Translate this page into:
Phytochemical derivatives and secondary metabolites rich Rhizophora mucronata as an active anti-oxidant and anti-bacterial agent against multi drug resistant bacteria
⁎Corresponding author at: Department of Biomedical Engineering, Noorul Islam Centre for Higher Education, Kumaracoil, Kanyakumari, Tamil Nadu 629180, India. muruganbt@niuniv.com (Manavalan Murugan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, the phytochemicals and anti-oxidant ability rich marine mangrove plant Rhizophora mucronata (R. mucronata) was evaluated. The UV-bio spectrometer result of O.D value of phenolic content was shown 0.986 as well as flavonoid content were shown 0.994 at 100 µg/mL concentration. The high yields of phenol and flavonoid contents were observed compared with standard gallic acid and rutin. Concisely, the more phenols and flavonoids and some bioactive derivatives were clearly exhibited by LC-MS scanning report. In addition, the anti-oxidant activity result shown with 85 % and DPPH scavenging assay result shown 66% in crude extract of R. mucronata were observed and it was very higher than the rate of standard gallic acid and ascorbic acid. Further, the liquid–liquid extraction of available bioactive compounds were purified and shown excellent anti-bacterial activity against multi drug resistant bacteria. The anti-microbial activity result indicated that the 250 µg/mL of R. mucronata extract was shown 24 and 26 mm zone of inhibition against K. pneumoniae and A. baumannii. Then, the concentration dependent inhibition of R. mucronata extract was shown against both the tested pathogens and 250 µg/m concentrations was fixed as a minimum inhibition concentration range. Finally, the induction of outer cellular layer morphology effect and damaged size and shapes of the R. mucronata extract was shown against K. pneumoniae and A. baumannii. Altogether, the present study results were deliberately recommended that the R. mucronata extract as potential anti-oxidant and anti-bacterial agent. Hence, the natural marine mangrove plant R. mucronata is the safe, eco-friendly, low cost source for discovery of potential drug against various infections.
Keywords
Mangrove region
Environmental parameter
Phytochemical screening
Anti-oxidant activity
Bioactive materials
1 Introduction
Each and every factor in the ecosystem is granting some advancement. Likewise, the fecal matter of mangrove crab influenced the growth of plants. The fecal matter present in the mangrove region acts as manure for that plant. During rainfall, the salinity of the water various and it impacts in the pH also, because of that organic matter and heavy metals which are driven here by tides makes some reaction (Alessandra et al., 2021). Mangrove environment additionally have the massive role in removing the carbon not only from the atmosphere and also from water by sediment along with it by presence of isotopes in the surface could trap the carbon particle. So it’s also called as carbon basket or carbon sink, it is being confirmed by two speculations.
Mangroves are shrubs and trees that grow in coastal intertidal zone with low- oxygen Soil. Mangrove forests are found in 116 countries in the tropical and sub-tropical regions. Mangroves act as a natural barrier, reducing the impact of the tsunami, cyclones, etc., through this Mangrove plays a pivotal role in protecting the shoreline. Although it’s not only protecting the shores but also prevents riverbank erosion (Chandra et al., 2015). It also has the ability to immobilize the heavy metals by acting as a buffer. Mangroves dominate most tropical and subtropical coastlines in the world. Nutrient availability can vary incredibly within the mangrove forest and nutrients are extremely low in the majority of mangrove soil (Shan et al., 2020). The local population along the coast is benefited by mangrove resources in terms of fishery and timber products. Mangroves are rich, extensive and complex ecosystems at the terrestrial, freshwater and marine ecosystems contributing variety of environmental goods like fish, mussels, edible oysters, condiments and honey, etc. (Syed Ali et al., 2021).
Among the mangrove, the plant Rhizophora mucronata (R. mucronata) belongs to the family verbenaceae is a blackish grey coloured plant grows as a shrub or tree in saline region. Its common name is black mangrove because of its colour (Han et al., 2007). R. mucronata is a cosmopolitan present all over the world, that can tolerate 90 ppt of salinity but its optimum requirement of salinity ranges from 3.5 to 17.5 ppt (Santini et al., 2015). It has higher tolerance and accumulative properties of heavy metals than any other mangroves (MacFarlane and Burchett, 2000). Similarly, Sayantani et al. (2021) reported that R. mucronata has various phytochemicals like tannins, unsaturated sterols, terpenes, flavonoids and organic acids. The available phytochemical derivatives including Isoquercitrin, luteolin, 5-Hydroxy-4′,7dimethoxyflavone are some flavonoids, lupeol, betulin, ursolic acid are triterpenoids, avicennone A, avicennone D, avicenol A are some naphthoquinone derivatives, oleic acid, stearic acid, lauric acid are fatty acid, stigmasterol, β-sitosterol and some chemical derivatives were reported by Leen et al. (2020). The extract of leaves, aerial parts, fruits and leaves possess anti-viral, anti-bacterial activity and the woods are used for snakebite. Some flavonoids show moderate cytotoxicity against breast cancer cell lines (Sharaf et al., 2000). Ethanolic root extract of R. mucronata have effective anti-bacterial activity against P. aeruginosa, B. subtilis, S. aureus, and E. coli and the leave extract show activity only against S. aureus, and E. coli among other microorganism (Okla et al., 2021). Recently, Asbin Mary et al. (2020) reported, 48.2 % of phenol content and 66.95 of flavonoid content of were available in R. mucronata, and confirmed analysis by GC–MS result including phenol, 2,4-bis(1,1-dimethylethyl), phytol, coumarine, pyran-4-one,7-hydroxy-2 phenyl, 3–92,4-dinitrophenyl. Similarly, phenol and flavonoid rich benzoic acid, penilpropanoid and isoflavone compounds were found from the extract of R. mucronata and they have excellent anti-oxidant activity (Yeni et al., 2019). In addition, the phenol and flavonoid rich derivatives of R. mucronata was utilized to analyse the NMR by Phuong et al. (2020), and the exhibited spectrums indicated that the more flavonoid compounds were present in the extracts such as flavone chrysin (5,7-dihydroxy flavone)-, Cinchonain Ib, Polystachyol, β-Sitosterol 3-O-β-D-glucopyranoside, 1,3,4-trisubstituted phenyl, β-sitosterol3-O-β-D-(6′-O-palmitoyl)epicatechin, methylene, phenylpropanoid, pyranone, cinchonanin and 4-hydroxyphenyl 1-O- β-D-[6-(p-hydroxybenzoyl)]glucopyranoside.
Based on the recent study reports, the current study was highlighted the screening of available phytochemical derivatives, phenol, flavonoid and available bioactive compounds from marine mangrove plant R. mucronata. In addition, the anti-oxidant and biological activities of the compounds were further performed against various invitro experiments.
2 Materials and methods
2.1 Chemical and glass wares
Basic chemicals of this work were used in this research was purchased from Suresh Scientific & Co. In addition, some solvent and media were procured from Merck India PVT limited, Sub organization of Ponmani, Tiruchirappalli, Tamil Nadu, India. Detection and identification of phytochemicals and anti-oxidant using various chemicals were chosen by Suresh Scientific & Co, Tiruchirappalli, Tamil Nadu, India.
2.2 Preparation of R. mucronata samples
Initially, look out and carefully pluck the fresh, undamaged, well grown R. mucronata leafs for detection of phytochemicals and anti-oxidant property from Muthupet Mangrove Region, Thiruvarur District, East Coast of Tamil Nadu, India. The surface contaminant was removed by tap water and followed by distilled water. Followed by alcohol wash made to remove surface contamination and free floating microbes which are abundantly present in surface of the plant. To finalize the powder formation, the dried samples were ground and taken safe and use for extraction (Rajivgandhi et al., 2021).
2.3 Extraction of crude extract preparation
Initially 1:1 ratio concentration, about 1 Kg of R. mucronata powder was mixed with 1L of ethanol solution to keep in soxhlet instrument with 100 ℃ with 12 h incubation. After incubation, the process was monitored and cools the process when the colour of grey or green turn to yellow, clear yellow in requested time interval. It may be taken 12–15 h for complete the extraction process. Then, the cooled solvent separated in upper portion and mixed R. mucronata leaves were settling down separately in bottom of the flask. Both the layers of the samples were separately collected in1, 000 mL beaker using what man No.1 filter paper as a filtrate material. After, the liquid phase of the organic phase was dried in hot air oven at 45 ℃ and finally use rotary evaporator to complete dry the samples. The yield of the dried product was detected universal formula which is available in previously reported evidence (Nur et al., 2018; Ukwubile et al., 2020).
2.4 LC-MS analysis of phytochemical and anti-oxidant properties of dried extract
Based on the NIST library comparison, the extracted R. mucronata extract was performed by LC-MS spectroscopy for detect the alkaloids, flavonoid, phenols, bioactive materials and other organic materials. The interpretation was followed by previously published articles of Jamal et al. (2021). The program was set at 60-120℃ for 5–30 min respectively and also, the 200–300 ℃ of constant temperature was also set, and crosscheck the injector coupled detector was attached with this instruments. After set these manually, the capillary column was checked thoroughly which it fixed automatically or not for 30 m × 200 μm × 0.30 μm. Manually set the flow rate that it maintains 1 mL/min and gas carrier of 30 cm/s with linier velocity was made. Finally, the sample was put in in the respective tray and run the LC-MS machine for detect the chemical molecules of R. mucronata extract. After scanning, the result was checked with NIST library with retention time and occupation percentage of the extract.
2.5 Detection of phenol and flavonoid content
For detection of the phenols and flavonoid content availability in R. mucronata extract was performed by UV-spectroscopy reading analysis and followed by universal method of folin-ciocalteu’s (Varaprasadham et al., 2010). 1:5 ratio of R. mucronata extract and 10 fold dilution of folin-ciocalteu’s reagent were taken together in 100 mL test tube. Followed by addition of Na2 CO3 into the same mixture tubes before the previous reaction was done in tube and shaken gently to mix equally. The mixture of the sample was shaken vigorously to mix each other using shaking incubator with 1 h time interval. Then, the mixture tube was performed to scanned by UV–vis spectroscopy for detection of presented phenols and flavonoid contents of the R. mucronata extract. For working control, the various concentration of 10–100 ug/mL of gallic acid was used, as same as without extract of the gallic acid was acted as a negative control. After O.D value, the result was checked that the 1ug gallic acid was equal to conversion of 1 mg tested sample or not.
Further, the flavonoid derivatives of R. mucronata content were detected by colorimetric analysis performed by earlier report of Atamgba et al. (2015). As like phenolic detection, the 100 mL tube was filled by 200 µl of extract and 75 µl of sodium nitrate and mixed slightly for generate the initial process. Followed by addition of 1: ratio of AlCl3 and 1% potassium iodide combination was taken into the same tube, which added drop by drop through wall of the tubes gradually. The mixture sample was kept in shaking incubator at 1 h for complete mixture of the sample. Then, the sample was performed by analysis of O.D value using colorimeter at 520 nm. For flavonoid content detection, the rutin was performed as a control and without Avicennia sp. extract of the rutin maintained for negative control. As same as the 1 ug of rutin concentration equal to 1 mg of the sample or not was calculated.
2.6 Anti-oxidant effect of R. mucronata extract
The extracted R. mucronata extract was utilized in Phosphor-molybdenum method for detection of their anti-oxidant activity based on the procedure of recent report of Trouillas et al. (2003). Each two tube were taken with 5 mL of sodium phosphate and ammonium molybdate, and then addition of 5 mL H2SO4 into the available tubes and it were mixed thoroughly. In one tube, the sample mixture of each 2 mL was taken and put in water bath at 100 ℃ for 1 h. The shaking water bath facility is good for the solution mixture and then cools after incubation. Then, 1 mL of the cooled solution was taken in new tube and mixed with 10 µl of R. mucronata extract and maintained at room temperature 15 min time interval. Between the time interval, the anti-oxidant property was formed and it monitored by spectrophotometer with the O.D value of 610 nm. For anti-oxidant activity, ascorbic acid as a positive control and it used to comparison of available anti-oxidant property of R. mucronata. Finally, the result was considered as 1 µg ascorbic acid is equal to 1 mg of R. mucronata extract.
2.7 Free radical scavenging method by using DPPH
To check the amount of free radical that present in the R. mucronata extract was detected by DPPH assay followed previously published article procedure of Tlili and Sarikurkcu (2020). The sterile tubes were filled by 150 µl of DPPH solution and various concentrations (10–100 µg/mL) of R. mucronata extract. As same as 5ul butylated hydroxyl toluene with DPPH without addition of extract was taken in separate tube for control. All the experimental tubes were maintained at room temperature for 1 h and formed scavenging properties were scanned by UV-spectrometer at O.D value of 610 nm. The anti-oxidant activity result was interpreted based on the control tube result using available formula,
2.8 Bacterial inactivation using agar well diffusion method
The disruption of bacterial growth in the muller hinton agar plate was observed with zone formation by agar well diffusion method followed by Khalid et al. (2021). Briefly, the 24 h matured K. pneumoniae and A. baumannii were spread on the fresh muller hinton agar plate and wells were punched on the agar surface. Then, 50–250 µg/mL of R. mucronata extract was added into the wells and maintained at 37 ℃ for 24 h incubation. After incubation, the zones around the wells were calculated to confirm the anti-bacterial ability of R. mucronata extract. The zones were calculated in diameter.
2.9 Microbroth dilution method
The confirmation of anti-bacterial ability of R. mucronata extract was further validated by microbroth dilution method to detect the effective concentration that has highest inhibition of tested K. pneumoniae and A. baumannii (Ramachandran et al., 2019). Shortly, the nutrient broth was prepared freshly and added into the separate 96-well polystyrene plates which have the quantity of 100 µg/mL concentration. Followed by both the pathogens with 10 µg/mL were added into in respective 96-well plates. Subsequently, 25–250 µg/mL concentration of R. mucronata extract was treated in all the wells except control wells as filled fresh broth with pathogens only. Then, all the plates were maintained at room atmosphere for 24 h time interval. Finally, the lowest concentration of the R. mucronata extract inhibit the bacteria in high level was noted and fixed as a minimum inhibition concentration and this concentration was used for further invitro experiments.
2.10 Outer morphology inactivation of R. mucronata extract
The outer morphology of K. pneumoniae and A. baumannii was disrupted by the R. mucronata extract through minimum inhibition concentration was proved by scanning electron microscope, the procedure was followed by earlier report of Famuyide et al. (2020). Initially, 48 h matured cultures of K. pnumoniae and A. baumannii were fixed by 4% glutaraldehyde using cover glass and allowed 6 h for complete fixation. After complete fixation, the cover glass was treated by dehydration using alcohol in the preparation of 30–100%, each process conducted with each 10 min time interval. Finally, the dehydrated cover glass was washed by n-butanol and treated by t-butanol until complete fixation into the pathogens. Then, the samples were put on deep freezer for 6 h. After the time interval, the samples were taken out and cool, then seen under scanning electron microscope under 40 × magnifications (Zeiss, Japan).
3 Result and discussion
3.1 Phytochemical screening of R. mucronata extract
Based on the LC-MS result, we have confirmed that the R. mucronata extract has more contents of phenols, terpens, alkaloids, flavonoids, essential oils, organic materials, some hydrocarbons and bioactive metabolites (Fig. 1). Based on the NIST Wiley, the confirmed bioactive ability and anti-oxidant activity of the phytochemical derivatives were screened from 35 peaks, and confirmed peak was 11 only, as per previous report of Ukwubilea et al. (2019); Casuga et al. (2016), the peaks related with phytochemicals were found based on the retention time and occupied percentages including coumarins, 3-hydroxy-4-phenyl, di-tert-butyl-2-hydroxybenzylidene-amino-2-indanol, tritriacontane, triterpenoid saponins, oxalates, nonyl, 1,4-diaza-2,5-dioxo-3-isobutyl bicyclo[4.3.0] nonane cycloicosane, tetracosahexaene, 2,3-propanetriol,diacetate, benzene, 1,1′-[oxybis(methylene)]bis, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpr, 1,2,3-trimethyl, hexadecanoic acid, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpr sitosterol and 12,15-octadecatrienoicacid. All those resulted compounds, derivatives and essential oils were conveyed the information about available properties of R. mucronata extract has excellent reservoir to biological properties. Also, it suggested that the R. mucronata is the best choice to discovery of some novel compounds which act as an inhibition role against pathogenic bacteria. Previously, Rajivagndhi et al. (2018) reported that the R. mucronata is the essential plant to synthesize biological properties. It exhibited some excellent bioactive compounds based on the different environmental parameters and fluctuated atmospheric nature. The undesirable environment may influence the plant stimulants and it produces some excellent biological molecules (Han et al., 2007). Previously, Jamal et al. (2021), reported that the extract of R. mucronata inhibit the quorum sensing molecules and interfere the group formed bacteria. LC-MS based phytochemical detection was very important method to find the bioactive metabolites and it act as an effective identification method.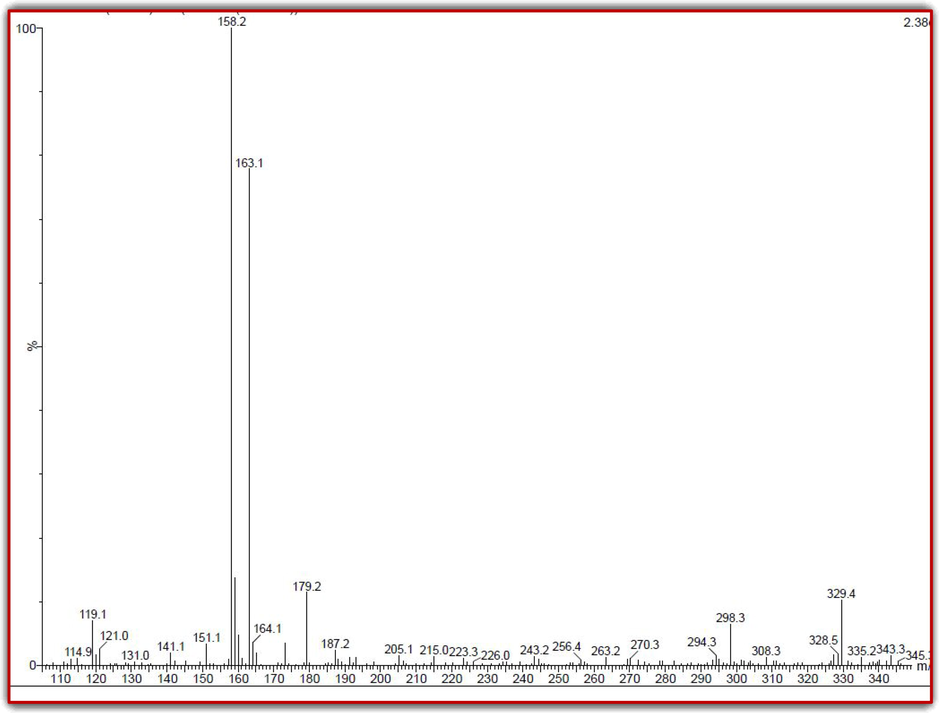
Detection of phytochemical and bioactive compounds of mangrove plant of R. mucronata extract by LC-MS analysis.
3.2 Measurement of phenolic and flavonoids contents of crude extract
The enormous amount of phenols and flavonoid contents available in the R. mucronata extract were confirmed after reading with UV-spectrometer. In addition, the result was also interpreted with controls result and confirmed that the R. mucronata extract has abundant phenols and flavonoid contents. Concisely, the R2 = 0.986 percentage of phenolic content yield was found from the R. mucronata extract as well as the respective values of R2 = 0.977 was found for gallic acid, their linearity of the calibration curve was started ranging 25–250 µg/mL concentration for both the samples. In addition, the values of R2 = 0.994 was obtained from R. mucronata extract for flavonoid content. Whereas the exhibited standard value curve of rutin was R2 = 0.969, it was compromised to the extract due to the lowest value yield of concentration. Based on the interpretation with control results, the available phenol and flavonoid contents were higher in R. mucronata extract instead of protein and sugar content. Based on the reported evidence, the R. mucronata extract was used for further study of this current research. The depicted photogram results of standard gallic acid (Fig. 2a), rutin (Fig. 2c) and available for phenols (Fig. 2b), flavonoid (Fig. 2d) contents were effectively available in Fig. 2. Similarly, marine mangrove plant of R. mucronata extract as an excellent reservoir for producing high level of phytochemical compounds including phenol and flavonoid derivatives (Santini et al., 2015). In addition, it also exhibited very high compared with terrestrial plant extract. It may be happened by influence of marine environmental parameter and nutrients. Because, marine has unpredictable environmental condition and also often fluctuated of intertidal capacity (MacFarlane and Burchett, 2000; Varaprasadham et al., 2010). Further, the protein and sugars percentages of this study were also clearly confirmed with lowest level, and it confirmed that the R. mucronata can be used as a future drug discovery target. Previously, Leen et al., 2020 and Aseer et al. (2015) reported that the R. mucronata is the excellent mangrove plant, and used for discovery of novel compound due to the unfavourable atmospheric nature including nutrients, minerals, temperature, carbon, pH and various stress conditions.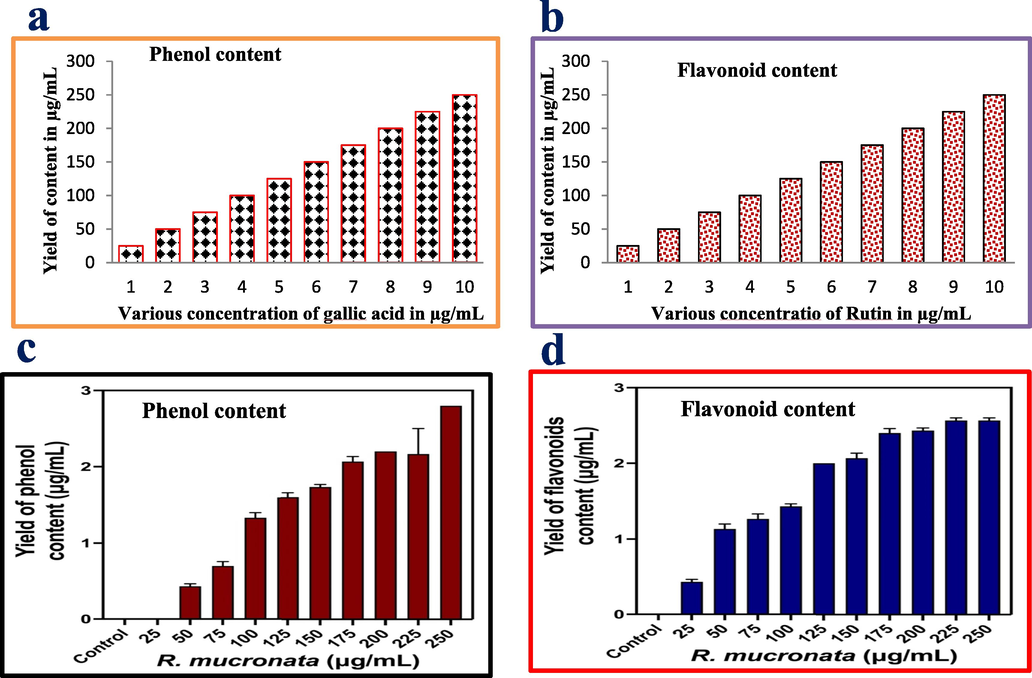
Measurement of the phenolic and flavonoid contents of the standard control values of gallic acid (a) and rutin, available phenol (c) and flavonoids (d) of the mangrove plant of R. mucronata extract by UV-spectroscopy.
3.3 Anti-oxidant and DPPH radical scavenging activities
As like as phenol and flavonoids, the anti-oxidant and formed free radical activities were shown excellent levels in R. mucronata extract, and the results were confirmed that the extract has more anti-oxidant and hydroxyl scavenging activity. Usually, the abundant level of carbohydrate present in the plant extract was usually produced more biomedical and pharmaceutical properties (Ramachandran et al., 2020). As like as, the present result was expected with excellent bioactive compounds synthesizers and it has rich anti-microbial anti-oxidant, larvicidal, immunomodulatory effect, anti-cancer, and other biological activities. In this study, the test sample was closely correlated with controls of gallic acid and rutin at 300 µg/mL. Recently, Suganthy and Pandima Devi (2016) reported, the high content of phenol and flavonoids rich plant extract of R. mucronata may produce excellent anti-oxidant activity (Fig. 3). As followed the previous report, the current study of marine mangrove plant R. mucronata extract was utilized to perform their total antioxidant and DPPH assay using invitro experiments. After comparison, the plant extract was shown very high in anti-oxidant activity and DPPH scavenging assay than the result of control ascorbic acid. The result was more favor to the inter relationship between the sample concentration and antioxidant values. Evidently, 86% of the antioxidant activity and 75% of DPPH scavenging activity were observed for plant extract as same as 76% and 68% were observed against ascorbic acid respectively at 350 and 300 µg/mL concentration (Fig. 4). All the results were proved, the concentration based anti-oxidant activity of control and test samples were more correlated. There were no significant differences for both the samples at tested concentration. Similar report of excellent anti-oxidant and DPPH assay results were reported by Gurudeeban et al. (2015) based on the tested concentration in control and tested samples. The result was agreed by Youssef et al. (2023), the reactive oxygen species and reactive nitrogen species rich plant bioactive compounds or chemical derivatives have played a role in increased anti-oxidant activities in the DPPH assay process, and also decreased the presence of active molecules, carbohydrates, proteins, lipids and protein contents. Therefore, the current results were more useful to the plant extract of R. mucronata for drug discovery research against various biomedical applications. Based on the all results, this study was proved that the mangrove plant Rhizophora mucronata has excellent phytochemical and anti-oxidant producer. It suggested that the research was more helped to future drug discovery research. The supportive result evidence was reported by Jairaman et al., (2019) and DPPH assay of R. mucronata was observed. Also, Vasanthakumar et al., (2019) documented that the unpredictable environmental factors were also influenced the phenols, flavonoids, anti-oxidant and DPPH radical scavenging activity. This statement was also supported by Shekhar Das et al. (2020), and mangrove region was very difficult to understand due to the fluctuated atmospheric nature.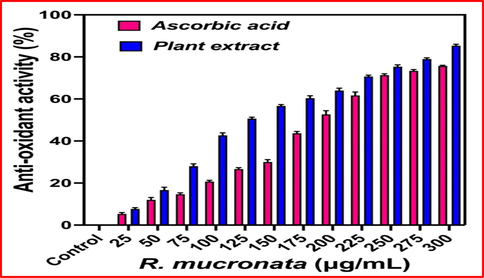
Detection of total antioxidant activity of R. mucronata extract by invitro analysis.
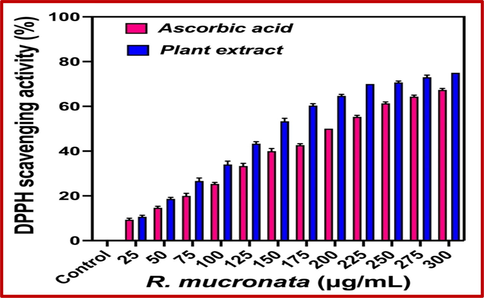
Detection of DPPH scavenging activity of R. mucronata extract by invitro analysis.
3.4 Anti-bacterial inactivation
The inactivation of bacterial growth using R. mucronata was exhibited 24 mm and 26 zones of inhibition for K. pneumonia and A. baumannii by agar well diffusion method (Fig. 5a, b). Initially, there are no any zones for below 25 µg/mL concentration. Based on the used concentration, the bacterial growth was arrested significantly in increasing concentration. The zone of inhibition against both the pathogens results were effectively presented in Fig. 5c. In addition, the 250 µg/ml concentration was more efficient to interact with bacterial surface through surface properties. As both the bacteria were gram negative bacteria, the thick theichoic acid layer and lipid contents were digested by R. mucronata extract through the charged particles. Previously, Zhou et al. (2022) reported that the R. mucronata has more phenol, flavonoid and bioactive compounds, all the compounds were efficiently bind with negative charges containing surfaces of the K. pneumonia and A. baumannii. So, the extract was entering into the bacteria and arrests the growth of the bacteria due to the influence of cytoplasmic granules, polysaccharide degradation and nucleolus deformation. Based on the previous report, the excellent permissible ability of the R. mucronata extract arrested the bacterial growth completely (Hardoko et al., 2016). Therefore, the initial screening of the anti-microbial activity result was more favour to R. mucronata extract, and it was excellent anti-bacterial agent against K. pneumonia and A. baumannii.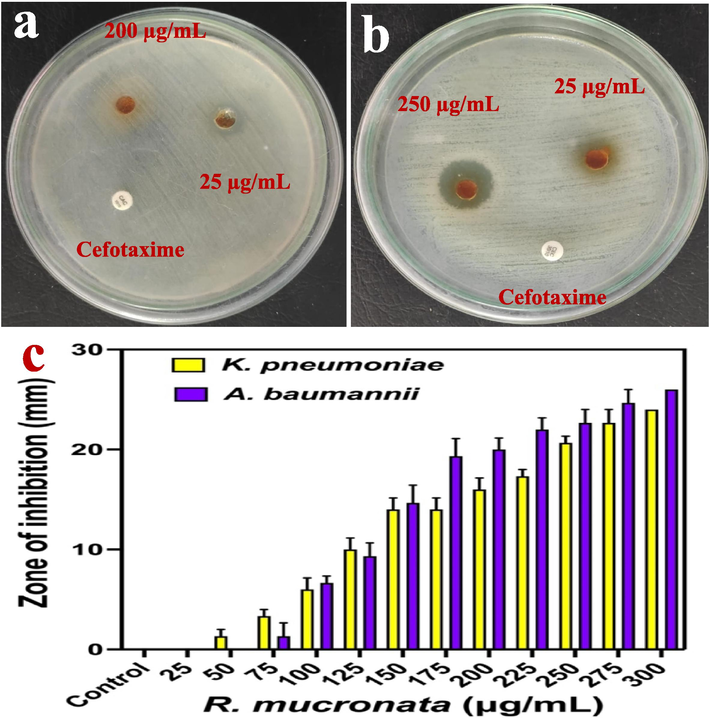
Anti-microbial activity of R. mucronata extract against K. pneumoniae (a) and A. baumannii (b). The various concentration of R. mucronata extract and their zone of inhibition level against K. pneumoniae and A. baumannii (c).
3.5 Microbroth dilution method
The 96-well plate method of minimum inhibition concentration detection was very efficient method to detect the inhibition concentration of bacteria (Rinto et al., 2022). In our result, the inhibition 90% and 92% of inhibition against K. pneumoniae at 200 µg/mL and 250 µg/mL (Fig. 6) were observed. The bacterial inhibition was confirmed as concentration-dependent due to the visible observation in the 96-well plate. The plates between before and after treatment, the more turbidity was shown at 200 µg/mL and 250 µg/mL treated wells for K. pneumoniae and A. baumannii. In addition, the turbidity was significantly increased in increasing concentration when compared with initial lowest concentration. So, weather the inhibition was based on the concentration dependent or not was confirmed by the result of O.D values. The calculated spectrophotometric O.D values were more favour to the R. mucronata extract, and shown the highest inhibition percentages were observed in higher concentration. The initial death cells were started at 25 µg/mL concentration for K. pneumoniae, 50 µg/mL concentrations for A. baumannii. The half inhibition of 56% and 52% were found at 100 µg/mL concentration for both the bacteria. Therefore, the result was confirmed that the 200 µg/mL and 250 µg/mL concentration for K. pneumoniae and A. baumannii were fixed as a minimum inhibition concentration. Also, these minimum inhibition concentrations were used to damage or cell cycle arrest or intracellular and extracellular destruction in gram negative bacteria. Recent reports of Luksamee et al. (2022); Yunos et al. (2021), the concentration dependent inhibition of R. mucronata extract as an excellent plant source to eradicate the multi-drug resistant bacteria. Similarly, Gurram et al. (2022) also reported that the R. mucronata has potential anti-bacterial components and it was confirmed by GC–MS analysis. Based on the supportive evidence of antimicrobial property results of agar well diffusion and minimum inhibition concentration experiments, the selected R. mucronata is an effective anti-bacterial agent.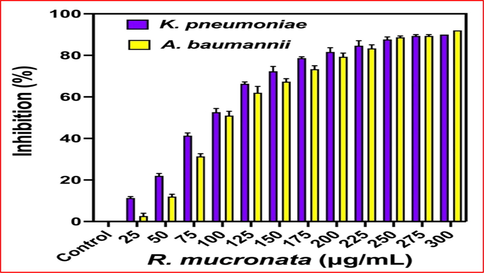
Detection of concentration dependent and lowest inhibition range effect of R. mucronata extract against K. pneumoniae and A. baumannii by microbroth dilution method.
3.6 Outer morphology damages by SEM
The anti-bacterial effect of R. mucronata was further evaluated against outer morphology destruction against both the K. pneumonia and A. baumannii. As per altered outer morphology result, the plant extract containing bioactive compounds were influenced the bacterial growth through surface charges. The positive charges of the active chemical components were effectively bound with negative charges on bacterial surface through electrostatic interaction. After, the outer cell membrane was degraded and it lost their original size and shape. If both the bacteria as gram negative, the outer lipid layer was compromised and thioctic acid was digested. When compared with control (Fig. 7a, c), the compromised cell membrane structure with changed morphology, size and shapes were found (Fig. 7b, d). As both bacteria are gram negative, rod shape, the control cells were clearly exhibited with original size and shape of the morphology after 24 h, whereas the belbing formation, crashed cells, destruction morphology, leakages of cytoplasmic materials with absence of rod shape was shown in R. mucronata treated K. pneumoniae and A. baumannii were clearly observed. The morphological changes of gram negative bacteria due to the plant bioactive compounds, chemical constituents were reported by Fenella et al. (2017). Recently, the unfavourable marine environment supplied the additional nutrients to R. mucronata to produce potential bioactive metabolites against various pathogenic infections (Zhou et al., 2022). The outer morphology damages due to the effect of the plant materials were agreed by Famuyide et al. (2020). Altogether, all the invitro anti-bacterial inhibition and morphological damages study results were clearly stated, the marine mangrove plant R. mucronata as an efficient plant materials which can be used for inhibition of various bacterial infections through drug discovery process.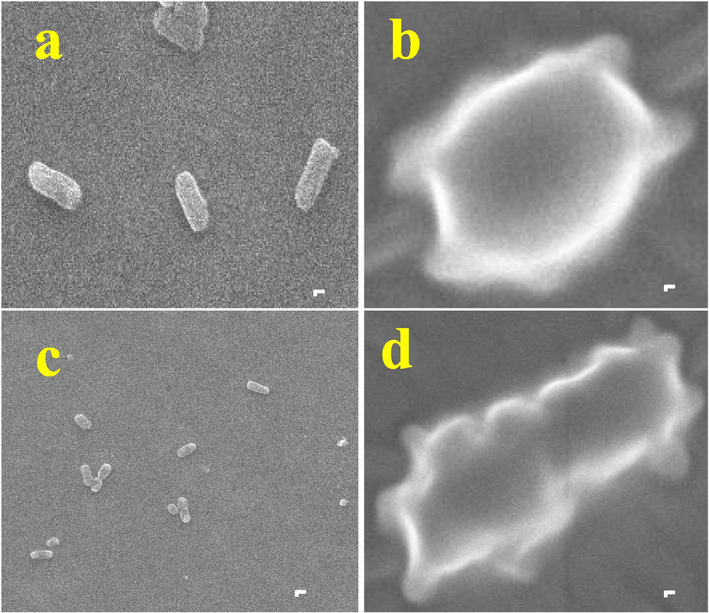
Outer cellular morphological differentiation of control and R. mucronata extract treated K. pneumoniae (a) and A. baumannii (b).
4 Conclusion
Marine is the excellent reservoir to supplied additional nutrients to living organisms worldwide. In addition, the fluctuated nutrient capacity, stress tolerance, unpredictable nitrogen, carbon sources were stimulated the plant responses against various pathogenic infections. As per positive information, the current study result of Rhizophora mucronata and synthesized extract has more efficient phytochemical derivatives and biopharmaceutical properties against various pathogenic infections. As same as, the phenol and flavonoid derivatives are containing more content in Rhizophora mucronata extract and confirmed by after comparison with standard control. Then, the yield of the phenol and flavonoids were more sufficient and shown excellent anti-oxidant and DPPH scavenging properties. Further, the antimicrobial properties of Rhizophora mucronata extract have excellent inhibition property against multi drug resistant K. pneumonia and A. baumanni. In addition, the inactivation of bacterial growth, inhibition efficiency, intracellular permeation ability, extracellular damages were conveyed, the mangrove plant Rhizophora mucronata has rich bio potential due to the influence of marine habitats including nutrients, stress, NaCl, carbon, nitrogen, minerals and et. Finally, the result was suggested, the natural, ecofriendly, toxic less, and socio-economic based mangrove plant Rhizophora mucronata can be used for drug discovery research in future.
Acknowledgment
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R70), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Local and meso-scale pressures in the eutrophication process of a coastal subtropical system: challenges for effective management. Estuar. Coast. Shelf Sci.. 2021;250:107109
- [Google Scholar]
- Identification of bioactive compounds from rhizophora mucronata methanolic leaf extract by GC-MS analysis. IJPSR. 2020;11:4598-4602.
- [Google Scholar]
- An in vitro antagonistic efficacy validation of Rhizophora mucronata. Asian Pacific J. Tropical Dis.. 2015;5:28-32.
- [Google Scholar]
- The biomedical significance of the phytochemical, proximate and mineral compositions of the leaf, stem bark and root of Jatropha curcas, Asian Pac. J. Trop. Biomed.. 2015;5:650-657.
- [Google Scholar]
- GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed. 2016:957-961.
- [Google Scholar]
- Distribution and dynamics of mangrove forests of South Asia. J. Environ. Manage.. 2015;148:101-111.
- [Google Scholar]
- The ultrastructural damage caused by Eugenia zeyheri and Syzygium legatii acetone leaf extracts on pathogenic Escherichia coli. BMC Vet. Res.. 2020;16:326.
- [Google Scholar]
- Microscopic study on colonization and antimicrobial property of endophytic bacteria associated with ethnomedicinal plants of Meghalaya. J. Microscopy Ultrastruct.. 2017;5:132-139.
- [Google Scholar]
- Purification and characterisation of phytochemicals extracted from Rhizophora mucronata: their efficacy against Pseudomonas aeruginosa infection in Catla catla. Rev. Anal. Chem. 2022;41:275-286.
- [Google Scholar]
- Antimicrobial and radical scavenging effects of alkaloid extracts from Rhizophora Mucronata. Pharm. Chem. J.. 2015;49:34-37.
- [Google Scholar]
- Unusual naphthoquinone derivatives from the twigs of Avicennia marina. J. Nat. Prod.. 2007;70:923-927.
- [Google Scholar]
- Antidiabetic and antioxidant activities of tannin extract of Rhizophora mucronata leaves. J. Chem. Pharm. Res.. 2016;8:143-148.
- [Google Scholar]
- Screening of phytochemical and antioxidant capacity of Rhizophora mucronata leaf extract from backwaters of muthukadu lake, Tamil nadu. IJRAR. 2019;6:1-10.
- [Google Scholar]
- Anti-biofilm activity of LC-MS based Solanum nigrum essential oils against multi drug resistant biofilm forming P. mirabilis. Saudi J. Biol. Sci.. 2021;28:302-309.
- [Google Scholar]
- Anti-bacterial effect of marine sea grasses mediated endophytic actinomycetes against K. pneumonia., Journal of King Saud University –. Science. 2021;33:101528
- [Google Scholar]
- Avicennia marina a natural reservoir of phytopharmaceuticals: curative power and platform of medicines. J. Ethnopharmacol.. 2020;263:113179
- [Google Scholar]
- Comparative analyses of saponin, phenolic, and flavonoid contents in various parts of Rhizophora mucronata and Rhizophora apiculata and their growth inhibition of aquatic pathogenic bacteria. J. Appl. Pharm. Sci.. 2022;12:111-121.
- [Google Scholar]
- Cellular distribution of copper, lead and zinc in the grey mangrove, Rhizophora mucronata (Forsk.) Vierh. Aquat. Bot.. 2000;68:45-59.
- [Google Scholar]
- An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process.. 2018;112:69-85.
- [Google Scholar]
- Antibacterial and antifungal activity of the extracts of different parts of Avicennia marina (Forssk.) Vierh. Plants (Basel). 2021;10:252.
- [Google Scholar]
- Secondary metabolites from the stem barksof rhizophora mucronatalam.kieu thi. Vietnam J. Sci. Technol.. 2020;58:653-664.
- [Google Scholar]
- Phytochemical screening and anti-oxidant activity of Sargassum wightii enhances the anti-bacterial activity against Pseudomonas aeruginosa. Saudi J. Biol. Sci.. 2021;28:1763-1769.
- [Google Scholar]
- Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens. Biocatal. Agric. Biotechnol.. 2019;17:750-757.
- [Google Scholar]
- Antibacterial activity of methanol extract Rhizophora mucronata leaves toward Salmonella typhi: leading the typhoid fever. Pharmaciana. 2022;12:364-371.
- [Google Scholar]
- The use of fresh and saline water sources by the mangrove Rhizophora mucronata. Hydrobiologia. 2015;745:59-68.
- [Google Scholar]
- A review on potential bioactive phytochemicals for novel therapeutic applications with special emphasis on mangrove species. Phytomedicine Plus. 2021;1:100107
- [Google Scholar]
- Anti-cancer activity of biosynthesized silver nanoparticles using Avicennia marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J. Biol. Sci.. 2020;27:3018-3024.
- [Google Scholar]
- Phytochemical profile and antibacterial activity of the mangrove plant avicennia officinalis L. Int. J. Curr. Res.. 2020;12(2020):9973-9977.
- [Google Scholar]
- In vitro antioxidant and anti-cholinesterase activities of Rhizophora mucronata. Pharm. Biol.. 2016;54:118-129.
- [Google Scholar]
- Spatial and temporal distribution of mosquito larvicidal compounds in mangroves. Asian Pacific J. Tropical Dis.. 2021;2:401-404.
- [Google Scholar]
- Bioactive compounds profile, enzyme inhibitory and antioxidant activities of water extracts from five selected medicinal plants. Ind. Crop. Prod.. 2020;151:112448
- [Google Scholar]
- Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem.. 2003;80:399-407.
- [Google Scholar]
- Cytotoxic effects of new bioactive compounds isolated from a Nigerian anticancer plant Melastomastrum capitatum Fern. leaf extract. Scientific African. 2020;8:00421.
- [Google Scholar]
- GC–MS analysis of bioactive compounds from Melastomastrum capitatum (Vahl) Fern. leaf methanol extract: an anticancer plant. Sci. African. 2019;3:59.
- [Google Scholar]
- Preliminary phytochemical and antimicrobial studies on a spike-moss Selaginella inaequalifolia (hook. & grev.) Spring. Asian Pac. J. Trop. Med. 2010:957-960.
- [Google Scholar]
- Total phenolic, flavonoid content and antioxidant capacity of stem bark, root, and leaves methanolic extract of Rhizophora mucronata Lam. World News Nat. Sci.. 2019;26:118-127.
- [Google Scholar]
- Phytochemistry and anticancer effects of mangrove (Rhizophora mucronata Lam.) leaves and stems extract against different cancer cell lines. Pharmaceuticals. 2023;16:4.
- [Google Scholar]
- Phytochemicals from Rhizophora mucronata propagules, its in vitro anti-cancer and in silico drug-likeness potential. Chemistry. 2021;3:979-990.
- [Google Scholar]
- Evaluation of the antimicrobial and cytotoxic potential of endophytic fungi extracts from mangrove plants Rhizophora stylosa and R. mucronata. Sci. Rep.. 2022;12:2733.
- [Google Scholar]







