Translate this page into:
Biodegradation of chromium by laccase action of Ganoderma multipileum
⁎Corresponding author. ash.dr88@gmail.com (Aisha Umar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Laccase is a fungal enzyme that play a crucial role in bioremediation. The purified laccase from Ganoderma multipileum and its effectiveness in bioremediation of Cr (VI) was determined in this study. Two strains of G. multipileum were identified by ITS sequences and their phylogeny was compared with G. multipileum taken from GenBank (KF494997, LC149613, MG739453, MG739455). The fungi were grown on guaiacol substrate for laccase optimization using different environmental and nutritional conditions. Laccase Glacc113 (75 kDa) was partially purified and characterized under different parameters. Glacc113 (GIAPTAD) was confirmed by using a Precise Protein Sequencing System to analyze sequence of N-terminal amino acid. Laccase exhibited maximum optimal activity (1355.5 ± 8.8 U/L) at pH 3.0 and can tolerate the maximum temperature upto 70 °C. During submerged fermentation, on 7th day after inoculum of 3 fungal discs at 100 rpm yielded maximum laccase. The production of laccase increased by optimization of inorganic and organic nitrogen and carbon sources. The purified laccase from G. multipileum was used to reduce (>94%) 100 μg/mL of Cr (VI) into less toxic chromium Cr (III). The catalytic kinetic parameters Vmax and Km for guaiacol were 1.817 (mM min−1) and 1.4617 (mM), respectively. This study determined the conditions that enhance production and an ecofriendly approach to bio remediate the Cr (VI) to Cr (III). The purified enzyme exerted maximum durability and reliability for industrial usage also.
Keywords
Bioremediation
Characterization
Guaiacol
Laccase
Nutritional parameters
Kinetics
- Cr
-
Chromium
- ITS
-
Internal Transcribed Spacer
- kDa
-
Kilo Dalton
- U/L
-
Enzyme Activity Unit/L
- CTAB
-
Cetyl Trimethylammonium Bromide
- BLAST
-
Basic Local Alignment Search Tool
- NCBI
-
National Center for Biotechnology Information
- MAFFT
-
Multiple Alignment using Fast Fourier Transform
- MEGA
-
Molecular Evolutionary Genetics Analysis
- OS
-
Organic Sources
- RA
-
Relative Activity
Abbreviations
1 Introduction
Ganoderma multipileum, commonly known as lingzhi or chizhi belongs to the family Ganodermataceae (Wang et al., 2009; Bhosle et al., 2010). The species of this genus are widely grown on a commercial scale due to its medicinal properties and commonly use in traditional medicines (Zhou et al., 2015). Multiple biological activities of the genus Ganoderma are due to its secondary metabolites including lanostane triterpenoids, meroterpenoids, ergostane steroids and farnesyl hydroquinones. Major lanostanes extracted from G. multipileum are ganoderic acid AM1, ganodermanondiol 24, 25-acetonide, lucidumol A, B, ganoderiol F, ganoderitriol M, ganodermanontriol and 7-oxoganoderic acid (Binh et al., 2018).
G. multipileum produces laccase, a ligninolytic and extracellular enzyme belonging to the family oxidoreductase (Alfarra et al., 2013). It accommodates a broad range of substrates viz: diphenols, polyaromatic amine and iodine as well as phosphates, ketones, ascorbate and lignin (Munk et al., 2017; Rodrigues et al., 2019). Laccase is a metalloenzyme with a wide range of activities such as azodye oxidation, xenobiotic degradation, pollutant detoxification, steroid transformation as well as pharmaceutical products formation and degradation (Tortella et al., 2013; Litwińska et al., 2019). Many other laccase producing wood rotting fungi with multiple applications are Pleurotus sajor-caju, P. ostreatus, P. ostreatus POXA1, Trametes trogii POXL3, Pycnoporous cinnabarius, Coriolus hirsutus, and Ganoderma lucidum (Shin and Lee, 2000a, 2000b; Soden et al., 2002).
In low quantities, a few heavy metals are necessary for life, but as concentrations rise, they become poisonous. Their high concentrations cause allergy, carcinogenicity and sometimes inhibit the enzymes activities (Koropatrick and Leibbrandt, 1995). Exposure to environmental or natural concentrations of chromium are hardly hazardous to human health. Natural occurrence in plants, soils and its inclusion in animal feed, Cr (III) is part of the human diet (Pavesi and Josino, 2020). Chromium heavy metal toxicity poses a great threat to the environment. Soil, air and water are heavily contaminated by Cr (VI) released by chrome-plating, steel manufacturing, anti-corrosion agents, leather tannery, textiles, dyes and pigments (Gu et al., 2015). The compounds contain Cr (VI) are mutagenic and carcinogenic; and poses serious injuries to the ecosystem with serious health issues in humans, animals and marine life (Sandana et al., 2015). Cr (VI) easily penetrates the red blood cells (RBCs) due to its bioavailability and gets converted to Cr (III), which sticks to the cellular components of RBC (Shekhawat et al., 2015).
It is critical to comprehend in-depth that the reduction conditions in order to reassemble the higher quality of chromium toxicity. A variety of functional groups in fungal species provide a great biosorbent capacity in heavy metal remediation. Moreover, fungi grow naturally in heavily polluted environments (Zapana-Huarache et al., 2020). A few fungal species especially laccase from filamentous fungi (Trichoderma viride, A. fumigatus, A. awamori, Fusarium proliferatum, Penicillium radicum, Beauveria bassiana, Phanerochaete chrysosporium etc.) indicated in literature with great potential for heavy metals bioremediation (Joshi et al., 2011). Similarly, Streptomyces sp. is a stronger candidate for the remediation of chromium containing effluents (Shazia et al., 2013). Isolates of dark septate endophytic fungi exhibited an efficient removal capacity (99% of 50 mg/L) of Cr (VI) (Melati et al., 2023). In literature, there are a few reports on laccase treated chromium tolerant wood rotters e.g., Phlebia brevispora and P. floridensis (white rot fungi) effectively removed the chromium from industrial wastewater (Sharma et al., 2023). Table 1 indicated the fungal species biodegraded the heavy metals via action of bio products e.g., laccase.
Sr No.
Fungal species
Heavy metals removal (%)
References
1
Aspergillus sp.
53.94 and 52.54% reduction of As
Tanvi et al., 2020
2
A. flavus
89.1% reduction of Cr
Kumar and Dwivedi, 2019
3
A. fumigatus
76.07% Pb, 69.6% Cu, 40.0% Cr
Shazia et al., 2013
4
A. candidus
60% As
Vala, 2010
5
A. fumigatus ML43
95% reduction of Cr
Hussain et al ., 2018
6
A. tamarii
58.60% Cr
Kamal et al., 2023
7
A. ustus
57.00% Cr
Kamal et al., 2023
8
Penicillium radicum
95% reduction of Cr
Hussain et al ., 2018
9
Beauveria bassiana
67.8% Zn(II), 74.135 Cu(II), 63.4% Cd(II), 61.13% Cr(VI), 75% Ni(II)
Gola et al., 2016
10
Trametes hirsuta
96% Cr (VI)
Liu et al., 2020a
11
Penicillium citrinum
Cr (VI) tolerance
Zapana-Huarache et al., 2020
12
Trichoderma viride
Cr (VI) tolerance
Zapana-Huarache et al., 2020
In view of the above literature and environmental problem, Ganoderma species are one of the most important ornamental degrader of heavy metals pollutants, but no or a very few works available on this achievement. The present investigation was taken to investigate the ability of a laccase from newly explored wood rotting fungal species to deal the chromium metal and to study the efficiency of metal removal from the liquid medium. This study also suggests its high potential for effluents bioremediation and biotechnological usage.
G. multipileum is a key player in the bioremediation process. The presence of a functional Cr (VI) reducing mushroom is a necessary pre-requisite for developing a bioremediation technique for Cr detoxification (VI). This study drop down the Cr (VI) to a less toxic Cr (III) via a novel purified laccase under less cost-effective and eco-friendly technique. The findings from this study will be a new report, which will decipher the significance of G. multipileum laccase in bioconversion of toxic Cr (VI) to less toxic Cr (III) state without any pollution.
2 Materials and methods
2.1 Sample collection and molecular identification
Samples were collected from Pakistan in 2018 during the monsoon season and dried by a dehydrator (Fig. 1A). Modified CTAB method was applied to extract the total genomic DNA of the specimen (Doyle and Doyle, 1987). The nuclear ribosomal ITS region was amplified using the primers ITS1 (5′ CTTGGTCATTTAGAGGAAGTAA‘3) and ITS4 (5′TCCTCCGCTTATTGATATGC‘3) (White et al., 1990). BioEdit version 7.2.5 used to create the consensus of sequences and BLASTn was used to examine homology at the National Center for Biotechnology Information (NCBI). The sequences generated during this study were deposited in GenBank and assigned accession numbers.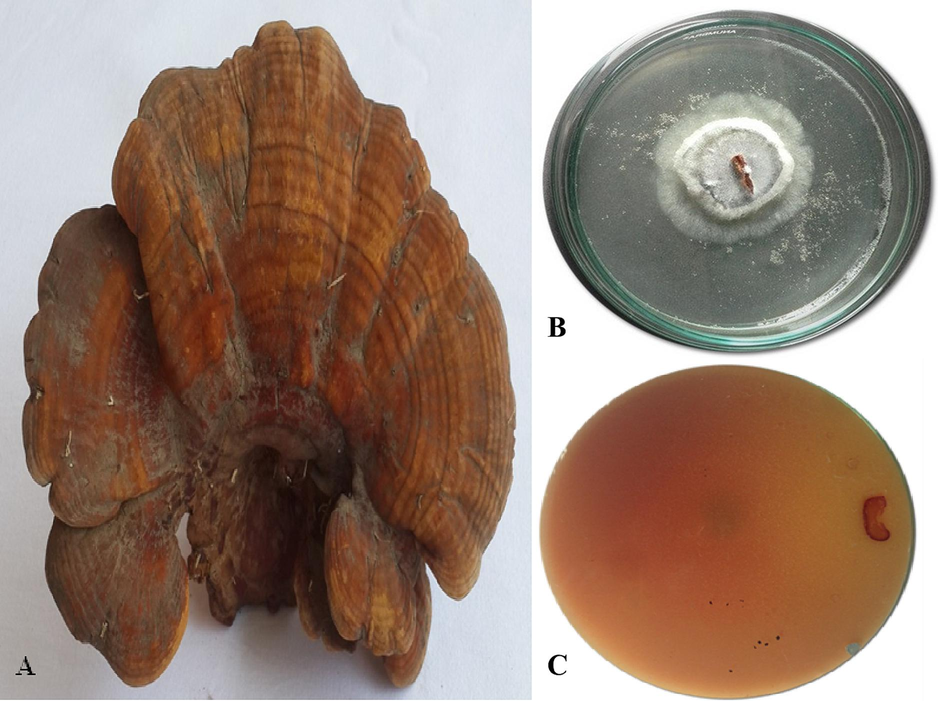
Pictures showing Ganoderma multipileum (CM10): A. Basidiome, B. Pure culture and C. Guaiacol plate medium (bottom view) (Photos taken by Aisha Umar).
2.2 Phylogenetic analysis
A dataset of ITS-based accessions was acquired from GenBank based on published literature. To align and edit the sequences, we utilized ClustalX 2.1 and BioEdit (Hall, 1999; Larkin et al., 2007). MAFFT v. 10 (Katoh and Standley, 2013) was used to manually align the downloaded and newly produced sequences at 593 location. The maximum likelihood technique with 1000 bootstrap replicates was used to create the phylogenetic tree using these sequences representing 15 taxa in MEGA 10.0 software. Out-group of Tomophagus colossus and the obtained tree less than 50% bootstrap was buckled.
2.3 Qualitative plate screening of laccase production
The MEA (Malt Extract Agar) media was made in g.L-1 by adding ME 7, Agar 10, MgSO4·7H2O 0.5, K2HPO4 0.5, KH2PO4 0.5, ZnSO4, 0.005, MnSO4 0.05, Peptone 2.5 and Glucose 15 at pH 5.0 (Fig. 1B) (White et al., 1990). Streptomycin (200 mg.L-1) an antibacterial agent was added and sterilized for 20 min at 121 °C, allowed to cool down for 15 min and then add 0.02% guaiacol for laccase screening (Fig. 1B). This media was transferred into Petri plates (to get hard) and 2 to 3 mm of pure fungal disc inoculated onto each plate for 5 days at 30 °C. The laccase-producing G. multipileum was screened by formation of a reddish brown oxidation zone. (Fig. 1C).
2.4 Quantitative analysis of extracellular laccase activity
Laccase activity was determined by “Kirk's medium” with little modification (Hall, 1999). For mycelial growth, the macronutrient and trace elements (g.L-1) was kept in the flasks. The macronutrients included (10 g.L-1) glucose, starch and yeast extract, while the trace elements were [MgSO4·7H2O, NaCl, FeSO4·7H2O, KH2PO4 0.046%, K2HPO4 0.1%, CaCl2·2H2O, ZnSO4, CuSO4·5H2O, H4PO4 (1.0%), Na4HPO4 (0.05%), MnSO4 (0.001%), ZnSO4 (0.001%)] (Larkin et al., 2007) regulated at pH 5.0. One-liter medium was autoclaved, allowed to cool and aliquots of 100 mL of each was put into 3 different flasks. Each of the flasks was inoculated with mycelial plugs (5 mm) at 27 ± 2 °C in the static condition for 3 days. The medium moved gently through shaker to “optimize the nutritional and environmental factors”.
The guaiacol substrate used to determine the enzyme activity by following Umar & Ahmed (2022). UV Spectrophotometer used to monitor the change in absorbance of the reaction solutions containing guaiacol for 3 min at 470 nm (Sharma et al., 2013). This activity was measured by following formula No. 1 in triplicate and expressed in U/L by measuring the absorbance for 3 to 5 min (Jhadav et al., 2009).
2.5 Optimization of environmental conditions
The culture growth conditions for hyper-production of laccase by selected Ganoderma strains were optimized by adjusting the pH, temperature, incubation time, quantity of fungal discs, and agitation speed of the media. Hundred milliliter separated from the culture flasks containing two mycelia discs were cultured at varied pH levels for seven days “(3.0, 5.0, 6.0)” and different temperature range (60 °C, 40 °C and 20 °C). A complete batch was set at different revolutions (50, 100, 150) per min for 7, 10 and 15 days with 2, 3 and 5 mycelial discs to maximize the laccase production.
2.6 Nutritional conditions and laccase production
The medium was amended by different concentration of the nutritional sources. Actively growing three mycelial discs were plugged out and inoculated in three different flasks comprising fermented broth of pH 5.0 on a shaker with 100 rpm at 35 °C. According to Revankar and Lele (2006), after 10 days of post inoculation (dpi), laccase activity was calculated. The filtrate was used for optimization of nutritional conditions. For the carbon optimization, different sources like “1: maltose, 2: glucose and 3: sucrose” at 20 g and 25 g concentration were evaluated. For nitrogen optimization, suitable sources (5 g.L-1 and 10 g.L-1) of beef extract, peptone, yeast extract; and inorganic sources like potassium nitrate, ammonium sulphate and sodium nitrate (“incubated for 10 days at 40 °C”) were selected for this study.
2.7 Laccase purification
The best optimized conditions were used to prepare 1000 mL broth and filtrate centrifuged for 15 min at 10 °C at “13,000 × g. The cold supernatant was thoroughly mixed with 60%−80% NH4SO4 till saturation level achieved (Das et al., 2001). The ground powder of NH4SO4 was added until the protein precipitated in liquid broth. The further protocol followed Umar & Ahmed (2022).
2.8 Determination of laccase mmolecular weight
SDS-PAGE (Criterion XT, Bio-Rad, CA, USA) gel apparatus was used to determine the yield of expressed protein. Estimated laccase's molecular weight (MW) was compared to conventional protein indicators (14.3–97.0 KDa). A native PAGE was performed and stained with guaiacol to assign the ∼67 kDa laccase. Incubated the gel in 50 mM sodium acetate buffer (pH 5.0) with 100 mM guaiacol, allowed the separated protein to be seen.
2.9 Analysis of “Nitrogent-terminal amino acid Sequence”
The protein sequence was determined on the “N-terminal amino acid of laccase” band by the “Precise Protein Sequencing System” (Applied Biosystem).
2.10 Characterization of laccase
The impact of pH on the partial purified laccase was evaluated at pH ranges 2.0–8.0 (in 50 mM citrate phosphate buffer) and temperature of 40 °C. Laccase activity and stability was measured after every 15 min. For temperature effect on laccase activity, the protein was incubated at optimal pH of 3.0 to 5.0. The thermo stability of the enzyme was measured at 10 °C to 80 °C temperature range. The readings were taken every 10 °C increase in temperature and various metal concentrations used to investigate the impact of metal ions on laccase activity (Cu2+, Ca2+, Zn2+) with sulfate donor in 1, 3, 6, and 9 mM of the solutions. Aliquots of the enzymes, 50 mM citrate–phosphate buffer (pH 3.0), and specific metallic ions were mixed in the right concentrations for enzymatic assays for 30 min at 40 °C.
For 10 min, the laccase band (10 µg) was incubated in the above-mentioned solution, 100 mM guaiacol added and assay activity was done at 470 nm. The initial activity before incubation was used to calculate the RA%.
Laccase kinetic parameters (Km and Vmax) were resoluted by guaiacol at various concentrations “1 mM, 2 mM, 3 mM, 5 mM and 10 mM in 100 mM” of citrate–phosphate buffer (pH 3.0). After 15 min, a spectrophotometer was used to detect the wavelength of the enzyme in the presence of guaiacol.
2.11 Quantification of chromium reduction
The effect of laccase was investigated on Cr (VI) reduction. About 0.5 μg of the partial purified laccase was homogenized in various concentration of Cr (VI) (100, 150, 200, 250, 300 “μg/mL”) in 1.5 mL Eppendorf. The inoculated tubes were incubated for 120 min at 35 °C with 200 rpm shaking. The cultures were taken out from each tube after 24 h time interval and centrifuged for 15 min at 8000 rpm. The Cr (VI) concentration in the supernatant and laccase activity was spectrophotometrically measured by guaiacol method. The direct UV–vis spectrophotometer scanned at 1100 nm was used to recognize the most profound Cr (VI) absorption wavelength. The following formula No. 2 (Mousavi et al., 2023) was used to calculate the Cr (VI) removal ratio (percentage):
2.12 Statistical analysis
The collected data from various parameters were analyzed. The vertical error bars represented the ± standard deviation (SD) less than 5% of triplicate assays. Statistical analysis was calculated by using 1-way ANOVA in SPSS18.0 software using Duncan’s LSD test at 5% level of significance.
3 Results and discussion
3.1 Molecular phylogeny
Phylogenetic analysis of the sequenced ITS region with other G. multipileum species from GenBank produced four major clades (A, B, C and D) with Tomophagus clossus as the outgroup. Two of our newly sequenced G. multipileum strains (MW349830 and MW349829) nexted with other Ganoderma species in clade A with 73% branch support (Fig. 2). Clade A comprised mainly of G. multipileum specie nexting with G. lucidium, G. parvulum, G. martinicense and G. destructants. No G. multipileum was found in clades B, C and D. These three clades were dominated by other Ganoderma species. Fungal species in clades B and D had very high branch support (88–99%), while clade C 69–98% branch support.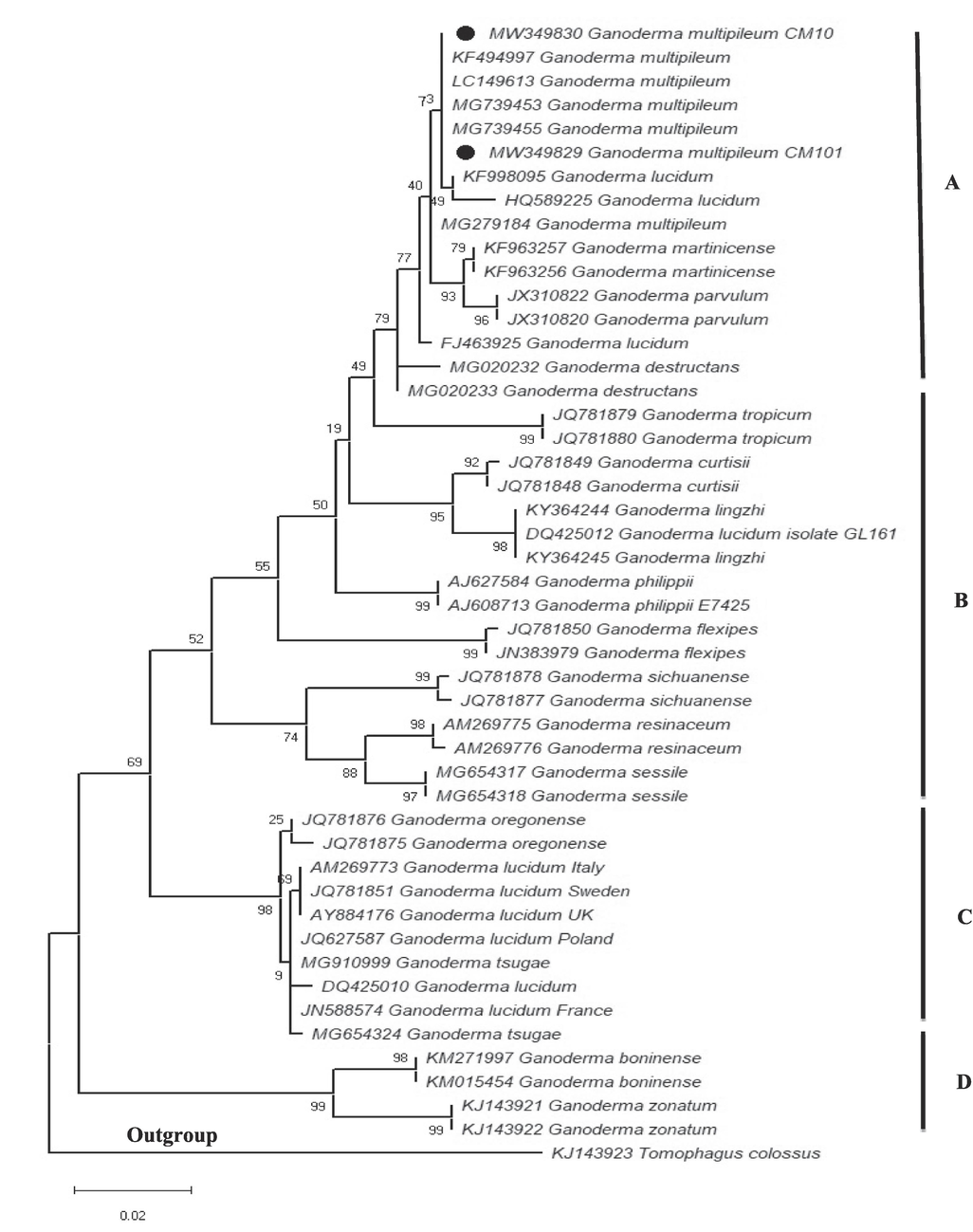
Phylogenetic tree of G. multipileum (CM10, CM101) and related species based on ITS sequences generated by maximum likelihood method in MEGA 10.0. Tomophagus colossus was chosen as the outgroup. Bootstrap values (>50%) are shown at the branches (Constructed by Aisha Umar).
In this study, G. multipileum was collected from tropical region of Pakistan. Species of this study analyzed by sequencing, molecularly identified and their phylogenetic relatedness with Ganoderma species from the other regions were conducted. Our sequenced were closely matrixed to Nepalian and Chinese G. multipileum. Furthermore, all G. multipileum formed a close cluster in the phylogenetic analysis was an indication of close evolutionary emergence.
3.2 Effect of environmental conditions on laccase production
The screening experiment is dependent on inexpensive method, so, Plate-method reported, where guaiacol utilized for laccase detection and quick visual expression. The laccase potential of G. multipileum was sorted out by using a preliminary differential screening procedure (by using appropriate growth media). The formation of brown, intense brown and reddish brown color below and around the fungal colony a positive indicator of guaiacol oxidation (Vantamuri et al., 2015). The development of reddish brown color below the fungal colony of this study was analogous to other reports based on Ganoderma species (Kiiskinen et al., 2004). Laccase synthesis is influenced by culture circumstances, which include differences in the kind and concentration of available nutrients that drive laccase formation (Lorenzo et al., 2002).
Extracellular laccase is formed in a very small proportion by default, but this can be enhanced by improving the fermentation parameters such as medium components, temperature, carbon–nitrogen ratio, and aeration rate (An et al., 2020a, 2020b). Temperature is a substantial environmental aspect for exudation of laccase isozymes (Li et al., 2016). In this work, temperature at which laccase produced at its best in G. multipileum (G113) was 30 °C (Fig. 3). At this temperature, 789 U/L ± 5.4 of the enzyme produced and activity decreased as the temperature increased from 35 °C to 40 °C. Laccases in fungi play a function as phenol oxidases prefer the temperature between 30 °C and 55 °C for catalytic activity. In this study, the optimal temperature for laccase activity was between 25 °C and 30 °C reported from other studies as well. In other fungal species like P. ostreatus, Cyathus bulleri, Trametes modesta, Phlebia brevispora, the optimal temperature for highest isozyme studies is 30 °C and 35 °C for T. versicolor (Šnajdr and Baldrian, 2007). Ganoderma lucidum MDU-7 secreted laccase isozymes in comparable patterns at 25 °C and 30 °C, with additional isoforms with larger molecular mass seen at 35 °C, in contrast to Ganoderma sp. kk-02 (25 °C) (Kumar et al., 2017). Temperature is a key factor in regulating the development of fungi, when grown with malt extract alone. Laccase activities were higher in cultures of T. versicolor and R. vitreus (Reyes et al., 2021).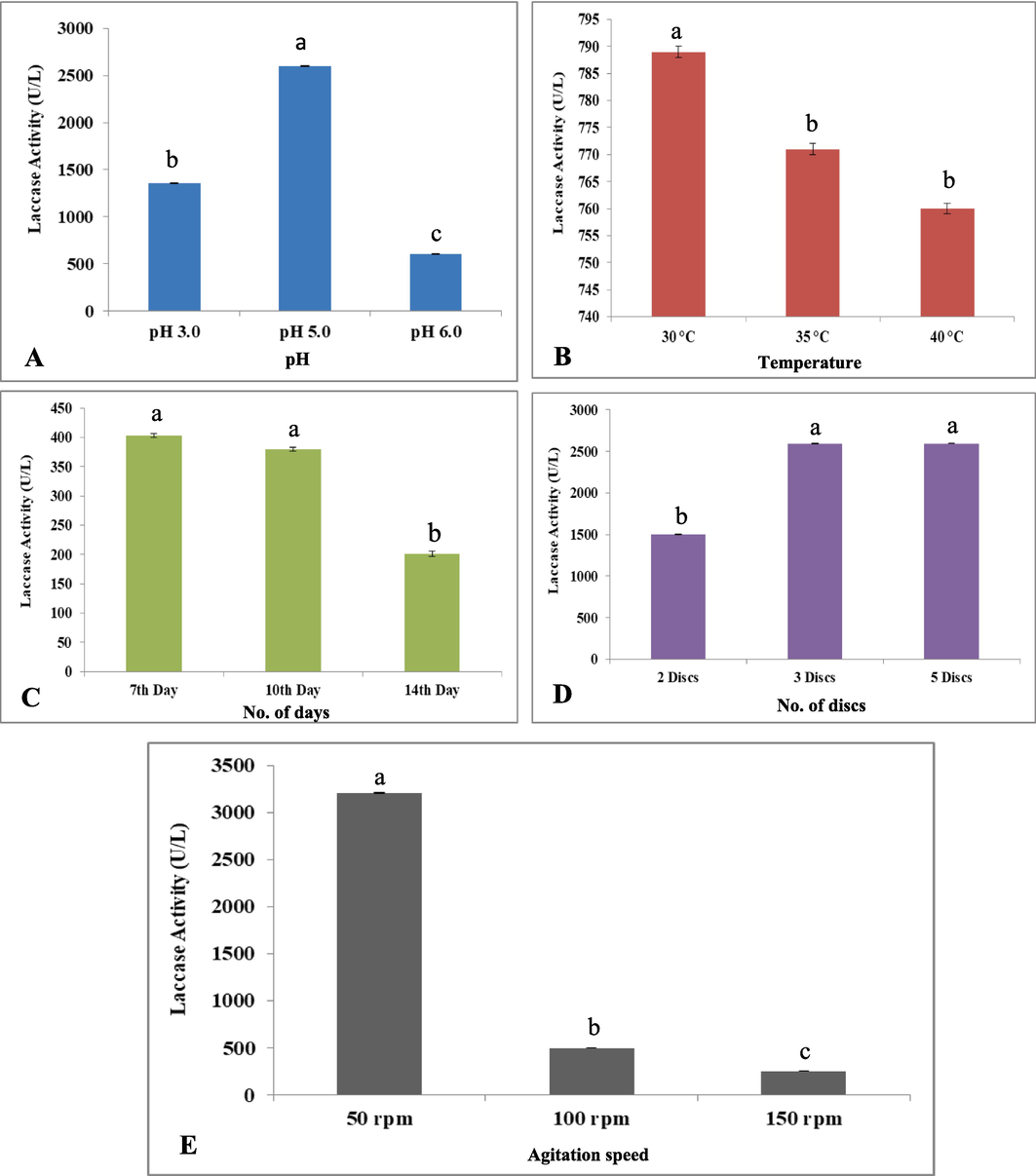
Optimization of culture growth conditions (A. pH; B. Temperature; C. No. of Days; D. No. of Fungal Growth Discs; E. rpm = revolutions per min) for maximum laccase production.
The excretion of laccase experienced a slight decline at 40 °C. The fungal laccase showed greater production at pH 4–6/3.6–5.2. The optimum pH for guaiacol (phenolic compounds) was 4.0 to 7.0 (Fig. 3). Ganoderma strains secreted the maximum laccase, when the pH was 5.0. The pH 5.0 was more promising than pH 3.0 and 6.0.
The laccase activity was minimum at 3.0 pH in this species and optimum at 5.0 pH (Fig. 3). A reduction in activity was observed at pH 3.0 (1355.5 ± 8.8 U/L) and pH 6.0 (605 ± 3.9 U/L), respectively. The fungal laccase exhibits the highest solidity in “acidic pH (pH 4–6/3.6–5.2)”. It also acts as phenol oxidases under acidic medium (Hailei et al., 2013). The polypeptide mobility enlarged at “pH 3.0 to 5.0” (Bonomo et al., 2001), while no activity was seen at neutral pH. Laccase isozyme regulatory patterns (G. lucidum MDU-7) at pH 5.2 have recently been published, which drop sharply as pH climbed from 5.0 to 7.0 in various studies. Shrestha et al. (2016) studied G. lucidum-CDBT1 to see maximal level of laccase secretion (92 U/mL) by adjusting the pH. Fomitopsis pinicola FP58527 SS1 secreted several laccases and two of them (FpLcc1 and FpLcc2) were acidic at pH 3.5 for guaiacol. At pH 5.0, the activation impact is substantially stronger than pH 3.0 (Csarman et al., 2021).
On the seventh day of incubation, G. multipileum produced the maximum laccase, whereas production level started on 4th day, while reached at peak on 7th day. The 80% laccase production was observed on 7th day of inoculum, but the activity declined as the days increased from 10 to 14 (Fig. 3). Songulashvili et al. (2007) studied four Ganoderma species and found that they have laccase activity ranging from 0.6 to 49.5 U/L. Comparatively, Trametes under submerged fermentation released higher extracellular laccase (9000–20000 U/L) on 7th to 14th day than Ganoderma species (Moldes et al., 2004). On the 10th day, maximum laccase produced in the liquid medium was 0.59 U/mL from G. lucidum in just six days (Fang et al., 2015). Wehaidya et al. (2018) observed maximum production on 7th day, however, the activity was lowered in Polyporus durus ATCC 26726 at this time point. The authors suggested that reduction in production may be due to nutrient depletion or proteolytic enzymes that may have caused cell digestion by autolysis.
Inoculating “5 mycelial” discs of G. multipileum in shake flasks exhibited maximum activity of laccase (2598 ± 0.7 U/L). No significant difference observed in activity, when 2 and 3 fungal discs were used (Fig. 3). Also, no significant difference was observed by using three and five discs, but 5 mycelial discs was greater than the use of two discs. The highest production of laccase in G. multipileum was on the10th day at 50 rpm (Fig. 3). The secretion levels were 3204.4 ± 9.3 U/L at 50 rpm, whereas 498.5 ± 1.4 U/L and 253.3 ± 5.41 U/L at 100 and 150 rpm, respectively. G. multipileum showed more secretion of laccase at 100 rpm (Fig. 3). Higher rpm imply to improve the oxygen transport to G. lucidum mycelium in the fermenting broth, because of the stirring situation, the highest enzyme ability in shake flask fermentation resulted in the creation of tiny pellets (Li et al., 2016).
3.3 Effects of nutritional variations on laccase yield
The laccase activity at 25 and 20 (g.L-1) sucrose concentrations were 2310 ± 1.5 UL-1 and 1525 ± 1.3 U L-1, respectively. Laccase yield in G. multipileum significantly declined with maltose addition (no significant difference was observed by using 20 g.L-1 and 25 g.L-1 maltose in the culture) (Fig. 4A). For the nitrogenous sources, 10 g.L-1 of the organic beef extract showed significant increase in laccase activity (Fig. 4B). The yeast extract had a greater impact on laccase production than the beef extract at 10 g.L-1 and secretion was also improved by yeast extract (5 g.L-1) (Fig. 4B). Organic peptone did not significantly influence the laccase activity at the two tested concentrations (Fig. 4B). For the inorganic nitrogen, the laccase activity was significantly increased at 5 g.L-1 KNO3 (Fig. 4B), while NaNO3, slightly improved the activity at 10 g.L-1 concentration than 5 g.L-1, whereas (NH4)2SO4 more enhanced the secretion at 5 g.L-1 than 10 g.L-1 concentration (Fig. 4B).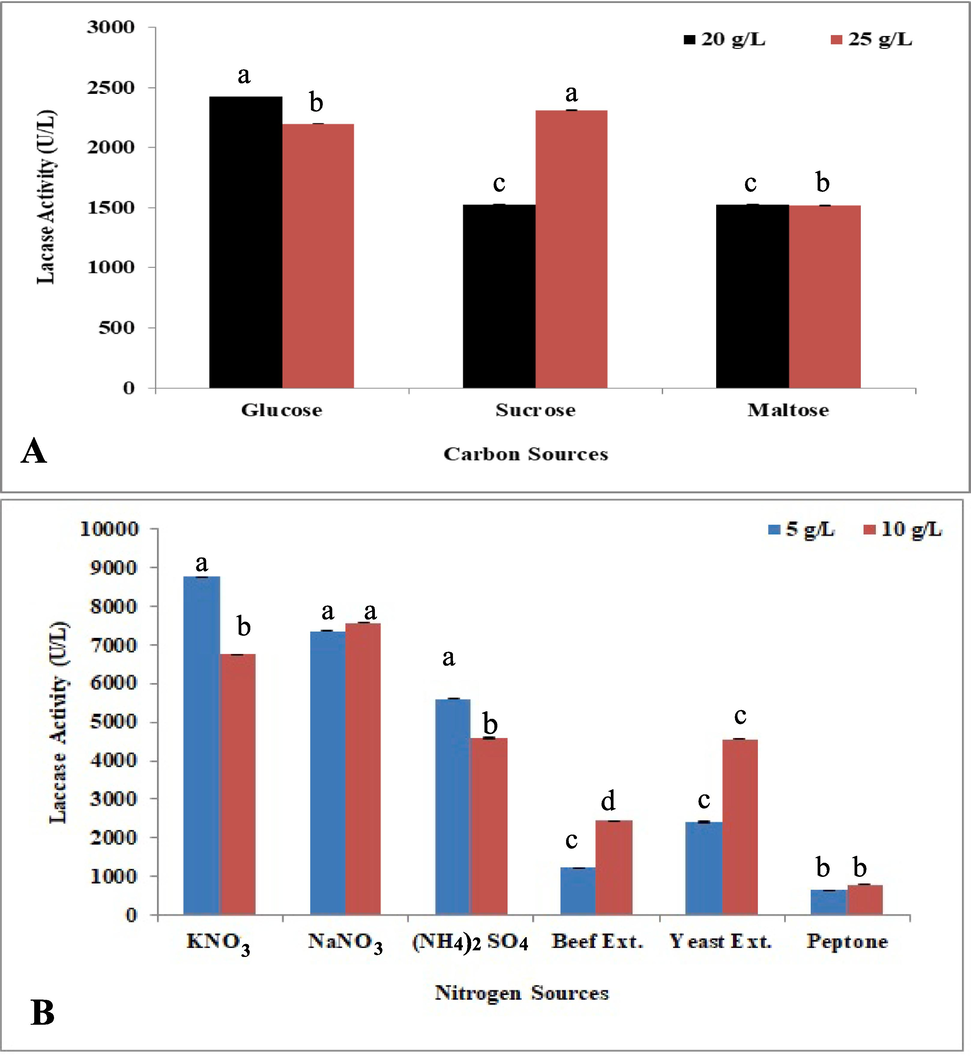
Optimization of nutritional conditions for laccase production: A-Organic carbon sources and B-organic and inorganic nitrogen sources.
Laccase activity is also influenced by the amount and kind of carbon sources used, as well as the mushroom species (Claudia et al., 2013). Similarly, different carbon sources have been tested in some experiments, where “20 g.L-1 glucose” was effective to stimulate maximum activity (Zhang, 2012), similarly the findings of this work.
Furthermore, laccase activity is reliant on nature and concentration of nitrogen sources in wood decaying fungi (Elisashvili et al., 2008). Organic nitrogen was more effectual than the inorganic sources and less effective on enzyme activity, whereas laccase yield increased in some fungal species (Kunamneni et al., 2007). Some authors have been suggested low carbon–nitrogen ratio for higher laccase production, while others shown the higher ratio with maximum yield (Dong et al., 2005). Zhang (2012) used different nitrogen sources to evaluate the laccase from G. lucidum (Garzillo et al., 2001). This result agreed with Teerapatsakul et al., (2007) for Ganoderma species. Many natural laccase-mediators including proteins and many other factors are secreted by mushrooms in shake flasks culture (Papinutti et al., 2008).
3.4 Purification, identification and N-terminal sequences of laccase
Separately, a complete culture broth setup (1000 mL) was created under optimal conditions. The best concentration (80%) for laccase production was ammonium sulphate, which yielded 65% laccase. Protein (Glacc 113) molecular weight of ∼75.0 kDa was estimated by SDS-PAGE and Native PAGE. A brown band of ∼75.0 kDa in a lane was stained by guaiacol, which designated the laccase of G. multipileum extract.
The molecular weight (75 kDa) of Glacc113 of G. multipileum was quite similar to other wood rotting fungal laccases. Single protein band appeared on SDS-PAGE, which mean Glacc113 comprised only N-glycosylation. The N-terminal “amino acid” sequence was same (GIAPTAD) and exhibited closest similarity to wood rotting fungi (Table 2).
Sr No.
Wood Rotting Fungi
N-terminal amino acid sequences
References
1
Ganoderma multipileum
GIAPTAD
This work
Ganoderma lucidum
GIGPT
Ko et al., 2001
2
Trametes versicolor 951,022
GIGPVAD
Han et al., 2005
3
Trametes versicolor ATCC 20869 laccase II
GIGPVAD
Bourbonnais et al., 1995
4
Trametes versicolor ATCC 20869 laccase I
AIGPVAS
Bourbonnais et al., 1995
5
Trametes villosa I
AIGPVAD
Yaver et al., 1996
6
Basidiomycete PMI
SIGPVAD
Han et al., 2005
7
Phlebia radiata
SlGPVTD
Saloheimo et al., 1991
8
Coriolus hirsutus
GICTKAN
Shin and Lee, 2000a, 200b
9
Pleurotus ostreatus POXAI
AlGPTGD
Palmieri et al., 1997
10
Phellinus ribis
AlVSTPL
Min et al., 2001
11
Agaricus bisporus
DTXKTFN
Perry et al., 1993
12
Ceriporiopsis subvermispora
AIGPVTD
Fukushima and Kirk, 1995
13
Pycnoporus cinnabarinus
AIGPVAD
Eggert et al., 1996
14
Coriolus hirsutus
AIGPTAD
Kojima et al., 1990
Laccases ranging from 30 to 300 kDa e.g., isoforms from G. lucidum were reported to be 40 kDa to 68 kDa (D’souza et al., 1999). Kuhar and Papinutti (2014) reported isozyme in G. lucidum, while GlLCCI of G. lucidum was 58 kDa (Sun et al., 2012). From literature findings, the molecular mass of laccase ranges from 34 to 85 kDa, 50–80 kDa (Thitinard et al., 2012), 55–90 kDa, 50–100 kDa, 40–66 kDa (Amit et al., 2017) and 38.3 kDa in Ganoderma sp (Manavalan et al.,2013). In other fungal species, the molecular mass is 45 and 90 kDa for C. versicolor, 61.7 kDa for Mycena purpureofusca and 66 kDa in Lentinus squarrosulus (Mukhopadhyay and Banerjee, 2015). The Glacc113 show 7–10% glycosylation. The glycoproteins lose their activity, when carbohydrate moieties are removed, so that proteins denature first to eliminate the carbohydrates from the fungal laccase. This is impossible to estimate the deglycosylated proteins activity, when endoglycosidase H (which removes all glycosylations) or N-glycosidase F (which removes N-glycosylation) applied to the Glacc113. Carbohydrate moieties of Glacc113, where each moiety exhibited the identical band, furthermore, the N-terminal amino acid sequence of Glacc113 was similar to other wood rotter laccases.
3.5 Characterization of laccase
In this experiment, pH profile aided in the identification of Ganoderma strains and highest relative activity (%) was found in the pH of 3.0–5.0, while activity dropped at pH 6.0–8.0. At pH 3.0, highest laccase activity was observed under standard conditions, when purified laccase stability index retained at pH 3.0–5.0. The relative activity of Glacc113 was 77.12% at pH 3.0 (Fig. 5A). As pH changed from acidic to basic, the relative activity decreased and stability plummeted at the pH range of 6.0–8.0.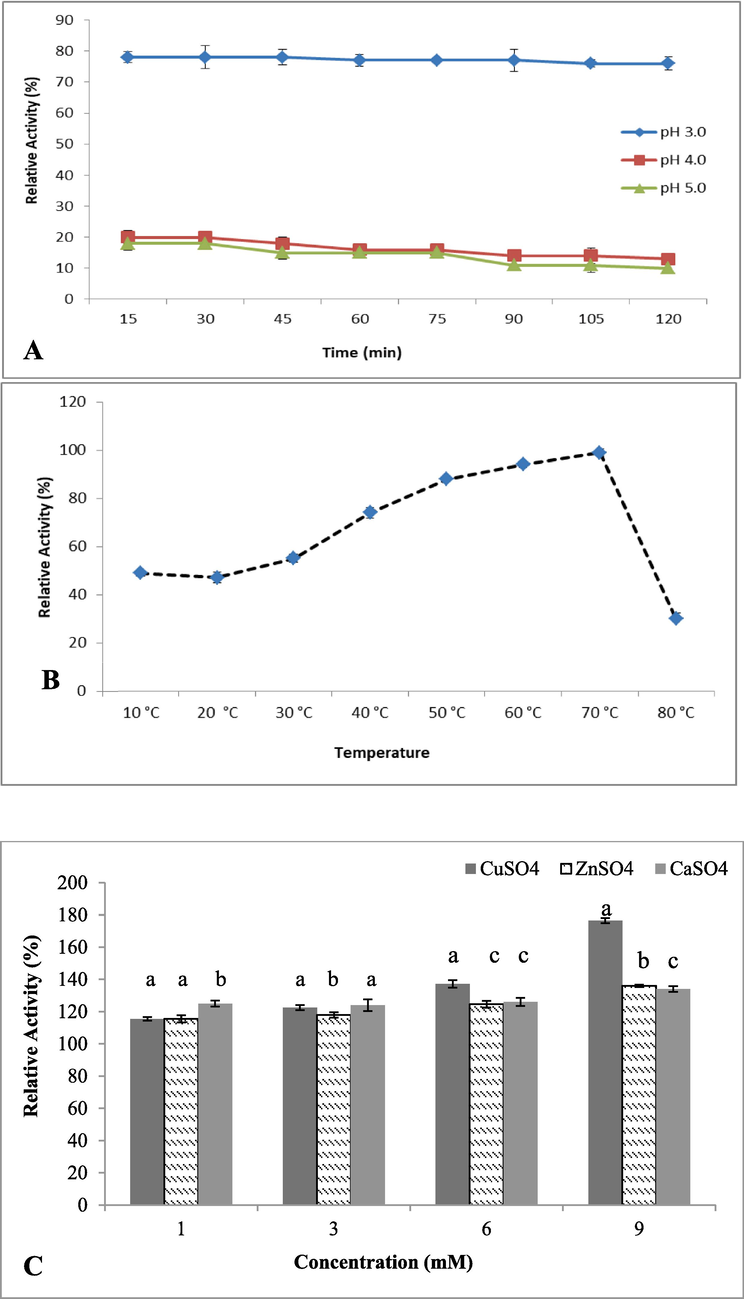
Determination of the effect of environmental parameters on the activity of the purified laccase, Glacc113: A-Effect of pH, B-Effect of temperature, and C-Effect of metallic ions.
The purified laccases was analyzed at temperature range 10–80 °C to evaluate its tolerance and maximal activity after incubation for 60 min at pH 3.0 (Fig. 5B). Favorable stability temperature range of the laccase was 40–70 °C for 60 min, while activity nearly inactivated at 80 °C. Laccase activity increased dramatically from 60 to 70 °C, then declined at 70 °C, according to the temperature tolerance profile.
Laccase has been characterized by scientists to check its stability and reported optimal pH for laccases (2.0–3.0) (Garzillo et al., 2001). Thermostable laccase has been reported in Pycnoporus sp., P. ostreatus and G. lucidum (Wang et al., 2010a, 2010b), while thermal transitions (87 and 92 °C) in laccase have been examined in Coriolus hirsutus and C. zonatus using scanning calorimetric curves (Koroleva et al., 2001). The optimum, stable and inactivated temperatures of laccase in Trametes sp. LS-10C were “40 °C, 20 °C and > 60 °C”, respectively (Li et al., 2016). The purified laccase from Ganoderma strains was constant at 30 °C and retained 100% residual activity after 150 min. Sharma et al. (2013) shown the optimal temperature in Ganoderma species (purified laccase) was 50 °C. Similarly, the optimum temperature in G. lucidum was 50 °C and 70 °C, whereas uppermost laccase activity established at 25 °C (Sandana et al., 2015).
3.6 Effects of ion modulators on laccase activity
The purified laccase exhibited 100% relative activity by addition of 1 mM Cu2+, also 9.0 mM CuSO4 significantly increased the laccase relative activity. The maximum laccase relative activity (174.4%) obtained in Ganoderma Glacc113 at 9 mM CuSO4 (Fig. 5C). The highest concentration of Ca2+ (9 mM) generated a pronounced effect on Glacc113 and RA increased abruptly from “1 mM to 9 mM”. The selected concentrations of Ca2+ exerted more than 100% positive effect on this species (Fig. 5C). For Zn2+modulators, 9.0 mM ZnSO4 considerably increased (136%) the laccase RA, whereas sharply decreased at 6 mM to 1 mM (Fig. 5C).
Metallic ions regulate the manifestation of laccase in fungi/mushrooms and tolerance to metal ions in laccase expression is an outstanding property. Murugesan et al., (2009) explained the effects of some metalic ions in laccase expression, a major obstacle for practical application in biotechnology industries. Metalic ions induce changes in enzyme (Okamoto et al., 2000) and Liu et al. (2020b) found that by addition of 12.5 mg.L-1 Cu2+ to a G. lucidum provided the maximum laccase stimulation and increased laccase activity by 1.6 times. The highest laccase activity from P. ostreatus in basal media with and without seven different metal ions e.g., Cu2+ (Media 1), Mn2+ (Media 2), Cu2+ and Mn2+ (Media 3), Fe2+ (Media 4), Mn2+ and Fe2+ (Media 5), Fe2+ and Cu2+ (Media 6), and Cu2+, Mn2+, Fe2+ (Media 7) were increased approximately 21.5-fold, 4.7-fold, 14.9-fold, 16.9-fold, 4.0-fold, 12.7-fold, and 24.8-fold higher, respectively (An et al., 2020a, 2020b).
Peniophora lycii a white-rot basidiomycete fungus was studied for laccase synthesis under copper induction (Glazunova et al., 2020). Laccase from T. hirsuta exhibited specific activity was 978.34 U/mg at 4–6 pH and 20–40 °C temperatures, where laccase remained stable for 16 h. Except for Fe2+ and Hg2+, the isolated enzyme displayed substantial stability for 10 metal ions (10 mM). Laccase activity was up to 142% greater in Cu2+-treated cells than in control cells.
3.7 Kinetic studies
The kinetic characteristics of the Glacc113 were evaluated by using guaiacol to assess the effect of substrate concentration on “laccase activity” (470 nm). Guaiacol concentration range was 1 mM, 2 mM, 3 mM, 5 mM and 10.0 mM in 100.00 mM citrate–phosphate buffer (pH 3.0). Km value is different form laccase to laccase and Lineweaver-Burk plot generated after adjusting kinetic information (data) to hyperbolae of Michaelis Menten’s equation. The effect of substrate on laccase activity, and the Km 1.4617 mM and Vmax 1.817 mM min−1 of G. multipileum is presented in Fig. 6.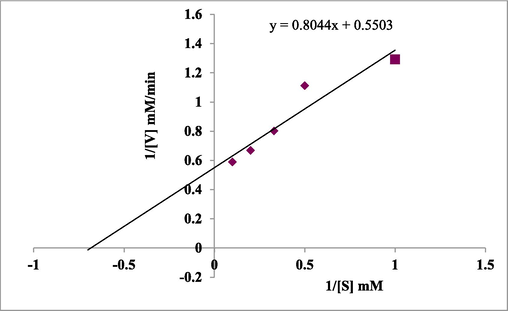
The Lineweaver-Burk plot of purified “Glacc113” of G. multipileum.
Km was 400 ± 60 µM and Kcat was 80.20 ± 1.59/s for guaiacol (Navada and Kulal, 2021). As the temperature lowered from 28 to 4 °C or increased upto 40 °C in Cerrena unicolor, the increasing quantities of copper and manganese in the medium induced the biggest change in laccase gene expression and laccase transcription (Pawlik et al., 2021).
P. ostreatus LAC-Yang1 demonstrated a high resilience to severely acidic conditions and a high level of stability under strong alkaline conditions (pH 9–12). This LAC-Yang1 also shown a high resistance to inhibitors (EDTA, SDS), metal ions (Mn2+, Cu2+, Mg2+, Na+, K+, Zn2+, Al3+, Co2+), and metal ion mixtures (Liu et al., 2021).
The Km (mM) values of purified laccase of Pleurotus sajor-caju, P. ostreatus, P. ostreatus POXA1, T. trogii POXL3, G. lucidum, G. lucidum GaLc3 (pH 5.0) were 2.50, 0.28, 0.09, 0.03, 0.107 and 0.037, respectively (Soden et al., 2002). Km of T. hirsutus was 10.9 μM, whereas the highest Km of laccase was 0.107 mM from G. lucidum (Zinnai et al., 2013). Laccase of Phoma herbarum KU4 reported in submerged fermentation (1590 U/mL) and Km, Vmax and Kcat of this species was 0.216 mM, 270.27 U/mg and 506.69 s−1, respectively (Debnath et al., 2021).
3.8 Percentage inhibition of Cr concentration by purified Glacc13
Environmental chromium exist in two oxidation states i.e., Cr (III) and Cr (VI), where “Cr(VI)” is highly alarming, mutagenic, carcinogenic, and toxic contaminant in natural environment (Peng et al., 2018; Hamilton et al., 2018). Chromium enter in the environment by weathering of Cr-containing rocks, leaching of soils, and direct ejection from industrial processes. This is widely used in multiple applications e.g., metal plating and tanneries. Under all pH values of water, the forms of Cr (VI) chromate and dichromate are soluble. The concentration of Cr in soils differ according to sediments, rocks and increase through anthropogenic deposition (Kimbrough et al., 1999). Chromium in soil (fastly movable) is presented as a mixture of both Cr (III) and (VI), while in aquatic environment, soil or sediment, this Cr undergoes a variety of transformations (Kimbrough et al., 1999). The MnO2 and dissolved oxygen (oxidants) present in the “soil can oxidize Cr (III) to Cr (VI) (Wang et al., 2010a, 2010b).
Laccase of G. multipileum effectively eliminated (>94%) the Cr (VI) concentration (100 μg/mL) from 72.18% to 14.46% into Cr (III), and Cr (III) concentration increased from 150 μg/mL to 300 μg/mL. At lower Cr (VI) concentrations of 100.00, 150.00, and 200.00 g/mL, a full decrease in Cr (VI) was seen for 20, 40, and 80 h, respectively (Fig. 7), as the concentration of Cr (III) increased, the consumed time was maximum for Cr (VI) to reduce it completely. After 120 h of incubation, G. multipileum was able to completely decreased the Cr (VI) at a concentration of 250 g/mL (82.3%) (Fig. 7). In this study, laccase of G. multipileum effectively eliminated the Cr (VI) at 100 μg/mL. Increased Cr (VI) concentration, the effectiveness of Glacc113 reduced at 150 μg/mL to 300 μg/mL. Maximum Cr (VI) concentration alter the physiological reactions, metabolic activities, and the growth of living organism.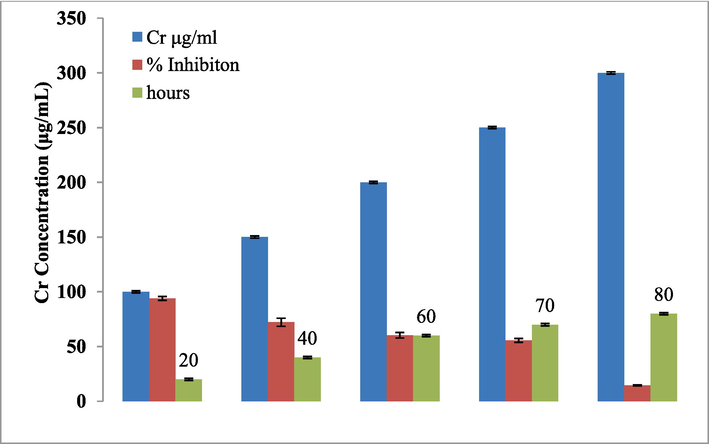
Removal of Cr(VI) concertation by Glacc113.
Cr (VI) is toxic and mutagenic at 100 μg/mL concentration (Liu et al., 2020b). Oves et al., (2013) reported that different Cr (VI) concentration could affect the growth of microbes. Trametes hirsuta TH315 eliminated the Cr (VI) (>96%) at 0.5 and 1 mM (Liu et al., 2020a), while removal ratio decreased from 76.78% to 16.56% at 2 to 5 mM concentration of Cr (VI), respectively (Baldrian, 2003). The heavy metals toxicity directly or indirectly influenced the growth and cellular components by the generation of free radicals. The study has identified the conditions that enhance the optimal production of laccase in G. multipileum and also the purified laccase by N-terminal amino acid sequences. The laccase produced in this work has interesting characteristics like thermo stability at higher temperature and acidic pH also with ability to reduce the toxic level of chromium.
4 Conclusion and future prospects
During this investigation, a new strain of Ganoderma multipileum was isolated, and its laccase was found to effectively remediate chromium, a hazardous agent. This discovery opens up a new avenue for industrial and biotechnological applications. This study can widely be used for numerous laccase based products, though care must be taken to ensure methodological consistency, when making comparisons with a different goal and scope. However, this study is limited to the lab scale due to a lack of resources, because assessment via bio-based systems is complicated from the real world with multiple methodological challenges. This study is also limited to future challenges like “system boundary definition, allocation methods, functional units, carbon accounting and storage, inventory data collection, and impact assessment methods”. Researchers worldwide are focusing on 'greener' technology-based concepts. The laccase found in this study can be used in advanced biotechnical tasks like the “Fungal Fuel Cell” due to the limited availability of organic reservoirs of bioenergy, biofuel, and bio products (Umar et al., 2023). Furthermore, the findings of this study can determine the life cycle analysis of various pretreatment strategies for power generation. Greener technologies generate many bioproducts with multiple environmental advantages compared to their fossil counterparts. Favorable conditions are also identified by energy, emergy, exergy, LCA, or a combination of these energy based assessment tools. ‘Life cycle assessment (LCA)’ aims to understand the environmental performance of bio production from lignocellulosic feedstock. This analysis includes the use of raw materials, treatment processes, purification steps, energy consumption and generation rates, and any waste produced during production. The pretreatment of biomass in the life cycle analysis of biofuel production is given less emphasis than other stages. While a generalized comparison of various fungal production strategies may not be conclusive, there are global efforts to develop economically viable methods for commercial biofuel synthesis through innovative technological advances. To obtain practical and validated results, it is necessary to focus on optimizing and analyzing a single type of feedstock. LCAs are also useful in highlighting the problems produced during various production stages and thus help to avoid negative environmental impacts. The life cycle analysis of multiple production strategies, developed over several years of research, can often assist in creating sustainable and feasible synthesis pathways (Gheewala, 2023). As we discover more environmental impacts, we are also creating and improving methods to assess them. Sensitivity analysis and uncertainty analysis tools enhance the assessment's strength to provide better support for decisions.
Availability of data and materials
The data set generated and analyzed during the current study is available from the corresponding author on personal request.
CRediT authorship contribution statement
Maha A. Alshiekheid: Methodology, Analysis, Writing the draft article. Aisha Umar: Conceptualization, Methodology, Software, Validation, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision. Fuad Ameen: Conceptualization, Methodology, Validation, Supervision. Sami A. Alyahya: Software, Validation, Resources, Supervision. Laurent Dufossé: Investigation, Writing – review & editing.
Acknowledgement
This research was funded by Researchers Supporting Project number (RSP2023R364), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Lignolytic fungi with laccase activity isolated from Malaysian local environment for phytochemical transformation purposes. Int. Res. J. Biol. Sci.. 2013;2(2):51-54.
- [Google Scholar]
- Enhanced laccase activity of white rot fungi induced by different metal ions under submerged fermentation. BioResources. 2020;15(4):8369.
- [Google Scholar]
- Comparative study on laccase activity of white rot fungi under submerged fermentation with different lignocellulosic wastes. BioResources. 2020;15(4):9166.
- [Google Scholar]
- Interactions of heavy metals with white-rot fungi. Enzym Microb Technol.. 2003;32:78-91.
- [CrossRef] [Google Scholar]
- Taxonomy and diversity of Ganoderma from the Western parts of Maharashtra (India) Mycosphere. 2010;1:249-262.
- [Google Scholar]
- Lanostane-type Triterpenoids from Ganoderma lucidum and G. multipileum Fruiting Bodies. Nat. Prod Commun.. 2018;13(11):1934578X1801301107
- [Google Scholar]
- Comparison of three fungal laccases from Rigidoporus lignosus and Pleurotus ostreatus: correlation between conformation changes and catalytic activity. J. Inorgan. Biochem.. 2001;83(1):67-75.
- [CrossRef] [Google Scholar]
- Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2, 2’-azinobis (3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol.. 1995;61(5):1876-1880.
- [CrossRef] [Google Scholar]
- Functional expression and characterization of two laccases from the brown rot Fomitopsis pinicola. Enzyme Microb Tech.. 2021;148:109801
- [CrossRef] [Google Scholar]
- Lignin-modifying enzymes of the white rot basidiomycetes Ganoderma lucidum. Appl. Environ. Microbiol.. 1999;65:5307-5313.
- [CrossRef] [Google Scholar]
- Purification and characterization of a growth regulating laccase from Pleurotus florida. J. Basic Microbiol.. 2001;41:261-267.
- [CrossRef] [Google Scholar]
- Partial purification and characterization of a thermophilic and alkali-stable laccase of Phoma herbarum isolate KU4 with dye-decolorization efficiency. Prep. Biochem. Biotech.. 2021;20:1-8.
- [Google Scholar]
- Influence of culture conditions on laccase production and isozyme patterns in the white-rot fungus Trametes gallica. J. Basic Microbiol.. 2005;45:190-198.
- [CrossRef] [Google Scholar]
- Doyle, J.J., Doyle, J.L., 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue.
- The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol.. 1996;62:1151-1158.
- [CrossRef] [Google Scholar]
- Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J. Indus Microbiol. Biotechnol.. 2008;35:1531-1538.
- [CrossRef] [Google Scholar]
- Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotech Biofuel.. 2015;8:54.
- [CrossRef] [Google Scholar]
- Laccase component of the Ceriporiopsis subvermispora lignin-degrading system. Appl. Environ. Microbiol.. 1995;61(3):872-876.
- [CrossRef] [Google Scholar]
- Structural and kinetic characterization of native laccases from Pleurotus ostreatus, Rigidoporus lignosus, and Trametes trogii. J. Protein Chem.. 2001;20:191-201.
- [CrossRef] [Google Scholar]
- Life cycle assessment for sustainability assessment of biofuels and bioproducts. Biofuel Res. J.. 2023;10(1):1810-1815.
- [Google Scholar]
- Purification and Characterization of Two Novel Laccases from Peniophora lycii. J Fungi. 2020;6(4):340.
- [CrossRef] [Google Scholar]
- Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Biores. Technol.. 2016;218:388-396.
- [CrossRef] [Google Scholar]
- Mechanism of Cr(VI) reduction by Aspergillus niger: enzymatic characteristic, oxidative stress response, and reduction product. Environ. Sci. Pollut. Res. Int.. 2015;22:6271-6279.
- [CrossRef] [Google Scholar]
- A novel membrane-surface liquid co-culture to improve the production of laccase from Ganoderma lucidum. Biochem Engineer J.. 2013;80:27-36.
- [CrossRef] [Google Scholar]
- Hall, T.A., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series No. 41: Oxford University Press, pp. 95–98.
- Chromium speciation in foodstuffs: a review. Food Chem.. 2018;250:105-112.
- [CrossRef] [Google Scholar]
- Purification and characterization of laccase from the white rot fungus Trametes versicolor. J. Microbiol.. 2005;43(6):555-560.
- [Google Scholar]
- Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere. 2018;211:653-663.
- [CrossRef] [Google Scholar]
- Optimization of production and partial purification of laccase by Phanerochaete chrysosporium using submerged fermentation. Int J Microbiol Res.. 2009;1(2):09-12.
- [Google Scholar]
- Bioremediation of heavy metals in liquid media through fungi isolated from contaminated sources. Indian J. Microbiol.. 2011;51:482-487.
- [CrossRef] [Google Scholar]
- Ecosystem protection through myco-remediation of chromium and arsenic. J Xenobiot.. 2023;13(1):159-171.
- [CrossRef] [Google Scholar]
- MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Bio Evol.. 2013;30(4):772-780.
- [CrossRef] [Google Scholar]
- Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiology. 2004;150(9):3065-3074.
- [CrossRef] [Google Scholar]
- A critical assessment of chromium in the environment. Crit. Rev. Environ. Sci. Technol.. 1999;29(1):1-46.
- [CrossRef] [Google Scholar]
- Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol.. 2001;57(1):98-102.
- [CrossRef] [Google Scholar]
- Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J. Bacteriol.. 1990;265:15224-15230.
- [CrossRef] [Google Scholar]
- Temperature-induced changes in copper centers and protein conformation of two fungal laccases from Coriolus hirsutus and Coriolus zonatus. Bioch Et Biophy Acta (BBA)-Prot Str. Mol. Enzy.. 2001;1547(2):397-407.
- [CrossRef] [Google Scholar]
- Koropatrick, J. Leibbrandt, M.E.I. 1995. Effects of Metal on Gene Expression. Tn R.A. Goyer and M.G Cherian,eds., Eds., handbook of experimental Pharmacology, Toxicology of metals, Biochem. Aspects, Springer, 115, pp. 93-120.
- Optimization of laccase production by two strains of Ganoderma lucidum using phenolic and metallic inducers. Rev. Argent. Microbiol.. 2014;46:0144-0209.
- [CrossRef] [Google Scholar]
- Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere. 2019;237:124567
- [CrossRef] [Google Scholar]
- Gel-based purification and biochemical study of laccase isozymes from Ganoderma sp. and its role in enhanced cotton callogenesis. Front. Microbiol.. 2017;8:674.
- [CrossRef] [Google Scholar]
- Kunamneni, A., Ballesteros, A., Plou, F. J., Alcalde, M., 2007. Fungal laccase e a versatile enzyme for biotechnological applications. In: Mendez-Vilas, A. (Ed.), Communicating Current Research and Educational Topics and Trends in Applied Microbiology. Formatex, pp. 233–245.
- ClustalW and ClustalX version 2.0. Bioinformatics. 2007;23(21):2947-2948.
- [CrossRef] [Google Scholar]
- High-level production and characterization of laccase from a newly isolated fungus Trametes sp. LS-10C. Biocat Agri Biotech.. 2016;8:278-285.
- [CrossRef] [Google Scholar]
- Characterization of recombinant laccase from Trametes versicolor synthesized by Arxula adeninivorans and its application in the degradation of pharmaceuticals. AMB Exp. 2019;9:102.
- [CrossRef] [Google Scholar]
- Characterization of a Novel Laccase LAC-Yang1 from White-Rot Fungus Pleurotus ostreatus Strain Yang1 with a strong ability to degrade and detoxify chlorophenols. Molecules. 2021;26(2):473.
- [CrossRef] [Google Scholar]
- Response of Trametes hirsuta to hexavalent chromium promotes laccase-mediated decolorization of reactive black 5. Ecotox Environ. Safety. 2020;205:111134
- [CrossRef] [Google Scholar]
- Inducing laccase activity in white rot fungi using copper ions and improving the efficiency of azo dye treatment with electricity generation using microbial fuel cells. Chemosphere. 2020;243:125304
- [CrossRef] [Google Scholar]
- Improving laccase production by employing different lignocellulosic wastes in submerged cultures of Trametes versicolor. Bioresour. Technol.. 2002;82(2):109-113.
- [CrossRef] [Google Scholar]
- Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem. Eng. J.. 2013;70:106-114.
- [CrossRef] [Google Scholar]
- Chromium (VI) bioremediation potential of dark septate endophytic (DSE) fungi. IOP Conf. Ser.: Earth Environ. Sci.. 2023;1201:012077
- [CrossRef] [Google Scholar]
- Characterization of a novel laccase produced by the wood-rotting fungus Phellinus ribis. Arch. Biochem. Biophys.. 2001;392(2):279-286.
- [CrossRef] [Google Scholar]
- Different proportion of laccase isoenzymes produced by submerged cultures of Trametes versicolor grown on lignocellulosic wastes. Biotechnol. Lett. 2004;26:327-330.
- [CrossRef] [Google Scholar]
- Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: adsorption and kinetic study. Biomass Conv. Biorefin.. 2023;13(9):7927-7936.
- [CrossRef] [Google Scholar]
- Purification and Biochemical Characterization of a Newly Produced Yellow Laccase from Lentinus Squarrosulus MR13. 3 Biotech. 2015;5:227-236.
- [CrossRef] [Google Scholar]
- Direct rate assessment of laccase catalysed radical formation in lignin by electron paramagnetic resonance spectroscopy. Enzyme Microb. Technol.. 2017;106:88-96.
- [CrossRef] [Google Scholar]
- Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater.. 2009;168:523-529.
- [CrossRef] [Google Scholar]
- Kinetic characterization of purified laccase from Trametes hirsuta: a study on laccase catalyzed biotransformation of 1, 4-dioxane. Biotech Lett. 2021;43(3):613-626.
- [CrossRef] [Google Scholar]
- Purification and characterization of extracellular laccase from Pleurotus ostreatus. Mycoscience. 2000;41:7-13.
- [CrossRef] [Google Scholar]
- Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur. J. Soil Biol.. 2013;56:72-83.
- [CrossRef] [Google Scholar]
- A novel white laccase from Pleurotus ostreatus. J. Biol. Chem.. 1997;272(50):31301-31307.
- [CrossRef] [Google Scholar]
- Stabilization studies of Fomes sclerodermeus laccases. Bioresour. Technol.. 2008;99:419-424.
- [CrossRef] [Google Scholar]
- Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol... 2020;1–15
- [CrossRef] [Google Scholar]
- Cerrena unicolor Laccases, Genes Expression and Regulation of Activity. Biomolecules. 2021;11(3):468.
- [CrossRef] [Google Scholar]
- High-efficient recovery of chromium (VI) with lead sulfate. J. Taiwan Inst. Chem. Eng.. 2018;85:149-154.
- [CrossRef] [Google Scholar]
- Identification of two laccase genes in the cultivated mushroom Agaricus bisporus. Microbiology. 1993;139(6):1209-1218.
- [CrossRef] [Google Scholar]
- Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochem.. 2006;41:581-588.
- [CrossRef] [Google Scholar]
- Enzyme activities of five white-rot fungi in the presence of nanocellulose. J Fungi. 2021;7(3):222.
- [CrossRef] [Google Scholar]
- Evaluation of laccase production by Ganoderma lucidum in submerged and solid-state fermentation using different inducers. J. Basic Microbiol.. 2019;59:784-791.
- [CrossRef] [Google Scholar]
- Isolation and structural analysis of the laccase gene from the lignin degrading fungus Phlebia radiata. Microbiology. 1991;137(7):1537-1544.
- [CrossRef] [Google Scholar]
- Inducible chromate reductase exhibiting extracellular activity in Bacillus methylotrophicus for chromium bioremediation. Microbiol. Res.. 2015;170:235-241.
- [CrossRef] [Google Scholar]
- Efficient bioremediation of metal containing industrial wastewater using white rot fungi. Int. J. Environ. Sci. Technol.. 2023;20(1):943-950.
- [CrossRef] [Google Scholar]
- Middle-redox potential laccase from Ganoderma sp.: its application in improvement of feed for monogastric animals. Sci. Rep.. 2013;3:1299.
- [CrossRef] [Google Scholar]
- Bioremediation of heavy metals using isolates of filamentous fungus Aspergillus fumigatus collected from polluted soil of Kasur. Pakistan. Int. J. Biol. Sci.. 2013;2:66-73.
- [Google Scholar]
- Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch Biochem Biophy.. 2000;384(1):109-115.
- [CrossRef] [Google Scholar]
- Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch. Biochem. Biophys.. 2000;384:109-115.
- [CrossRef] [Google Scholar]
- Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in Nepal. Biomed Res. Int.. 2016;3238909:1-10.
- [CrossRef] [Google Scholar]
- Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbio.. 2007;52:498-502.
- [CrossRef] [Google Scholar]
- Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiol.. 2002;148(12):4003-4014.
- [Google Scholar]
- Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb Technol. 2007;41(1–2):57-61.
- [Google Scholar]
- Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris. Mol Biol Rep.. 2012;39:3807-3814.
- [Google Scholar]
- Biosorption of heavy metal arsenic from Industrial Sewage of Davangere District, Karnataka, India, using indigenous fungal isolates. SN Appl. Sci.. 2020;2:1860.
- [CrossRef] [Google Scholar]
- Improvement of laccase production from Ganoderma sp. KU-Alk4 by medium engineering. World J. Microbiol. Biotechnol.. 2007;23:1519-1527.
- [CrossRef] [Google Scholar]
- Are white-rot fungi a real biotechnological option for the improvement of environmental health? Crit Rev Biotech.. 2013;85(51):1-8.
- [CrossRef] [Google Scholar]
- Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci. Rep.. 2022;12(1):2416.
- [CrossRef] [Google Scholar]
- The role of fungal fuel cells in energy production and the removal of pollutants from wastewater. Catalysts. 2023;13(4):687.
- [Google Scholar]
- Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus. Biores. Technol.. 2010;101:2565-2567.
- [CrossRef] [Google Scholar]
- Isolation and characterization of laccase producing fungi from different environmental samples. Int J Recent Sci Res.. 2015;6:6853-6857.
- [Google Scholar]
- Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Proc. Biochem.. 2010;45(10):1720-1729.
- [CrossRef] [Google Scholar]
- Stable isotope fractionation during chromium (III) oxidation by δ-MnO2. InAGU Fall Meeting Abstracts. 2010;2010:H53F-H.
- [Google Scholar]
- Two records of Ganoderma new to mainland China. Mycotaxon. 2009;108:35-40.
- [CrossRef] [Google Scholar]
- Comparative study on crude and partially purified laccase from Polyporus durus ATCC 26726 in the decolorization of textile dyes and wastewater treatment. Egyp Pharm J.. 2018;17:94-103.
- [Google Scholar]
- White, T.J., Bruns, T.D., Lee, S., Taylor, J., 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ eds. PCR protocols, a guide to methods and applications. San Diego, California: Academic Press, pp. 315–322.
- Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl. Environ. Microbiol.. 1996;62(3):834-841.
- [CrossRef] [Google Scholar]
- Chromium (VI) bioremediation potential of filamentous fungi isolated from Peruvian tannery industry effluents. Brazilian J. Microbiol.. 2020;51:271-278.
- [CrossRef] [Google Scholar]
- Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. African J Microb Res.. 2012;6(6):1147-1157.
- [CrossRef] [Google Scholar]
- Global diversity of the Ganoderma lucidum complex (Ganodermataceae Polyporales) inferred from morphology and multilocus phylogeny. Phytochemistry. 2015;114:1-7.
- [CrossRef] [Google Scholar]
- Chemical and laccase catalysed oxidation of gallic acid: Determination of kinetic parameters. Res J Biotech.. 2013;8(7):62-65.
- [Google Scholar]
Further reading
- Purification and characterization of fungal laccase from Mycena purpureofusca. Chiang Mai J. Sci.. 2013;40(2):151-160.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102948.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







