1219-1229.
- [Google Scholar]
- Detection of avian influenza virus H9N2 based on self-driving and self-sensing microcantilever piezoelectric sensor. Chin. Chem. Lett.. 2023;34(4):107700
- [Google Scholar]
- A genosensor based on the modification of a microcantilever: a review. Micromachines. 2023;14(2):427.
- [Google Scholar]
- Progress in graphene-based optical and electrochemical aptasensors. Surf. Chem. Nanobiomater. 2016:393-431.
- [Google Scholar]
- Novel sensitive impedimetric microsensor for phosphate detection based on a novel copper phthalocyanine derivative. Anal. Lett.. 2018;51(3):371-386.
- [Google Scholar]
- Vitamin D and cardiovascular disease: an updated narrative review. Int. J. Mol. Sci.. 2021;22(6):2896.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102951.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







