Translate this page into:
Interbreed differences in iron concentration in cattle organs and tissues
⁎Corresponding author. k.n.narozhnykh@gmail.com (Kirill Narozhnykh),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
This study aimed to evaluate the differences in iron accumulation in organs and tissues of cattle bred in ecologically robust zones of Western Siberia.
Methods
The authors presented 486 samples of the muscle, hair, heart, liver, kidneys, and spleen from three breeds of bulls. The authors examined 84 Black Pied, Hereford, and Holstein bulls aged 16–18 months. The Black Pied bulls were bred in the Ubinsky district of the Novosibirsk region, Russia, the Hereford bulls were bred in the Maslyaninsky district of the Novosibirsk region, and the Holstein bulls were bred in the Promyshlensky district of the Kemerovo region, Russia. The authors studied the chemical composition of organs and muscle tissue using atomic absorption spectrometry with flame and electrothermal atomization using an MGA-1000 spectrometer.
Results
The median iron concentrations in the muscle, hair, heart, liver, kidneys, and spleen were 16.5–49.1, 22.3–130, 42–70, 56.5–68.3, 39.3–52.2, and 252.2–472 mg/kg, respectively. The authors recorded the highest iron concentration in the spleen, where its concentration was significantly higher than in other organs, regardless of the breed.
Conclusion
The breed significantly affected the iron content in muscle tissue, heart, kidneys, hair, and spleen. The iron concentrations in muscle tissue, heart, and spleen were higher in Black Pied cattle, and iron concentrations in hair were lower in Holstein and Hereford cattle. Multivariate statistical methods showed high phenotypic similarity in iron accumulation in organs and tissues between Hereford and Holstein cattle. Animals’ breed and age should be considered when developing regional standards for iron content.
Keywords
Iron
Black Pied
Hereford
Holstein
Organ
Tissue
- AAS
-
atomic absorption spectrometry
- ATP
-
adenosine triphosphate
- BP
-
Black Pied (cattle breed)
- CAS
-
chemical abstracts service
- CHA
-
concentrated hydrochloric acid
- EC
-
European Commission
- HF
-
Hereford (cattle breed)
- HPEG
-
high-purity ethylene glycol
- HS
-
Holstein (cattle breed)
- IQR
-
interquartile range
- mg/kg
-
milligrams per kilogram
- MGA-1000
-
the model name of the spectrometer used in the study
- NRC
-
National Research Council
- PCA
-
principal component analysis
- SF
-
scale factor
Abbreviations
1 Introduction
Iron is one of the most abundant trace elements and accounts for approximately 5 % of the Earth's crust (Haynes, 2016). Iron is vital for all animal species (Lieu et al., 2001) and is the most abundant trace element in the body (Suttle, 2022). In adult animals, iron concentration is, on average, 0.005–0.006 % in natural moisture (Suttle, 2022). Most iron takes the form of organic compounds. These compounds can be divided into two groups: porphyrin (heme) iron (70–75 %) and nonheme iron (25–30 %). Heme iron is represented by hemoglobin, myoglobin, and heme-containing enzymes: cytochromes, cytochrome oxidase, catalase, and peroxidase. Non-heme iron is represented by transferrin, ferritin, hemosiderin, and some iron proteins, including ferroflavoproteins (Khaleghnia et al., 2021).
Peptide hepcidin mainly regulates systemic iron homeostasis in vertebrates. Iron is released through interaction with the transmembrane protein ferroportin. Hepcidin circulates freely in the blood and weakly binds to albumin and α-2-macroglobulin (Itkonen et al., 2012; Peslova et al., 2009). It is synthesized and secreted by hepatocytes and is found in the lungs, kidneys, and muscle tissue (Basbugan et al., 2019). Hepcidin production is affected by iron concentration in the body. Hepcidin synthesis increases with iron levels, and peptide production decreases with iron stores.
Approximately 5–15 % of iron is usually absorbed with feed, but it can be twice as efficient with iron deficiency. The intensity of iron absorption is inversely proportional to the ferritin concentration in the serum (Souza et al., 2019). Iron absorption from the gastrointestinal tract is influenced by endogenous factors, including age, iron levels, gastrointestinal environment, and overall health. Exogenous factors, such as chemical form, amount of iron, and other feed components, also influence iron absorption in the intestine. After the destruction of red blood cells, most of the iron is reabsorbed and used to form new hemoglobin. Therefore, only a tiny proportion of iron is excreted from the body relative to the volume of iron in the feed (NRC, 2005).
Iron is involved in many biochemical processes, including oxygen transport, blood production, energy metabolism, immune functions, etc. Iron accumulation in the muscle tissue of cattle is closely related to the biochemical processes occurring in the body (Narozhnykh, 2023). Despite its crucial role, excessive iron intake has harmful effects and causes poisoning symptoms (Wysocka et al., 2020). Iron, like some other metals, can cause oxidative stress. Iron, along with copper, participates in the Fenton reaction and other reactions producing hydroxyl radicals from hydrogen peroxide and reactive oxygen species. The accumulation of reactive oxygen species leads to significant animal tissue damage (Wysocka et al., 2020). One hypothesis about the etiology of cell and organ damage from iron overload is that excess iron selectively targets mitochondria and possibly the mitochondrial genome (Eaton and Qian, 2002). Experimental exposure of liver and myocardial cells in laboratory animals to high iron concentrations results in decreased polyunsaturated fatty acids and ATP (adenosine triphosphate) (Chen et al., 2022; Videla and Valenzuela, 2022). An alternative approach assumes that iron accumulation strongly sensitizes lysosomes to damage and rupture, leading to autophagocytosis and necrosis. This approach is not a mutually exclusive mechanism for the toxic effects of iron on cells and organs (Eaton and Qian, 2002).
This work aimed to evaluate the differences in iron accumulation in organs and tissues of cattle bred in ecologically robust zones in Western Siberia.
2 Materials and methods
2.1 Animals and experimental design
Animals were bred on large livestock farms in rural areas more than 100 km away from megacities and industrial enterprises. Hereford (HF) cattle were bred in the Maslyaninsky district of the Novosibirsk region, Russia (coordinates 54.545861 N, 84.217811 E), Holstein (HS) cattle were bred in the Promyshlennovsky District of the Kemerovo Region, Russia (coordinates 54.720527 N, 85.262930 E), and Black Pied (BP) cattle in the Ubinsky District of the Novosibirsk Region (coordinates 54.904551 N, 79.318958 E). By selecting a single, well-managed farm for each breed, we ensured that the environmental and management variables were consistent, thus isolating the breed as the primary variable. This design allowed for a clear assessment of how the breed influences iron concentration under similar environmental conditions.
Animals of each breed were kept under standard conditions. Typical cattle feeding was carried out using a complete and balanced diet, depending on the type of productivity, age, and live weight according to the norms and recommendations. At the time of the study, the animals were in the same age group of 16–18 months and received diets containing 60 mg/kg iron, which corresponds to the norm for fattening bulls (Nutrient Requirements of Beef Cattle, 2015).
We did not consider the farm effect, as dairy cattle were housed in large industrial complexes with a uniform tethered housing system and diet standards for microelement composition. Conversely, beef cattle were kept in year-round loose housing, but the microelement balance in the diet, including iron, did not significantly differ from that of dairy cattle. Therefore, the main effect we aimed to assess was the influence of the breed on the iron content in cattle organs and muscle tissue.
It is important to acknowledge that the farm effect, encompassing a multitude of random effects, cannot be entirely ruled out. Subtle variations in environmental conditions, such as microclimate, water quality, and soil mineral content, may exist even within well-managed farms. However, given the standardized feeding practices and rigorous management protocols implemented on the selected farms, we believe that the breed factor remained the primary driver of observed differences in iron concentration.
Samples were collected from June to July 2023. Internal organ and muscle tissue samples were collected from 84 HF, HS, and BP animals (28 bulls of each breed). Hair (HF and HS n = 26, BP n = 28) samples weighing 10 g were taken from the area of the withers. We selected diaphragm muscles for muscle tissue samples (HF, BP, and HS n = 28). The weight of the organ samples was 100 g. We also sampled myocardial tissue (BP and HS n = 28, HF n = 26) from the apex of the heart. Liver samples (BP and HF n = 26, HS n = 28) were taken from the left lateral lobe; kidney samples (BP and HF n = 28, HS n = 26) were taken predominantly from the cortical layer; and spleen samples (BP n = 27, HS n = 23, HF n = 28) were taken from the posterior end. All animals were clinically healthy at the time of slaughter. The animals were slaughtered according to the rules prescribed in the European Commission's regulations CE 853/2004, 854/2004, and 1099/2009.
2.2 Sample analysis
Organ and tissue samples were taken immediately after slaughter, frozen, and stored at −18 °C. The samples were thawed at room temperature before analysis. The chemical composition of organs and muscle tissue was studied using atomic absorption spectrometry with flame and electrothermal atomization with an MGA-1000 spectrometer.
For the analysis, the samples were homogenized by removing connective tissue and fat. The resulting mass was dried in an oven at 60–70 °C for approximately 12 h until a constant weight was reached. Next, a 3 g sample was taken from the obtained dry residue, which was ozonized in a muffle furnace at 500–550 °C for 10–15 h. Mineralization was completed when the ash became gray or white. After cooling to room temperature, the ash residue was dissolved in 3 ml of concentrated hydrochloric acid (CHA) 20-4 (CAS 7647-01-0) and evaporated to a dry residue on an electric stove. Next, the wet sediment was transferred to a volumetric flask and diluted to 25 ml with distilled water. Finally, iron concentration in the prepared solution was determined.
A 10 mg hair sample was cleaned of contaminants and placed in a flask with bidistilled water. Then, the sample was stirred for 1 min with a 1,000 rpm mixer, and the water was changed up to 10 times; this procedure was repeated. Subsequently, hair was washed with acetone high-purity ethylene glycol (HPEG) 9–5 (CAS 67–64-1) for 2 min, after which the residue was washed three times with deionized water and dried at room temperature. The sample was then dissolved in 2 ml of nitric acid, pure grade 27–5 (CAS 7697–37-2), and placed in a standard MARS-5 microwave autoclave (CEM). The autoclave was gradually sealed for 40 min, after which the temperature was increased to 180 °C. Finally, the obtained solution was transferred to a volumetric flask. The solutions were analyzed after 10- and 100-fold dilutions using calibration solutions prepared based on multielement standards (Tsygankova et al., 2017).
2.3 Statistical analysis
Statistical analysis was performed using the R programming language (R Foundation for Statistical Computing, Vienna, Austria) (Racine, 2012). The null hypothesis of a normal distribution was tested using the Shapiro-Wilk test. The homoscedasticity was assessed using the Fligner-Killeen test. The Kruskal-Wallis rank sum test (Bower et al., 2023) and Dunn’s test (Boos and Duan, 2021) of multiple comparisons using rank sums and adjustment of the p-value for multiple comparisons using Holm (Gou, 2022) were used to detect differences in iron concentration between the breeds. Principal component analysis (PCA) was used for multivariate statistical data analysis. The number of main components was selected using the broken stick model and Kaiser's rule.
3 Results
3.1 Iron concentration in organs and tissues
The median iron concentrations varied significantly across the studied organs and tissues (Table 1). As expected, the spleen demonstrated the highest iron accumulation, with median concentrations ranging from 252.2 mg/kg in Holstein cattle to 472 mg/kg in Black Pied cattle. The spleen plays a crucial role in iron recycling from senescent red blood cells, and the observed high iron levels are consistent with its physiological function. The liver, another organ involved in iron metabolism, displayed relatively consistent iron concentrations across all three breeds, ranging from 56.5 mg/kg to 68.3 mg/kg. Note: Me – Median; Min – Minimum; Max – Maximum; Q1 – First quartile; Q3 – Third quartile; IQR – Interquartile range; SF – Scale factor; SF.p – Scale factor percentile. Source: compiled by the authors.
Sample
Breed
n
Me
Min
Max
Q1
Q3
IQR*
SF
SF.p
Muscles
Black Pied
28
49.1
30.3
79.5
36.6
62.2
25.6
0.947
0.145
Hereford
28
24.5
9.7
43
19.3
29.8
10.5
0.982
0.809
Holstein
28
16.5
9
27
13.4
19
5.58
0.938
0.091
Hair
Black Pied
28
22.3
9.37
147.6
17.1
37.1
19.9
0.679
<0.001
Hereford
26
29
13
87
22.9
51.2
28.3
0.884
0 009
Holstein
26
130
23
290
107.8
160
52.2
0.938
0.109
Heart
Black Pied
28
70
31
150
64.4
82.8
18.3
0.796
<0.001
Hereford
26
43.6
32
71.4
37.4
49.9
12.5
0.911
0.030
Holstein
28
42
27
52
41
46.6
5.58
0.943
0.119
Liver
Black Pied
26
68.3
37.9
122.8
55.5
77.5
21.9
0.914
0.034
Hereford
26
59.2
39.2
102
52.3
72.5
20.2
0.906
0.024
Holstein
28
56.5
33
219
42.8
66.6
23.8
0.654
<0.001
Kidneys
Black Pied
28
52.2
33.1
92.6
44.4
67
22.6
0.962
0.328
Hereford
28
39.3
24.7
82.9
33.5
49.7
16.2
0.869
0.003
Holstein
26
50
33
64
44
55
11
0.976
0.699
Spleen
Black Pied
27
472
139
1251
423.5
677.7
254.2
0.902
0.017
Hereford
28
252.2
91.2
535.9
164.6
351.3
186.8
0.97
0.490
Holstein
23
255
120
1,000
171.7
334.2
162.5
0.704
<0.001
Muscle tissue exhibited the lowest median iron concentrations, with values ranging from 16.5 mg/kg in Holstein cattle to 49.1 mg/kg in Black Pied cattle. Interestingly, Black Pied cattle also displayed the lowest median iron concentration in hair (22.3 mg/kg), even lower than that in muscle tissue, while Holstein cattle had the highest iron concentration in hair (130 mg/kg).
The Shapiro-Wilk test revealed that the iron concentrations in several samples deviated significantly from a normal distribution. Furthermore, the Fligner-Killeen test indicated heterogeneity of variances in most cases. Therefore, we employed nonparametric statistical methods, specifically the Kruskal-Wallis and Dunn’s tests, to compare the iron concentrations across breeds while accounting for these deviations from normality.
The boxplots visually demonstrate these differences in iron concentration (Fig. 1). Black Pied cattle generally exhibit higher concentrations of iron in muscle tissue, heart, and spleen compared to Hereford and Holstein cattle. However, their iron concentration in hair is notably lower than in the other two breeds. Holstein cattle tend to have the lowest iron concentrations in most organs except for kidneys and hair, while Hereford cattle display intermediate values.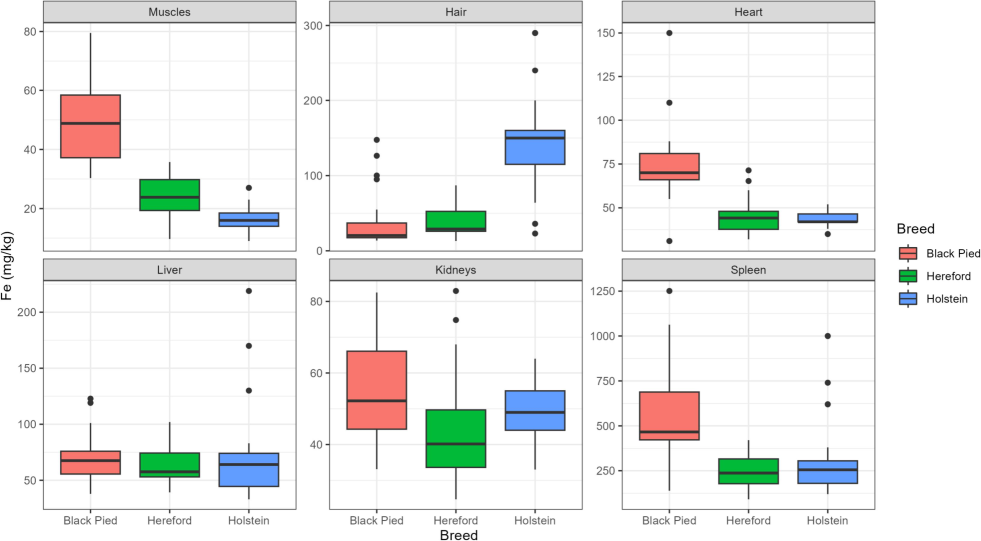
Distribution of iron concentrations in various organs and tissues of Black Pied, Hereford, and Holstein cattle. Source: compiled by the authors.
3.2 Interbreed differences in iron concentration
Statistical analysis revealed that the breed of cattle significantly affected the iron concentration in all studied organs except the liver (Table 2). This indicates a strong influence of breed-specific genetic and physiological factors on iron metabolism and tissue distribution. Source: compiled by the authors.
Sample
df
Fligner-Killeen median chi-squared
p
Kruskal-Wallis chi-squared
p
Muscles
2
27.518
>0.001
59.887
<0.001
Hair
2
15.423
>0.001
39.776
<0.001
Heart
2
8.815
0.012
44.221
<0.001
Liver
2
1.311
0.519
5.238
0.07
Kidneys
2
6.354
0.042
13.082
<0.001
Spleen
2
3.781
0.151
27.897
<0.001
Pairwise comparisons using Dunn’s test further clarified these interbreed differences (Table 3). Black Pied cattle consistently demonstrated significantly higher iron concentrations in muscle tissue, heart, and spleen compared to both Hereford and Holstein cattle (p < 0.001 for all comparisons). This finding aligns with the Black Pied breed's high milk production, which likely necessitates increased iron demand and mobilization to support lactoferrin synthesis in milk. Interestingly, the iron concentration in hair was significantly lower in Black Pied cattle compared to the other two breeds, suggesting a potential trade-off in iron allocation between milk production and other physiological processes. Source: compiled by the authors.
Sample
List of pairwise comparisons
Z statistic
P*
Muscles
Black Pied – Hereford
4.825
<0.001
Black Pied – Holstein
7.652
<0.001
Hereford – Holstein
2.827
0.023
Hair
Black Pied – Hereford
−0.750
0.2267
Black Pied – Holstein
−5.849
<0.001
Hereford – Holstein
−5.008
<0.001
Heart
Black Pied – Hereford
5.391
<0.001
Black Pied – Holstein
6.038
<0.001
Hereford – Holstein
0.534
0.296
Kidneys
Black Pied – Hereford
3.496
<0.001
Black Pied – Holstein
0.908
0.182
Hereford – Holstein
−2.522
0,006
Spleen
Black Pied – Hereford
4.795
<0.001
Black Pied – Holstein
4.258
<0.001
Hereford – Holstein
−0.303
0.381
Hereford cattle exhibited intermediate iron concentrations in most organs. In muscle tissue and spleen, their iron levels were significantly lower than those in Black Pied cattle but not significantly different from Holstein cattle. This finding may reflect the Hereford breed's selection for muscle growth, which could prioritize iron storage in muscle over other tissues. However, their significantly lower iron concentration in the kidneys compared to Holstein cattle (p = 0.006) warrants further investigation to elucidate potential breed-specific differences in renal iron handling.
Holstein cattle displayed the lowest iron concentrations in muscle tissue, heart, and spleen, consistent with their dairy breed status and a potentially lower emphasis on iron storage in these tissues. While their kidney iron levels were significantly higher than those in Hereford cattle, they did not differ significantly from Black Pied cattle.
Overall, these pairwise comparisons highlight distinct patterns of iron accumulation across the three cattle breeds, underscoring the influence of breed on iron metabolism and tissue distribution. Further research is needed to elucidate the specific genetic and physiological mechanisms underlying these interbreed variations, including the role of sex hormones and potential interactions between breed and sex effects.
3.3 Principal component analysis
To reduce dimensionality and identify general regularities in iron accumulation, the principal component method was applied. First, the eigenvalues for each piece were calculated (Table 4). One of the most popular heuristic approaches for estimating the number of necessary principal features is the broken stick rule (Fig. 2). According to this rule, two main components should be retained. The exact number of components should also be chosen according to the Kaiser rule because the eigenvalue of the first two principal components is more significant than unity. The first two main components explained 56.5 % of the variance in the original variables (Table 4). Source: compiled by the authors.
Index
PC1
PC2
PC3
PC4
PC5
PC6
Eigenvalue
2.2230
1.1667
0.9651
0.6725
0.6149
0.3579
Proportion explained
0.3705
0.1944
0.1608
0.1121
0.1025
0.0597
Cumulative proportion
0.3705
0.5649
0.7258
0.8379
0.9403
1.0000
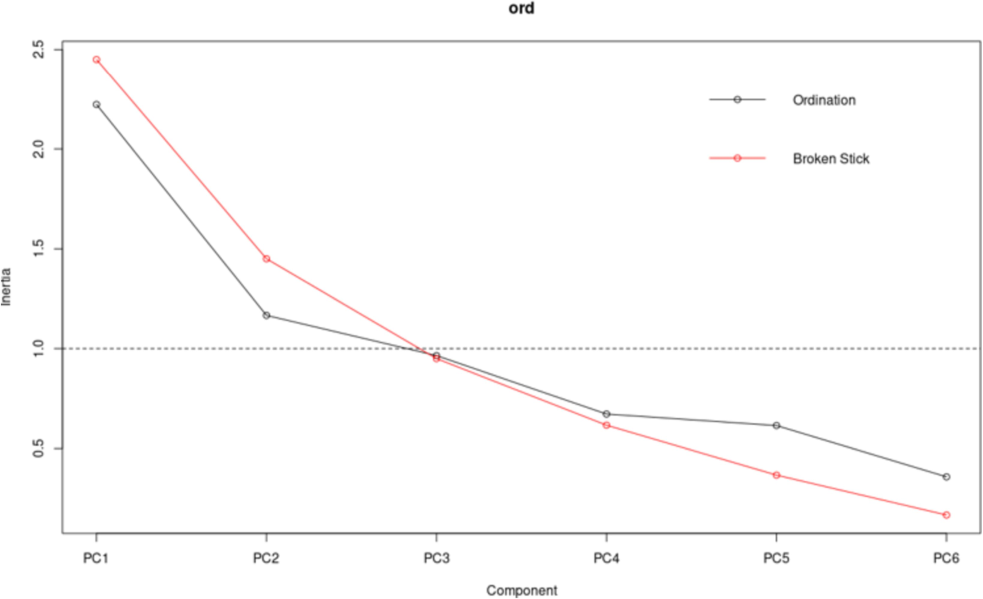
The plot of the eigenvalues of the principal components. Source: compiled by the authors.
Table 5 shows the factor loadings on the significant components. The most important contribution in the first principal component was in muscle tissue, heart, spleen, and hair and in the second, in the liver and kidneys. In the first component, the factor load in hair had the opposite direction, so animals along this axis had high iron concentrations in muscle tissue, heart, and spleen. However, iron levels in hair were low. High values of the second component occur in animals with increased iron concentrations in the kidneys and liver. Source: compiled by the authors.
Factor
PC1
PC2
PC3
PC4
PC5
PC6
Muscles
0.4482
−0.1282
0.0535
−0.1045
0.1271
−0.2329
Hair
−0.2980
−0.0382
−0.3795
0.0083
0.2562
−0.0388
Heart
0.4244
−0.2079
−0.0527
−0.0873
0.1315
0.2247
Liver
0.1293
0.4505
0.1546
0.0600
0.2304
0.0360
Kidneys
0.2371
0.2956
−0.2878
−0.2180
−0.1661
−0.0017
Spleen
0.3572
0.0169
−0.1861
0.3647
−0.0751
−0.0189
Principal component analysis (PCA) revealed a high phenotypic similarity between Hereford and Holstein cattle in terms of iron accumulation in organs and tissues (Fig. 3). Since the initial data were standardized before the principal component analysis; each ellipse's size equals one standard deviation for each breed. The most remarkable interbreed differences in iron accumulation in cattle organs and tissues were observed in the space of the first component. Thus, BP cattle had higher iron concentrations in muscle tissue, heart, and spleen and lower iron concentrations in hair than HS and HF cattle. HS and HF cattle had more similarities. HS cattle accumulated less iron in muscle tissue, heart, and spleen. BP cattle showed the most significant spatial variability in the first component. For the second principal component, we observed the greatest variability in HS cattle. However, no interbreed differences were observed in this component. The highest values for this component were observed in some HS cattle with an increased iron concentration in the kidneys and liver. HF cattle showed minor variability in the space of the second component.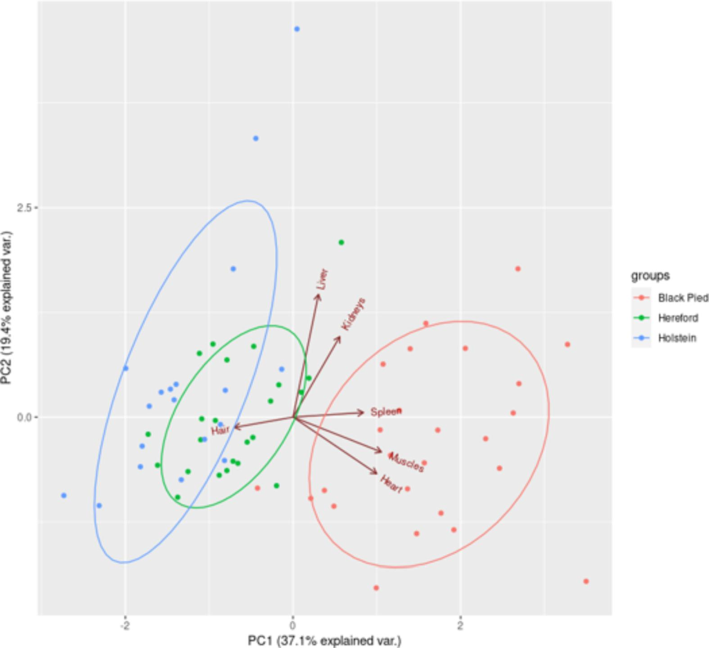
Distribution of iron levels in cattle organs and tissues (compiled by the authors).
4 Discussion
Sufficient iron in the diet is necessary to maintain physiological levels of red blood cells and hemoglobin (Mohri et al., 2010). The NRC established iron yield requirements for cattle diets in 1971 (Weiss, 2017). Age, sex, growth rate, and the bioavailability of dietary iron sources affect the iron requirements of cattle (Heidapour Bami et al., 2008; Prodanovic et al., 2014). The iron requirement for calves is estimated to be approximately 100 parts/million, and it is generally believed that young cattle require more iron than adult animals (Volker and Rotermund, 2000).
Heavy metal levels in cattle organs and tissues are influenced by many factors (Patel et al., 2020), and researchers study the effects of sex, breed, and farms on trace element concentrations. The contribution of factors to concentration variability differs for different trace elements. The breed has a relatively high effect of approximately 45 % on the iron concentration. Another study examines the effect of the breed and skeletal muscle type on iron levels in cattle (Miranda et al., 2018). Both factors and their interactions significantly (P < 0.001) affected the levels of this trace element in cattle. However, interbreed differences were only noted in one of the four skeletal muscle types (semimembranosus). The authors found interbreed differences in four of the six organs studied. This difference was especially noticeable in the muscle tissue, where each of the studied breeds differed from the others. However, in the other organs, representatives of one breed usually differed, and the two remaining breeds had no significant differences (Table 3). Domaradzki et al. (2016) report no differences in the iron content in muscle tissue among six cattle breeds. This study selected small groups of eight muscle tissue samples from each breed. Similar results were obtained (Duan et al., 2015) for larger samples (n = 24–45); the authors found no significant effect of the breed on the iron level in muscle tissue. In contrast, other studies have shown interbreed differences in the iron content of muscle tissue in cattle (Pereira et al., 2018; Pilarczyk, 2014a, 2014b). Anatomical and metabolic features can explain these differences (Baldwin et al., 2004; Bellmann et al., 2004; Fiems, 2012). Other organs (liver, kidney, and spleen) do not show differences in iron content between cattle of different productivity types and their crosses (Pereira et al., 2018). The phenotypic variability of iron levels in cattle organs and tissues os generally relatively low in contrast to the concentrations of pollutants, such as cadmium and lead (Narozhnykh et al., 2013, 2018; Skiba et al., 2017).
The iron level in the muscle tissue of cattle has been studied in sufficient detail (Table 5) because beef is one of the most important foods for humans. In our study, the iron level in muscle tissue varied considerably (16.5–49.1 mg/kg on average) depending on the breed. However, in most of the described cases, no effect of the breed on muscle iron concentration was found (Domaradzki et al., 2016; Duan et al., 2015; Pilarczyk, 2014a, 2014b). In contrast, Pereira et al. (2018) report significant interbreed differences in iron concentration, specifically in muscle tissue. Interbreed differences may only be evident in some types of muscle tissue. Miranda et al. (2018) find that some muscles (m. Semimembranosus) show differences in iron levels depending on the cattle breed, while others (m. Trapezius, Diaphragm, Cardiac) show no such differences. The intercostal space we studied can be characterized by high variability in iron levels depending on the breed. The data on iron levels in muscle tissue in our study are comparable to the results of other works carried out in different countries (Alonso et al., 2004; Bazargani-Gilani et al., 2016; Blanco-Penedo et al., 2006; Cabrera et al., 2010; Counotte et al., 2019; Czerwonka and Szterk, 2015; Domaradzki et al., 2016; Duan et al., 2015; Erdogan et al., 2004; Koréneková et al., 2002; Marshinskaia et al., 2021; Miedico et al., 2016; Miranda et al., 2018; Patel et al., 2020; Pereira et al., 2018; Pilarczyk, 2014a, 2014b; Puls, 1988; Rodríguez-Marín et al., 2019; Seiyaboh et al., 2018; Sharaf et al., 2020). According to Puls (1988), the adequate iron level in the muscle tissue of cattle should be in the range of 45–54 mg/kg, which is slightly higher than that reported in most of the papers. However, Puls does not specify the type of muscle tissue, breed, or breeding location where the values were obtained. The influence of such factors may have biased the estimation of iron concentration.
The observed interbreed variations in iron concentration likely reflect a complex interplay of genetic, physiological, and environmental factors, shaped by breed-specific selection pressures for distinct production traits and environmental adaptations. Different breeds of cattle have been selectively bred for diverse purposes, such as high milk production (e.g., Black Pied), superior meat quality (e.g., Hereford), or adaptability to specific climates. This selection process may have inadvertently shaped their iron metabolism and tissue distribution, leading to the observed variations in iron accumulation across breeds.
For instance, Black Pied cattle, renowned for their high milk production, may have evolved mechanisms for prioritizing iron allocation towards milk synthesis. This is supported by the observation that they exhibit higher iron levels in organs like the spleen and heart, potentially reflecting increased iron turnover and storage to support the high demand for iron in milk production. Lactoferrin, a crucial iron-binding protein found in milk (Sanchez et al., 1992), plays a vital role in the immune system and iron absorption in calves (Sanchez et al., 1992; Lonnerdal, 2003). Conversely, beef breeds like Hereford, primarily selected for muscle growth and meat quality, may prioritize iron storage in muscle tissue. This contributes to the characteristic red color of their meat, due to the high concentration of myoglobin, the primary iron-containing protein in muscle (Henriott et al., 2020). Myoglobin is essential for oxygen transport and storage, influencing both meat color and tenderness (Henriott et al., 2020; Mancini and Hunt, 2005).
These breed-specific adaptations are likely mediated by variations in the expression and activity of genes involved in iron metabolism. Studies have shown that polymorphisms in genes such as DMT1, responsible for intestinal iron absorption (Mateescu et al., 2013), and ferroportin (FPN), which regulates iron export from cells (Ganz, 2013), can influence iron status in cattle. It is plausible that the genetic backgrounds of Black Pied, Hereford, and Holstein cattle harbor distinct allelic variations in these and other iron-related genes, contributing to their unique iron accumulation profiles. Furthermore, hormonal factors, such as hepcidin, a key regulator of systemic iron homeostasis (Ganz and Nemeth, 2012), may also play a role in modulating interbreed differences. Breed-specific variations in hepcidin expression or responsiveness could contribute to the observed variations in iron storage across tissues.
Data on iron concentrations in the organs of cattle are less widely available than data for muscle tissue. We need to be aware of data on the iron content in the cattle heart. In our study, iron concentration in the myocardium was slightly greater than in muscle tissue. However, the myocardium is a type of muscle tissue, so their concentrations of iron are comparable. The most studied byproducts are the liver and kidneys, which are actively used for human food. Therefore, they are of great interest to researchers (Table 6). Source: compiled by the authors.
Country
Muscles
Liver
Kidneys
Lungs Heart
Spleen
Hair
Reference
Russia
49.1
68.3
52.2
70
472
22.3
This study
Russia
24.5
59.2
39.3
43.6
252.2
29
This study
Russia
16.5
56.5
50
42
255
130
This study
Canada
45–54
45–300
30–150
450–750
20–40
Puls, 1988
Italy
14.26
Patel et al., 2020
Italy
254
87.2
197
Miedico et al., 2016
Poland
17.3–22.8
66
Czerwonka and Szterk, 2015
Poland
17.66–21.77
Domaradzki et al., 2016
Spain
12–47
Miranda et al., 2018
USA
31.5–36.6
Duan et al., 2015
Poland
13.7–17
Pilarczyk, 2014b
Poland
13.3–15.7
Pilarczyk, 2014a
Spain
13.3–20
33.5–38.3
41–43.3
286.7–331.7
Pereira et al., 2018
Spain
19.5
80.9
Rodríguez-Marín et al, 2019
Slovakia
49.13–51.8
125.2–146.8
Koréneková et al., 2002
Polish
18.81–21.77
Domaradzki et al., 2016
Spain
38.7
70.3
51.3
Alonso et al.,2004
Russia
129–695
Marshinskaia et al., 2021
Iran
101.99
116.15
Bazargani-Gilani et al., 2016
Netherlands
465
Counotte et al., 2019
Pakistan
40.22–46.85
61.96–63.32
97.86–104.27
Sharaf et al., 2020
Nigeria
43.82
654.65
Seiyaboh et al., 2018
Turkey
65.4
57.4
Erdogan et al., 2004
Spain
43.6
58.9
Blanco-Penedo et al., 2006
Uruguay
14.2–48.2
Cabrera et al., 2010
Russia
80.2–1291
Miroshnikov et al., 2020
Russia
17.1–524.8
Miroshnikov et al., 2017
The liver is the only organ with no interbreed differences in iron levels. Similarly, a lack of breed effects on liver iron content was noted by Pereira et al. (2018). However, in the spleen and kidneys, there were no interbreed differences in this trait, in contrast to our data, where the differences were statistically significant. These differences can be explained by the fact that we studied different breeds, and interbreed differences in metal concentrations can be characteristic of different combinations when comparing breeds. The results we obtained for liver iron content are comparable to those of many studies (Alonso et al., 2004; Bazargani-Gilani et al., 2016; Blanco-Penedo et al., 2006; Czerwonka and Szterk, 2015; Erdogan et al., 2004; Pereira et al., 2018; Rodríguez-Marín et al., 2019). Iron levels significantly higher (4–10-fold) than ours are usually characteristic of cattle bred in environmentally disadvantaged areas (Counotte et al., 2019; Miedico et al., 2016; Seiyaboh et al., 2018). The reference values suggested for liver and kidney iron concentrations are 50–300 and 30–150 mg/kg, respectively (Puls, 1988). Iron levels in the liver and kidneys of cattle from Western Siberia were at the lower limits of these values. Data on the iron content in the spleen are limited. Although the spleen is associated with byproducts, it is usually used in animal feed. The spleen is the most generative organ for iron content, confirming its leading role in the metabolism of this metal (Bronte and Pittet, 2013; Li et al., 2015). Our findings are comparable to those of Pereira et al. (2018). However, their work showed no interbreed differences in iron accumulation in the spleen, in contrast to our study, where BP cattle had 47 % higher glandular levels than HF and HS cattle. However, according to Puls (1988), the reference range for metal content in organs is 450–750 mg/kg. In our work with HF and HS cattle, the iron level in the spleen was 40 % lower than this range. This discrepancy may be due to the features of paratypes and genetic factors that influence the level of the spleen in West Siberian cattle. Iron concentration in the spleen of cattle strongly depends on age. Thus, in animals aged 7–9 years, the iron level was more than 12 times greater than that in young cattle aged 1–3 years. The elemental composition of hair is of interest as a source for estimating the accumulation of metabolic pools of chemical elements in animals (Kalashnikov et al., 2019a, 2019b; Miroshnikov et al., 2019). The literature on the iron content in hair varies widely. For example, early studies indicated a narrow reference interval of 20–40 mg/kg in bovine hair (Puls, 1988). We obtained similar results for BP, HS, and HF cattle. However, recent works have established broader iron intervals. Thus, reference values of 80.2–1291 mg/kg have been proposed for lactating BP cows (Miroshnikov et al., 2020); values of 17.1–524.8 mg/kg have been reported for HF cattle (Miroshnikov et al., 2017). Our results for HS cattle satisfy this range. Another study showed that depending on the breeding region, the iron concentration in the hair of HS cattle varies significantly (Marshinskaia et al., 2021). Thus, it is logical to develop regional reference intervals of iron content in cattle organs and tissues, considering their age and breed.
Our study expands upon existing knowledge by investigating a wider range of organs and tissues and providing a detailed comparison of three distinct cattle breeds commonly raised in Western Siberia. Furthermore, our results highlight significant interbreed differences in iron accumulation in muscle tissue, heart, and spleen, which have not been extensively studied in previous research. Our findings emphasize the importance of considering breed-specific variations in iron metabolism when assessing animal health and nutritional requirements, as well as developing strategies for improving the iron content of beef products for human consumption.
5 Conclusion
This study demonstrates significant interbreed differences in iron content across various organs and tissues of cattle raised in Western Siberia. Black Pied cattle consistently exhibited higher iron levels in muscle tissue, heart, and spleen compared to Hereford and Holstein cattle, highlighting potential breed-specific adaptations in iron metabolism and allocation. These findings underscore the importance of considering breed as a factor influencing iron status in cattle.
From a practical standpoint, these results have implications for tailoring dietary iron management strategies for different breeds, particularly in dairy cows with high iron demands during lactation. For example, ensuring adequate iron intake through dietary supplementation or mineral mixes could be crucial for maintaining optimal health and productivity in high-yielding Black Pied dairy cows. Furthermore, understanding breed-specific iron accumulation patterns in beef can be valuable for optimizing the nutritional quality of beef products for human consumption. By selecting breeds with naturally higher iron content in muscle tissue, or by implementing dietary strategies to enhance iron levels, the beef industry can contribute to improving dietary iron intake among consumers, potentially mitigating the prevalence of iron deficiency. Future research should investigate the underlying genetic and physiological mechanisms responsible for these interbreed differences and explore strategies to optimize iron status in cattle to enhance both animal health and the nutritional value of beef. For instance, further studies could focus on identifying specific genes and regulatory pathways involved in iron metabolism in different breeds, allowing for the development of targeted breeding programs to enhance iron content in beef. Additionally, research on the bioavailability of iron from beef derived from different breeds could inform dietary guidelines and recommendations for optimizing iron intake from beef consumption.
Each analyzed breed was represented with samples from only one farm. Although these farms implement standards and breed-specific management practices, this sampling strategy limits the generalizability of our results.
When making consumption choices, especially for people with iron deficiency anemia, it is important to consider the differences in iron content between different organ types depending on the breed.
CRediT authorship contribution statement
Kirill Narozhnykh: Writing – review & editing, Visualization, Project administration, Methodology, Conceptualization. Valeriy Petukhov: Writing – review & editing, Validation, Resources, Project administration, Formal analysis. Tatiana Konovalova: Validation, Supervision, Resources, Methodology, Formal analysis. Olga Sebezhko: Writing – review & editing, Writing – original draft, Resources, Formal analysis, Data curation. Olga Korotkevich: Writing – review & editing, Writing – original draft, Resources, Formal analysis, Data curation.
Funding
The study was supported by the Russian Science Foundation (grant no. 22-76-00003, https://rscf.ru/project/22-76-00003/ (accessed on 05 April 2024)).
Acknowledgements
The study was supported by the Russian Science Foundation Grant No. 22-76-00003, https://rscf.ru/project/22-76-00003.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Interactions between toxic (As, Cd, Hg, and Pb) and nutritional essential (Ca Co, Cr, Cu, Fe, Mn, Mo, Ni, Se, Zn) elements in the tissues of cattle from NW Spain. Biometals. 2004;17(4):389-397.
- [CrossRef] [Google Scholar]
- Visceral tissue growth and proliferation during the bovine lactation cycle. J. Dairy Sci.. 2004;87:2977-2986.
- [CrossRef] [Google Scholar]
- Relation between hepcidin levels and hematologic parameters in cattle with theileriosis. Med. Wed.. 2019;75(6):355-359.
- [CrossRef] [Google Scholar]
- Heavy metals and trace elements in the livers and kidneys of slaughtered cattle, sheep, and goats. Iran. J. Toxicol.. 2016;10(6):7-13.
- [CrossRef] [Google Scholar]
- Beef versus dairy cattle: a comparison of metabolically relevant hormones, enzymes, and metabolites. Livest. Prod. Sci.. 2004;89(1):41-54.
- [CrossRef] [Google Scholar]
- Influence of copper status on the accumulation of toxic and essential metals in cattle. Environ. Int.. 2006;32(7):901-906.
- [CrossRef] [Google Scholar]
- Pairwise comparisons using ranks in the one-way model. Am. Stat.. 2021;75(4):414-423.
- [CrossRef] [Google Scholar]
- The spleen in local and systemic regulation of immunity. Immunity. 2013;39(5):806-818.
- [CrossRef] [Google Scholar]
- Selenium, copper, zinc, iron, and manganese contained seven meat cuts from Hereford and Bradford steers-fed pasture in Uruguay. Meat Sci.. 2010;84(3):518-528.
- [CrossRef] [Google Scholar]
- Effects of oxidative stress caused by iron overload on arachidonic acid metabolites in MES23. 5 cells. J. Biosci.. 2022;47(4):83.
- [CrossRef] [Google Scholar]
- Levels of trace elements and potentially toxic elements in bovine livers: a trend analysis from 2007 to 2018. PLoS One. 2019;14(4):e0214584
- [CrossRef] [Google Scholar]
- The effect of meat cuts and thermal processing on selected mineral concentration in beef from Holstein–Friesian bulls. Meat Sci.. 2015;105:75-80.
- [CrossRef] [Google Scholar]
- Evaluation of the mineral concentration in beef from polish native cattle. Biol. Trace Elem. Res.. 2016;171(2):328-332.
- [CrossRef] [Google Scholar]
- Sire breed effect on beef longissimus mineral concentrations and their relationships with carcass and palatability traits. Meat Sci.. 2015;106:25-30.
- [CrossRef] [Google Scholar]
- Molecular bases of cellular iron toxicity. Free Rad. Biol. Med.. 2002;32(9):833-840.
- [CrossRef] [Google Scholar]
- Seasonal and locational effects on dairy cows' serum, milk, liver and kidney chromium, manganese, copper, zinc, and iron concentrations. Biol. Trace Elem. Res.. 2004;98(1):51-61.
- [CrossRef] [Google Scholar]
- Double muscling in cattle: genes, husbandry, carcasses, and meat. Animals. 2012;2(3):472-506.
- [CrossRef] [Google Scholar]
- Hepcidin and iron homeostasis. Biochim Biophys Acta.. 2012;1823(9):1434-1443.
- [CrossRef] [Google Scholar]
- Quick multiple test procedures and p value adjustments. Stat. Biopharm. Res.. 2022;14(4):636-650.
- [CrossRef] [Google Scholar]
- Haynes, W.M., 2016. Abundance of elements in the Earth's crust and the sea. In: CRC Handbook of Chemistry and Physics, 97th ed. CRC Press, Boca Raton, pp. 14–17.
- Effects of parenteral supply of iron and copper on hematology, weight gain, and health in neonatal dairy calves. Vet. Res. Commun.. 2008;32(7):553-556.
- [CrossRef] [Google Scholar]
- Impact of myoglobin oxygenation level on color stability of frozen beef steaks. J Anim Sci.. 2020;98(7):skaa193
- [CrossRef] [Google Scholar]
- Binding of hepcidin to plasma proteins. Clin. Chem.. 2012;58(7):1158-1160.
- [CrossRef] [Google Scholar]
- The total content of toxic elements in horsehair is given the level of essential elements. Environ. Sci. Pollut. Res.. 2019;26(24):24620-24629.
- [CrossRef] [Google Scholar]
- Assessment of gender effects and reference values of mane hair trace element content in English thoroughbred horses (North Caucasus, Russia) using ICP-DRC-MS. Biol. Trace Elem. Res.. 2019;191(2):382-388.
- [CrossRef] [Google Scholar]
- The effects of parenteral iron administration on thyroid hormones, hematology, oxidative stress characteristics, performance, and health in neonatal Holstein calves. Biol. Trace Elem. Res.. 2021;199(5):1823-1832.
- [CrossRef] [Google Scholar]
- The concentration of some heavy metals in cattle reared in the vicinity of a metallurgic industry. Veterinarski Arhiv. 2002;72(5):259-268.
- [Google Scholar]
- Transcriptomic profiling of spleen in grass-fed and grain-fed Angus cattle. PLoS One. 2015;10(9):e0135670.
- [Google Scholar]
- The roles of iron in health and disease. Mol. Aspects Med.. 2001;22(1–2):1-87.
- [CrossRef] [Google Scholar]
- Bioactive proteins in breast milk. J Paediatr Child Health.. 2003;39(6):426-430.
- [CrossRef] [Google Scholar]
- Elemental status of farm animals from different regions with different environmental loads. IOP Conf. Ser. Earth Environ. Sci.. 2021;624(1):012199
- [CrossRef] [Google Scholar]
- Genome-wide association study of concentrations of iron and other minerals in longissimus muscle of Angus cattle. J Anim Sci.. 2013;91(8):3593-3600.
- [CrossRef] [Google Scholar]
- Environmental monitoring of the area surrounding oil wells in Val d'Agri (Italy): element accumulation in bovine and ovine organs. Environ. Monit. Assess.. 2016;188(6):338.
- [CrossRef] [Google Scholar]
- Importance of breed aptitude (beef or dairy) in determining trace element concentrations in bovine muscles. Meat Sci.. 2018;145:101-106.
- [CrossRef] [Google Scholar]
- The reference intervals of hair trace element content in Hereford cows and heifers (Bos taurus) Biol. Trace Elem. Res.. 2017;180(1):56-62.
- [CrossRef] [Google Scholar]
- The reference values of hair content of trace elements in the Holstein Breed dairy cows. Biol. Trace Elem. Res.. 2020;194(1):145-151.
- [CrossRef] [Google Scholar]
- The content of toxic elements in hair of dairy cows indicates animal productivity and elemental status. Environ. Sci. Pollut. Res.. 2019;26(18):18554-18564.
- [CrossRef] [Google Scholar]
- Effects of parenteral supply of iron on RBC parameters, performance, and health in neonatal dairy calves. Biol. Trace Elem. Res.. 2010;136:33-39.
- [CrossRef] [Google Scholar]
- Prediction models of iron level in beef muscle tissue toward ecological well-being. Glob. J. Environ. Sci. Manag.. 2023;9(4):833-850.
- [CrossRef] [Google Scholar]
- The content of lead in some organs and tissues of Hereford bull-calves. E3S Web Conf.. 2013;1:15003.
- [CrossRef] [Google Scholar]
- Lead content in the soil, water, forage, grains, organs and the muscle tissue of cattle in Western Siberia (Russia) Indian J. Ecol.. 2018;45(4):866-871.
- [Google Scholar]
- NRC (National Research Council), 2005. Mineral Tolerance of Domestic Animals. National Academy of Press, Washington, DC, 510 p.
- Nutrient Requirements of Beef Cattle, 2015. Nutrient Requirements of Beef Cattle, 8th Revised Edition. National Academies Press. https://doi.org/10.17226/19014.
- Exploration of the effect of farm, breed, sex, and animal on the detailed mineral profile of beef and their latent explanatory factors. Int. J. Food Sci. Technol.. 2020;55(3):1046-1056.
- [CrossRef] [Google Scholar]
- Trace element concentrations in beef cattle related to the breed aptitude. Biol. Trace Elem. Res.. 2018;186(1):135-142.
- [CrossRef] [Google Scholar]
- Hepcidin, the hormone of iron metabolism, is explicitly bound to alpha-2-macroglobulin in the blood. Blood. 2009;113(24):6225-6236.
- [CrossRef] [Google Scholar]
- Concentrations of toxic and nutritional essential elements in meat from different beef breeds reared under intensive production systems. Biol. Trace Elem. Res.. 2014;158(1):36-44.
- [CrossRef] [Google Scholar]
- Elemental composition of muscle tissue of various beef breeds reared under intensive production systems. Int. J. Environ. Res.. 2014;8(4):931-940.
- [Google Scholar]
- Relationship between serum iron and insulin-like growth factor-I concentrations in 10-day-old calves. Acta Vet. Brno. 2014;83(2):133-137.
- [CrossRef] [Google Scholar]
- Mineral Levels in Animal Health. Diagnostic Data. Clearbrook: Sherpa International; 1988. p. :168.
- RStudio: a platform-independent IDE for R and Sweave. J. Appl. Econ.. 2012;27(1):167-172.
- [CrossRef] [Google Scholar]
- Toxic (Al, Cd, and Pb) and trace metal (B, Ba, Cu, Fe, Mn, Sr, and Zn) levels in tissues of slaughtered steers: risk assessment for the consumers. Environ. Sci. Pollut. Res.. 2019;26(28):28787-28795.
- [CrossRef] [Google Scholar]
- Level of selected heavy metals in liver and muscles of cow meat sold in Yenagoa Metropolis, Bayelsa State, Nigeria. Int. J. Pub. Health Safe.. 2018;3(2):154.
- [Google Scholar]
- Comparative study of heavy metals residues and histopathological alterations in large ruminants from selected areas around industrial waste drain. Pak. Vet. J.. 2020;40(1):55-60.
- [Google Scholar]
- Direct determination of copper, lead and cadmium in the whole bovine blood using thick film modified graphite electrodes. J. Pharm. Sci. Res.. 2017;9(6):958-964.
- [Google Scholar]
- Serum biochemical profile of neonatal buffalo calves. Arq. Bras. Med. Vet. Zootec.. 2019;71(1):187-196.
- [CrossRef] [Google Scholar]
- Mineral Nutrition of Livestock. Wallingford; New York: CABI Pub.; 2022.
- Analysis of trace elements in hair of farm animals by atomic emission spectrometry with Dc Arc excitation sources. J. Pharm. Sci. Res.. 2017;9(5):601-605.
- [Google Scholar]
- Perspectives in liver redox imbalance: toxicological and pharmacological aspects underlying iron overloading, nonalcoholic fatty liver disease, and thyroid hormone action. Biofactors. 2022;48(2):400-415.
- [CrossRef] [Google Scholar]
- Possibilities of oral iron supplementation for maintaining health status in calves. Dtsch. Tierarztl. Wochenschr.. 2000;107:16-22.
- [Google Scholar]
- A 100-year review: from ascorbic acid to zinc – mineral and vitamin nutrition of dairy cows. J. Dairy Sci.. 2017;100:10045-10060.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103581.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







