Translate this page into:
The molecular effects of Asperuloside against thermogenesis and anti-inflammatory process through multiple recent obesity pathways: An anti-obesity drug discovery by in-silico analysis
⁎Corresponding author at: Health Information Technology Department, The Applied College Studies, King Abdulaziz University, Jeddah, Saudi Arabia. aftab786sa@hotmail.com (Aftab Ahmad) abdulsalam@kau.edu.sa (Aftab Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Adenosine receptor signaling and suppressing potential pathways such as the aryl hydrocarbon receptor (AHR) and takeda G-protein-coupled receptor-5(TGR5) have been identified as potential targets for enhancing metabolic health. Certain adenosine receptor (AR) ligands have been suggested to reduce inflammation and improve thermogenesis in adipose tissue.
Methods
This study employed in-silico biomolecular fractions of adenosine receptors and other potential targets to understand the mechanism of action of Asperuloside. Additionally, the anti-obesity potential of Asperuloside, a dual-acting ligand with A2A adenosine receptor (A2AAR) agonist and A3 adenosine receptor (A3AR) agonist activities, were examined using computational analysis in the obesity model. The impact of Asperuloside on inflammation and thermogenesis was studied through diverse protein structures such as the A2AAR complex with agonist/A2AAR complex with antagonist, the rhodopsin mutant with bound galphact peptide (as A3 adenosine receptor), The Human TGR5 complex with synthetic agonist 23H, and AHR receptors antagonism.
Results
The study found that Asperuloside has therapeutic affinity for the binding site of adenosine receptors and revealed a novel binding interaction that helps reduce inflammation and improve thermogenesis-mediated obesity.
Conclusion
Asperuloside may have anti-obesity effects through its dual-acting ligand with A2AAR and A3AR agonist activities. This study provides a major step towards understanding the mechanism of action of Asperuloside and its potential use as an anti-obesity drug. In vivo tests will help ascertain its pharmacokinetic characteristics, metabolite production in animals, and the effects of chronic daily absorption.
Keywords
In silico
Asperuloside
Obesity
A2AAR
A3AR
1 Introduction
Obesity is a worldwide health burden linked to an increased risk of cardiometabolic illness and death. Obesity causes chronic low-level inflammation linked to several negative health outcomes, including cardiovascular events, diabetes and cardiorespiratory fitness, several forms of cancer, and, as we've found lately, issues from the coronavirus disease 2019 (COVID-19) virus. Obesity results from an energy deficit caused by excessive eating and insufficient physical activity. The first layer of defense is a change in lifestyle that emphasizes eating less and exercising more. It has been suggested that time-bound feeding, intermittent fasting and fasting-mimicking diets can help prevent and treat obesity (Waldman et al., 2019). Adherence to such programs is often low, though, and weight reduction is typically just moderate. Weight reduction with bariatric surgery is significant and long-lasting, but not all patients who meet the criteria for the procedure can have access to or tolerate it. Therefore, immediate treatment is necessary for permanent weight loss.
Excessive fat storage, or obesity, is a major contributor to the development of various pathological conditions. These abnormalities include nonalcoholic fatty liver disease, hypertension, heart attacks and type 2 diabetes mellitus, etc. (Huang 2009). It's critical to comprehend the differences between brown and white adipose tissue (BAT and WAT, respectively). While BAT has many mitochondria and is involved in non-shivering thermogenesis, WAT focuses on fat storage and mobilization. Therefore, increasing energy expenditure by activating and recruiting BAT is a strategy that could be further investigated as a potential anti-obesity approach.
An increased focus on pharmacologically stimulating adenosine receptor signaling as a possible goal for enhancing metabolic status has been widely reported. Scientists have found four different types of adenosine receptors. These are designated as A1, A2A, A2B, and A3. Inhibition of cAMP synthesis and PKA signaling by adenylyl cyclase (AC) is mediated through the A1 and A3 adenosine receptors (A1AR and A3AR, respectively) connected to Gi proteins. But AC activity is facilitated by the A2A adenosine receptor (A2AAR) and the A2B adenosine receptor (A2BAR), which are connected to Gs proteins.
A2AAR is the most commonly expressed adenosine receptor in BAT compared to WAT. Several studies have shown that A2AAR signaling prevents obesity and is essential for BAT's full physiological function. Although A3AR is expressed in WAT, its precise roles in regulating adipocyte metabolism remain unclear.
Adenosine has been linked to obesity because of the several ways in which it has been shown to play a role in the development and maintenance of the condition. These include thermogenesis, inflammatory process suppression, improvement in insulin resistance, glucose homeostasis, and the formation of fatty tissue, to name a few examples (Pardo et al., 2017; D’Antongiovanni et al., 2020). Indeed, adenosine receptors (ARs) are widely dispersed in tissues that play a crucial role in regulating metabolism, prompting researchers to speculate that pharmacological manipulation of adenosine pathways might be an effective strategy for combating obesity and its associated complications (Kotańska et al., 2020). According to this theory, therapies with particular AR ligands have been shown to reduce inflammation and insulin resistance in adipose tissue, which are promising preclinical findings.
The pathophysiology of obesity is thought to include abnormal glucose and lipid metabolism, although abnormal amino acid metabolism in obese people is often disregarded (Green et al., 2016). Increased circulation of Kyn has been linked to the preferential catabolism of the important amino acid tryptophan via the kynurenine pathway (KP) in people with obesity, as demonstrated by previous investigations (Wolowczuk et al., 2012, Laurans et al., 2018). The origin of the incremental Kyn, however, is still a mystery. In this paper, we established that attenuation of overstimulated AHR in mature adipocytes from obese people, regulated through tryptophan catabolism associated with excessive Kyn, exacerbates obesity and insulin resistance. Kyn initially stimulates AhR, which translocate signal transducer and activator of transcription 3 (STAT3) activity and upregulates interleukin-6 (IL-6) release. Here, obesity and insulin resistance are accompanied by elevated AHR/STAT3/IL-6 signaling in adipose tissues, which is associated with elevated Kyn concentrations due to elevated IDO1 transcription. Notably, mice supplemented with Vitamin B6 (a catalyst for Kyn degradation) were offered protection from obesity and insulin resistance brought on by a high-fat diet. Therefore, eliminating excess Kyn might be an effective technique for combating obesity.
Several studies were conducted to identify pharmacologic targets for obesity treatment. The gastrointestinal tract, BAT, spleen, and macrophages all exhibit a form of the bile acid transmembrane protein, which is G-protein coupled receptor 5 (TGR5) (also referred to as GPBAR1) (Maruyama et al., 2002). There is mounting evidence that activating TGR5 can, directly and indirectly, affect glucose and lipid metabolism. Evidence suggests that TGR5 is essential for the metabolic improvement brought on by gastric sleeve surgery. By stimulating cAMP-dependent iodothyronine deiodinase 2 and influencing the synthesis of mitochondrial uncoupling protein (UCP) 1, TGR5 reinforces BAT energy expenditure (Watanabe et al., 2006). Researchers have observed that activating TGR5 with vertical sleeve gastrectomy helps maintain weight reduction, mitigates liver fibrosis, and improves insulin resistance in a mouse model (Ding et al., 2016). However, cholic acid-7-sulfate, a TGR5 agonist only absorbed in the gut, has reduced excess body fat and type 2 diabetes in patients undergoing bariatric surgery (Boknik et al., 2021). Thus, developing ligands for TGR5 has been proposed as a therapeutic approach to obesity and its associated comorbidities.
In this work, the iridoid glycoside Asperuloside’s (Rubiaceae family) potential biological use has been observed from past research such as analeptic, analgesic, sedative, antihypertensive, and diuretic. Asperuloside, the principal active ingredient in Eucommia leaves, has been connected to its anti-obesity effects in recent years (Hosoo et al., 2015). Additionally, it has been shown in the iScience journal that Asperuloside reduces obesity and associated metabolic dysfunctions by altering the makeup of the gut microbiota and synthesizing secondary metabolites, consequently affecting whole-body signaling (Nakamura et al., 2020). A fascinating study published in the Journal of Nutritional Science demonstrates that the mRNA levels of uncoupling protein 1 (UCP1) in the BAT of HFD-fed rats were considerably elevated after Asperuloside injection (Fujikawa et al., 2012).
Docking is a technique used in molecular modeling to predict how a ligand and a target molecule will bind to one another to create a stable complex (Lengauer and Rarey 1996). Since molecular docking can accurately anticipate the binding conformation of small molecule ligands to the proper target binding site, it is one of the most widely utilized approaches in structure-based drug design. Binding behavior characterization is crucial for rational drug design and understanding underlying biochemical processes (Kitchen et al., 2004, Mostashari-Rad et al., 2019). In the early days, compound fits were scored based on approximations of form and electrostatic complementarities. The early phases of docking models, at least, continue to make heavy use of very simple scoring systems. Scoring techniques that take into account solvation and entropic effects, as well as electrostatic and van der Waals interactions in more detail and that are applied to already-selected conformers, are common (Gohlke and Klebe 2002). Therefore, the anti-obesity benefits of Asperuloside, a dual-acting ligand with A2A AR agonist and A3 AR agonist activities, were studied utilizing a Schrodinger software associated with in-silico computational analysis in the obesity model. The effects of Asperuloside on lipolysis, inflammation, and metabolism were studied through the diverse protein structures of the A2AAR complex with an agonist/A2AAR complex with an antagonist; the rhodopsin mutant with bound galphact peptide (as the A3 adenosine receptor); the human TGR5 complex with a synthetic agonist 23H and the AHR receptor antagonism, respectively.
2 Material & methods
2.1 Selection of literature review
This study performed a docking analysis by searching eight databases: Medline, Elsevier, PubMed, Google, Scholar, Mendeley, and Springer Nature. Many terminologies have been used separately and in combination in scientific reports. To evaluate the literature, the key terms were: Asperuloside, clinical aspects of Asperuloside, an overview of obesity, recent updates in the pathophysiology of obesity, and adenosine-associated clinical features of obesity. The study focused exclusively on English-language publications. Reference lists were also checked for relevant studies that weren’t discovered during the original search.
2.2 Selection of natural product
A study exhibits Asperuloside-mediated beneficial effects based on reducing serum inflammatory mediators and process levels, indicating hepatoprotective activity in obesity. Similarly, previous research indicated that Asperuloside treatment led to less weight gain and less visceral fat accumulation in the body. The exact method through which Asperuloside contributes to weight gain and impacts linked illnesses is, however, still unclear.
2.3 Prediction of biological interaction and activity utilizing the PAAS server
The PASS (Predicted Activity Spectrum for Substances) service (https://www.way2drug.com/PASSOnline/) predicted the activity spectra of a chemical substance as Pa (probably activity) and Pi (possible inactivity). The array of pharmacological properties, mechanisms of action, and distinct toxicities that a certain molecule may display in its interaction with bioactive molecules, as predicted by PASS (Shelley et al., 2007; Pogodin et al., 2018). The prediction of these spectra by PASS was derived from the relationship between structure and assessment of the training set, which encompassed more than 300,000 chemicals with over 4000 categories of biological activity and an average accuracy of greater than 95% (Filimonov et al., 2014; Abdou et al., 2017). The compounds with a higher Pa value than Pi are considered to have the potential to exhibit a certain pharmacological action.
2.4 Planning for protein preparation wizard
For docking studies, proteins from the obesity targets were chosen as receptors, and their crystal structures were retrieved from the RCSB PDB database. These proteins consist of the thermostabilized human receptor bound with the agonist Cgs21680 (PDB: 4UHR); The non-ribose partial agonist LUF5833 bound to the adenosine A2A receptor (PDB: 7ARO); the A2A AR-BRIL in complex with the antagonist ZM241385 (PDB: 6AQF); the adenosine A1 receptor A1AR-bRIL in complex with the covalent antagonist DU172 (PDB: 5UEN); the AHR PAS-B domain bound by the antagonist alpha-naphthoflavone (PDB: 7VNH); the human TGR5 complex with a synthetic agonist 23H (PDB: 7BW0); TNF-α in complex with the antagonist (PDB: 7JRA) and rhodopsin mutant with bound galphact peptide (PDB: 2X72). According to the protein data bank, these PDB structures have a 2.60 Å resolution with an R-value of 0.261; a 3.12 Å resolution with an R-value of 0.225; a 2.51 Å resolution with an R-value of 0.222; a 3.20 Å resolution with an R-value of 0.288; a 2.40 Å resolution with an R-value of 0.235; a 3.90 Å resolution with an R-value of 0.143; a 2.10 Å resolution with an R-value of 0.196; and a 3.00 Å resolution with an R-value of 0.212, respectively (observed). The ligand Asperuloside PubChem ID: 84,298 was saved as SDF files (Table 1).
PDB ID
PDB-bound Compound (Agonist/Antagonist)
R-Value
PDB: 4UHR
A2A Receptor bound with agonist Cgs21680
2.60 Å resolution with an R-value of 0.261
7ARO
adenosine A2A receptor partial agonist LUF5833
3.12 Å resolution with an R-value of 0.225
6AQF
A2A AR-BRIL in complex with the antagonist ZM241385
2.51 Å resolution with an R-value of 0.222
5UEN
Adenosine A1 receptor A1AR-bRIL in complex with the covalent antagonist DU172
3.20 Å resolution with an R-value of 0.288
7VNH
AHR PAS-B domain bound by the antagonist alpha-naphthoflavone
2.40 Å resolution with an R-value of 0.235
7BW0
Human TGR5 complex with a synthetic agonist 23H
3.90 Å resolution with an R-value of 0.143
7JRA
TNF-α in complex with the antagonist
2.10 Å resolution with an R-value of 0.196
2X72
rhodopsin mutant with bound galphact peptide
3.00 Å resolution with an R-value of 0.212
Before ligand docking, protein structures were processed to a phase of protonation, the deletion of water atoms beyond 0.00 Å of heteroatoms, and an energy minimization procedure, all of which were accomplished by OPLS4 at a pH of 7 ± 2. The Maestro interface (protein preparation and receptor grid generation) was employed for active site prediction, and a previously described approach was utilized for Lig prep and docking(Saxena et al., 2019).
2.5 Molecular docking
Docking of the compounds (Asperuloside) was carried out utilizing LigPrep of Maestro (Schrodinger Maestro 2021–22 version 12.8). After employing the OPLS4 force field to reduce the ligands' energy, we used Epik to induce protonation and tautomeric states at a pH of 7 ± 2 (Greenwood et al., 2010). After preparing the chosen proteins and ligands, molecular docking was utilized to examine the interaction between Asperuloside and obesity target proteins. The Maestro interface's standard precision (SP) docking was used to compute the docking score and glide hydrogen bond (Saxena et al., 2019). After docking, the Maestro was used to study and visualize residues of the protein involved in the ligand–protein interaction resulting from the docking procedure.
3 Result
The originality of the material under study affects the results of the prediction made utilizing an online pass server. The compounds suggested by the prediction may be analogues of already-approved pharmacological medications if we limit ourselves to those projected activity categories with the highest Pa values. For instance, when Pa greater than 0.70, there is a fairly high likelihood of finding compounds with experimental activity, however these compounds may be structural analogues of well-known drugs.
The probability of discovering experimental activity will be decreased, if we choose compounds with Pa values between 0.5 and 0.7, although the compounds will be less potent than recognized pharmacological drugs. The probability of discovering experimental activity is significantly lower for Pi < Pa < 0.5. the discovered molecule, however, might behave as the parent compound for a completely new chemical class for the biological activity being studied, if the prediction turns out to be correct. Table 2 displays the anticipated activity and inactivity values observed in the testing of Asperuloside, demonstrating a wide range of pharmacological effects. *Probability of activity, # probability of inactivity.
Pa*
Pi#
Predicted Pharmacological activity
0.938
0.003
Hepatic disorders treatment
0.924
0.002
Hepatoprotectant
0.899
0.004
Antiinflammatory
0.897
0.003
Antiprotozoal (Leishmania)
0.902
0.009
CDP-glycerol glycerophosphotransferase inhibitor
0.870
0.005
Antineoplastic
0.821
0.013
Sugar-phosphatase inhibitor
0.820
0.015
Alkenylglycerophosphocholine hydrolase inhibitor
0.796
0.003
Antioxidant
0.788
0.006
Immunosuppressant
0.778
0.006
Antifungal
0.758
0.005
Antithrombotic
0.753
0.004
Lactase inhibitor
0.750
0.006
Cholesterol antagonist
0.725
0.004
Antibacterial
0.716
0.004
Antiprotozoal
0.723
0.031
CYP2H substrate
0.694
0.004
Beta glucuronidase inhibitor
0.692
0.016
Fucosterol-epoxide lyase inhibitor
0.678
0.013
Mucinaminylserine mucinaminidase inhibitor
0.642
0.006
Licheninase inhibitor
0.648
0.012
Cytostatic
0.654
0.018
Hypolipemic
0.637
0.010
Aspartyltransferase inhibitor
0.633
0.008
Fructan beta-fructosidase inhibitor
0.625
0.012
IgA-specific metalloendopeptidase inhibitor
0.620
0.021
Respiratory analeptic
0.597
0.005
Levansucrase inhibitor
0.595
0.006
Free radical scavenger
0.605
0.016
Alkenylglycerophosphoethanolamine hydrolase inhibitor
0.619
0.038
Phosphatase inhibitor
0.582
0.003
Antibiotic
0.579
0.008
Caspase 8 stimulant
0.574
0.004
Alpha-N-arabinofuranosidase inhibitor
0.572
0.003
Topoisomerase I inhibitor
0.582
0.014
Levanase inhibitor
0.568
0.012
Bilirubin oxidase inhibitor
0.555
0.004
Endo-1,4-beta-xylanase inhibitor
0.571
0.022
Analeptic
0.551
0.005
Lactose synthase inhibitor
0.550
0.012
Chemopreventive
0.532
0.005
Sweetener
0.541
0.016
Anticarcinogenic
0.548
0.030
Immunostimulant
0.602
0.086
Membrane permeability inhibitor
0.529
0.016
Proliferative diseases treatment
0.512
0.005
Alpha-amylase inhibitor
0.525
0.024
Antinociceptive
0.506
0.007
RNA synthesis inhibitor
0.503
0.004
Maltose-transporting ATPase inhibitor
0.504
0.006
Glucan 1,6-alpha-glucosidase inhibitor
0.508
0.013
Myc inhibitor
0.500
0.005
4-Alpha-glucanotransferase inhibitor
3.1 Molecular docking analysis
See Table 2.
3.2 Result & discussion
Losing weight requires an increase in energy expenditure over time. Drugs that suppress appetite, increase thermogenesis, reduce inflammation, and alter the composition of gut microbes are the mainstays of modern obesity therapy. Asperuloside's potential to decrease inflammation and its associated metabolic consequences has been demonstrated in preclinical development (Manzione et al., 2020). Based on the docking analysis, we zeroed down on the interplay between adipocyte metabolism and novel obesity-related targets such as adenosine receptors (A2AAR and A3AR). We found a strong association between A2AAR (PDB ID: 4UHR) activation with docking and glide emodel energy −6.411 kcal/mole and −44.324 kcal/mole, respectively, which is highly expressed in BAT and associated with thermogenesis. In addition, Asperuloside concentration corroborates the findings of a recent study reporting that succinate buildup stimulates BAT thermogenesis (Mills et al., 2018). Notably, this is the first time we explored the one mechanism of Asperuloside that exhibits partial agonistic activity towards A2AAR (docking score −7.944 kcal/mol and glide emodel energy −58.710 kcal/mol) (Table 3). In addition, we believe there are inverse relationships between Asperuloside and A1AR & A3AR inhibition at WAT [Fig. 1 (a-f)] (Tables 3 and 4). In addition, our docking research found that Asperuloside did not have any attractive inhibitory action toward A2AAR (Tables 3 and 4). However, we found Asperuloside to have agonistic activity towards A3AR with the docking score −8.720 kcal/mol and glide emodel energy (-66.035 kcal/mol) [Fig. 2(a-f)] (Tables 3&4), that is associated with anti-inflammatory activity during obesity. Furthermore, our docking analysis of Asperuloside explored the binding affinity and interactions with other potential targets responsible for obesity, such as AHR, and TGR5 (Tables 3 and 4). Therefore, it has been suggested through in-silico analysis that Asperuloside has anti-obesity potential via activation of thermogenesis and suppression of low-grade inflammation [Fig. 3(a-d)].
COMPOUND
ASPERULOSIDE INTERACTION WITH VARIOUS THERAPEUTIC TARGETS FOR THE TREATMENT OF OBESITY
DOCKING SCORE IN STANDARD PRECISION
kcal/mol
GLIDE EMODEL ENERGY
kcal/mol
TYPES OF INTERACTION
Asperuloside
Pub Chem ID:
84,298
A2A Receptor bound with agonist Cgs21680
PDB ID: 4UHR
−6.411
−44.324
H-bond at PHE 168; TYR 271, and ASN 253 with a distance of 2.64 Å; 2.62 Å and 2.16 Å respectively
Non-ribose partial agonist LUF5833 bound to the adenosine A2A receptor
PDB ID: 7ARO
−7.944
−58.710
Two double H-bond at GLU 169; One H-bond at ALA 63 and TYR 9 with a distance of 1.59 Å/ 1.86 Å; and 1.90 Å and 2.69 Å, respectively.
A2A AR-BRIL in complex with the antagonist ZM241385
PDB ID: 6AQF
−4.684
−46.267
Only one H-bond at TYR 271 with a distance of 1.76 Å
Adenosine A1 receptor A1AR-bRIL in complex with the covalent antagonist DU172
PDB ID: 5UEN
−6.134
−66.570
H-bond at ASP 1002; ASP 1039, and LYS 1042 with a distance of 2.30 Å; 2.21 Å and 2.26 Å respectively
AHR PAS-B domain bound by the antagonist alpha-naphthoflavone
PDB ID: 7VNH
−7.607
−30.597
H-bond at LEU 281 and VAL 316 415 with a distance of 2.62 Å and 1.67 Å respectively
Human TGR5 complex with a synthetic agonist 23H
PDB ID: 7BW0
−7.549
−65.747
Two H-bond at SER 247 and one H-bond at ASN 93; ILE 160 with a distance of 1.65 /1.86 Å and 2.79 Å; 2.38 Å, respectively
TNF-α in complex with the antagonist
PDB ID: 7JRA
−7.889
−27.639
H-bond at SER C 136; TYR B: 195 and TYR C: 227 233A with a distance of 2.19; 1.81 Å and 2.76 Å, respectively
Rhodopsin mutant with bound galphact peptide (assumed as A3AR).
PDB ID: 2X72
−8.720
−66.035
Two H-bond at GLU 181; and one H bond at ILE 189; ALA 117 with a distance of 2.34/2.46; 2.33 Å and 2.58 Å respectively;
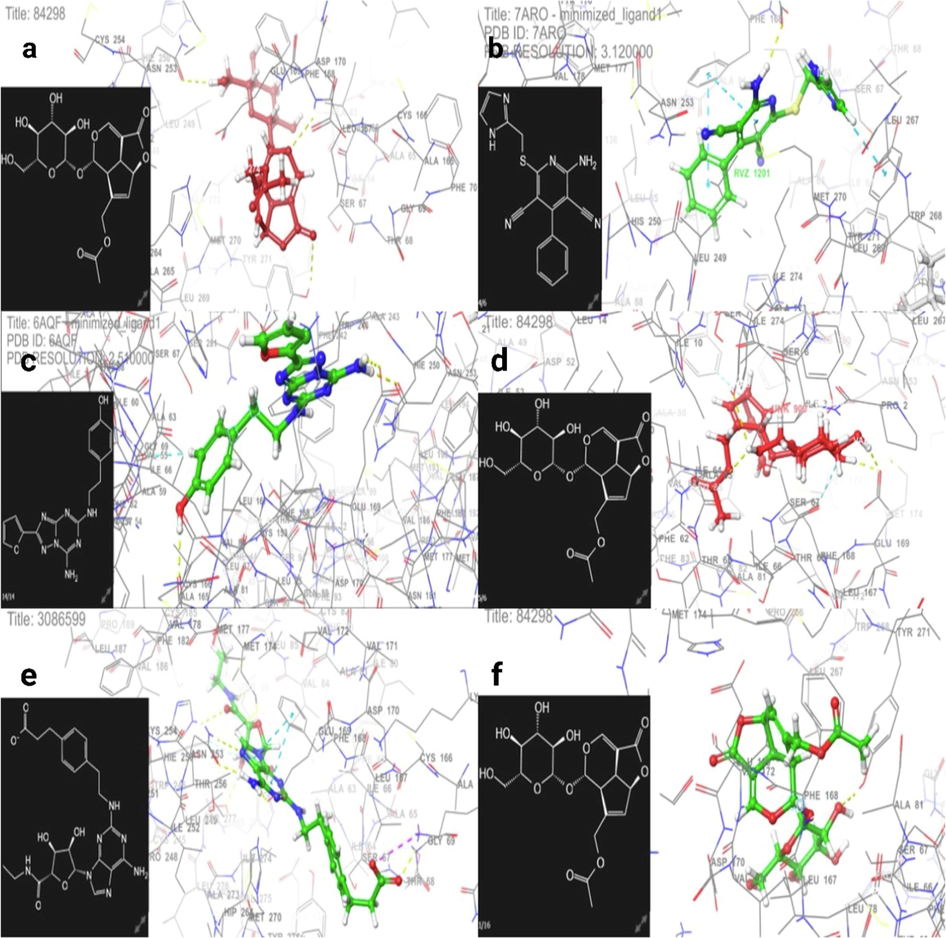
Fig. 1a and 1b show the 3D binding interaction of target compounds Asperuloside and Cgs21680 as A2A receptor agonists at the Cgs21680 active site, respectively, examined and investigated using molecular docking. Fig. 1c and 1d display the 3D binding interaction of Asperuloside and LUF5833 as A2A receptor partial agonists at the LUF5833 active site. Finally, Fig. 1e and 1f exhibit the 3D interaction of Asperuloside and ZM241385 as an A2A receptor antagonist at the ZM241385 active site.
COMPOUND
ASPERULOSIDE INTERACTION WITH VARIOUS THERAPEUTIC TARGETS FOR THE TREATMENT OF OBESITY
DOCKING SCORE IN STANDARD PRECISION
kcal/mol
GLIDE EMODEL ENERGY
kcal/mol
TYPES OF INTERACTION
Cgs21680
A2A Receptor bound with agonist Cgs21680
PDB ID: 4UHR
−13.349
−150.303
Single H-bond at various positions, including THR 88; LYS153; GLU 169; HIE 250; SER 277; HIS 278 and double H-bond at ASN 253 with a distance of 1.87 Å; 1.97 Å; 2.13 Å; 2.26 Å; 1.91 Å; 2.22 Å and 1.94 Å/2.21 Å respectively. Double Pi-Pi Stacking at PHE 168 with distance 3.93 Å /3.99 Å. Single salt bridge with LYS 153 at a distance of 3.64 Å.
LUF5833
Non-ribose partial agonist LUF5833 bound to the adenosine A2A receptor
PDB ID: 7ARO
−9.628
−94.020
Single H-bond at GLU 169 with a distance of 1.96 Å and two Pi-Pi Stacking at PHE168 and one Pi-Pi Stacking at TYR 271 with a distance of 4.12 Å/ 4.92 Å; and 5.03 Å, respectively.
ZM241385
A2A AR-BRIL in complex with the antagonist ZM241385
PDB ID: 6AQF
−10.132
−84.981
H-bond at ILE 80; GLU169, and ASN 253 with distance of 1.91 Å; 2.27 Å and 1.81 Å respectively
DU172
Adenosine A1 receptor A1AR-bRIL in complex with the covalent antagonist DU172
PDB ID: 5UEN
−7.470
−69.344
Only one H-bond at THR 225 with a distance of 1.75 Å
alpha-naphthoflavone
AHR PAS-B domain bound by the antagonist alpha-naphthoflavone
PDB ID: 7VNH
−11.185
−75.373
H-bond at TYR 305 with Pi-Pi stacking (distance of 1.83 Å and 5.48 Å, respectively). Two Pi-Pi stacking with HIS 275 (distance 5.07 Å and 5.27 Å) and Two with PHE 279 (distance 3.54 Å and 3.87 Å)
23H
Human TGR5 complex with a synthetic agonist 23H
PDB ID: 7BW0
−5.162
−55.138
H-bond at PHE 290 with a distance of 1.85 Å; and Halogen bond with ILE 288 with a distance of 2.84 Å
2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol
TNF-α in complex with the antagonist
PDB ID: 7JRA
−12.785
−120.350
H-bond at TYR C: 227; TYR B: 195 with a distance of 2.07; 2.48 Å respectively; one Pi-Pi stacking at TYR C: 135; TYR A: 135 with a distance of 4.01 Å and 4.89 Å respectively
GalphaCT peptide
rhodopsin mutant with bound galphact peptide (assumed as A3AR).
PDB ID: 2X72−9.033
−71.130
One H-bond at LYS 296 with a distance of 1.97 Å respectively;
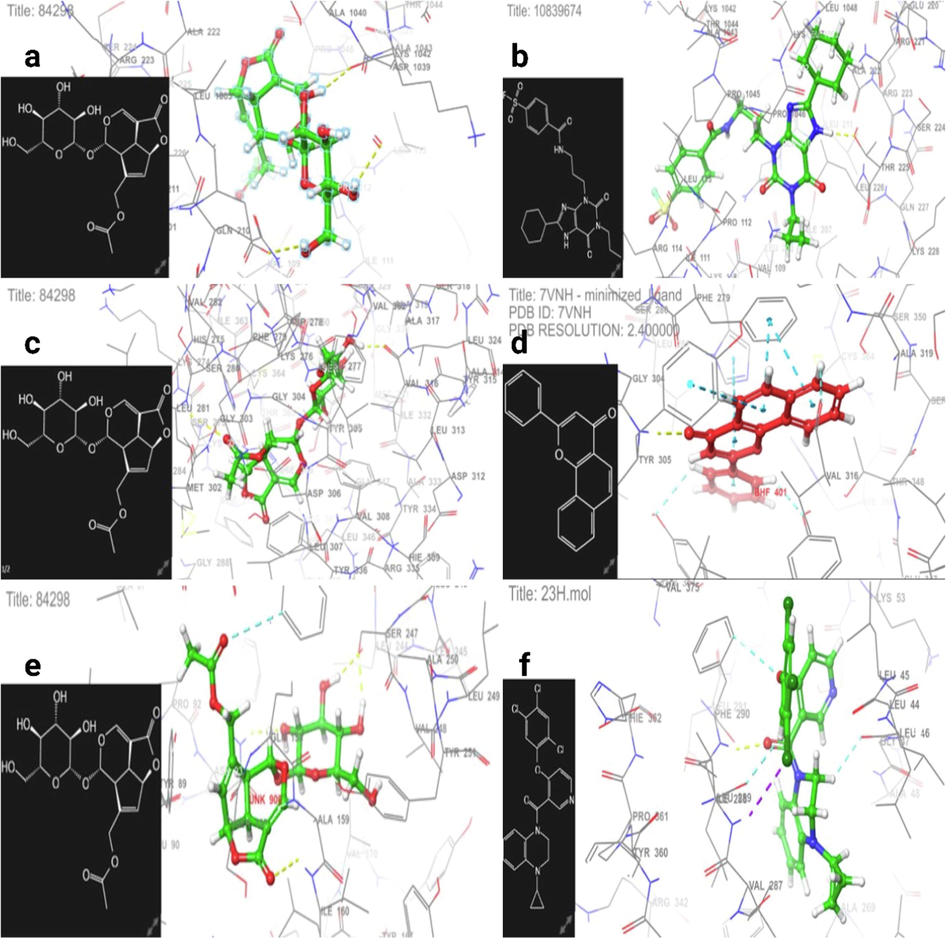
Fig. 2a and b exhibit the 3D interaction of Asperuloside and DU172 as A1 receptor covalent antagonists at the DU172 active site. Fig. 2c and d exhibit the 3D interaction of Asperuloside and alpha-naphthoflavone as antagonistic sites of the AHR receptor bound by the antagonist alpha-naphthoflavone. Fig. 2 e and f exhibit the 3D interaction of Asperuloside and 23H as an agonistic site and a synthetic agonist, respectively, with the human TGR5 complex.
![Fig. 3 a and b exhibit the 3D interaction of Asperuloside and 2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol as antagonist sites to human TNF-α in complex with antagonist, respectively. Similarly, Fig. 3 c and d exhibit the 3D interaction of Asperuloside and GalphaCT as agonist sites in rhodopsin mutants with bound galphact peptide, respectively.](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102897-fig3.png)
Fig. 3 a and b exhibit the 3D interaction of Asperuloside and 2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol as antagonist sites to human TNF-α in complex with antagonist, respectively. Similarly, Fig. 3 c and d exhibit the 3D interaction of Asperuloside and GalphaCT as agonist sites in rhodopsin mutants with bound galphact peptide, respectively.
Asperuloside exhibits an H-bond at PHE 168; TYR 271, and ASN 253 with a distance of 2.64 Å, 2.62 Å, and 2.16 Å, respectively, at A2AAR (PDB ID: 4UHR) 2D analysis. Moreover, Asperuloside exhibits two double H-bonds at GLU 169; one H-bond at ALA 63 and TYR 9 with a distance of 1.59 Å/1.86 Å, 1.90 Å, and 2.69 Å, respectively, (PDB ID: 7ARO) [Fig. 4(a-f)]. Molecular docking predictions for the LBD of A2AAR and Asperuloside suggested that the primary contacts would differ for full and partial agonists. The docked value for full agonist bounds A2AAR interaction with Asperuloside was less than that of a partial agonistic compound bound to A2AAR, a recognized as a partial agonistic activator of A2AAR instead of a full agonist (Tables 3 and 4). There has been some evidence linking the extent to which A2AAR is agonized and transactivated to the severity of the side effect(Chen et al., 2013, Boknik et al., 2019). To this end, the creation of partial agonists was an effort to combat or lessen such negative effects. Based on these results, Asperuloside seems like a promising lead molecule for creating new therapeutic candidates to combat obesity and its associated low-grade inflammation via A3AR activation with docking score (- 8.720 kcal/mole) glide emodel energy −66.035 kcal/mole) [Tables 3 and 4; Fig. 5 (a-d)].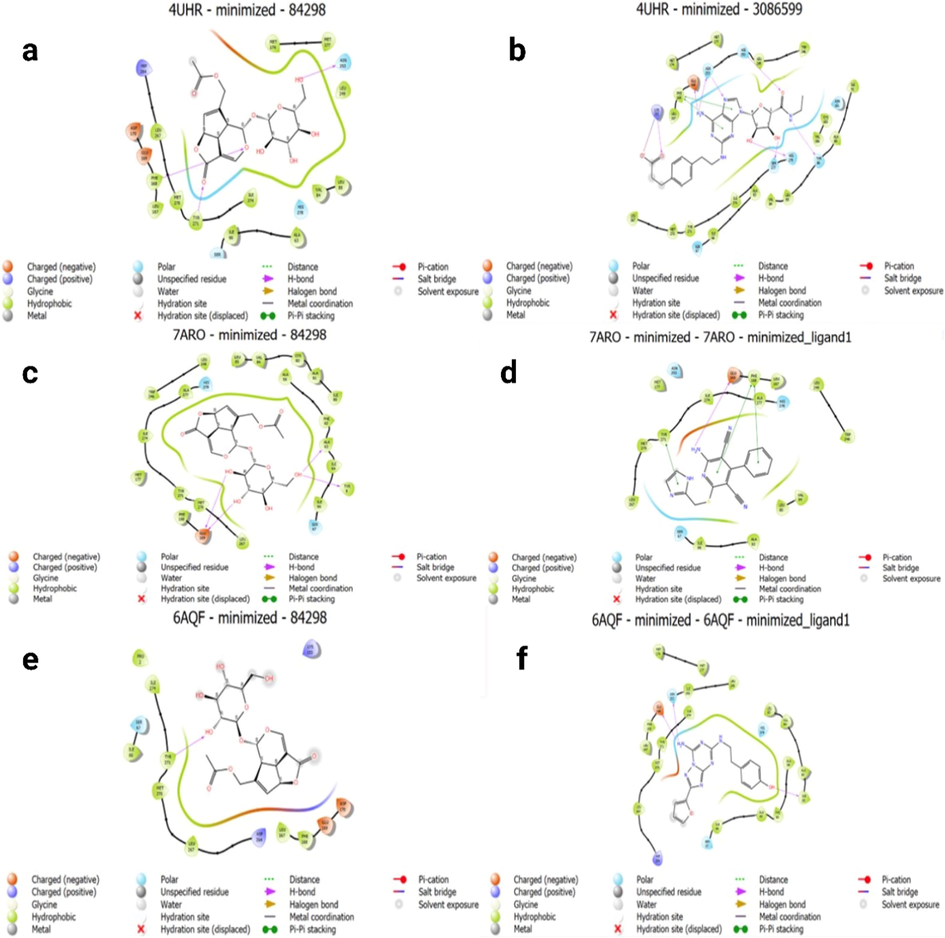
The molecular docking of target compounds Asperuloside and Cgs21680 as A2A receptor agonists at the Cgs21680 active site was examined as in Fig. 4 a and b, respectively. Fig. 4 c and d show the 2D binding interaction of Asperuloside and LUF5833 as A2A receptor partial agonists at the LUF5833 active site. Finally, Fig. 4 e and f demonstrate the 2D interaction of Asperuloside and ZM241385 as A2A receptor antagonists at the ZM241385 active site.
![Fig. 5 a and b exhibit the 2D interaction of Asperuloside and DU172, respectively, as A1 receptor covalent antagonists at the DU172 active site. Fig. 5 c and d show the 2D interaction of Asperuloside and alpha-naphthoflavone, respectively, at the antagonistic site of the AHR receptor bound by the antagonist alpha-naphthoflavone. Finally, Fig. 5 e and f demonstrate the 2D interaction of Asperuloside and [4-(2,4,5-Trichlorophenoxy)pyridin-3-yl]-(4-cyclopropyl-3,4-dihydro-2H-quinoxalin-1-yl)methanone (23H), respectively, as agonistic sites to the human TGR5complex with a synthetic agonist 23H.](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102897-fig5.png)
Fig. 5 a and b exhibit the 2D interaction of Asperuloside and DU172, respectively, as A1 receptor covalent antagonists at the DU172 active site. Fig. 5 c and d show the 2D interaction of Asperuloside and alpha-naphthoflavone, respectively, at the antagonistic site of the AHR receptor bound by the antagonist alpha-naphthoflavone. Finally, Fig. 5 e and f demonstrate the 2D interaction of Asperuloside and [4-(2,4,5-Trichlorophenoxy)pyridin-3-yl]-(4-cyclopropyl-3,4-dihydro-2H-quinoxalin-1-yl)methanone (23H), respectively, as agonistic sites to the human TGR5complex with a synthetic agonist 23H.
Numerous reports of A3AR partial agonists, agonists, allosteric modulators, and antagonists have been published, and their SARs have been investigated, leading to the development of potent and selective compounds with drug-like distinctive features. However, in our study, two H-bond at GLU 181; and one H bond at ILE 189; ALA 117 with a distance of 2.34/2.46, 2.33 Å, and 2.58 Å, respectively, seen in 2D interaction at PDB: 2X72 which is working as A3AR in previous research [Fig. 6(a-d)]. By blocking the NF-kappa B signaling pathway, which blocks the production of IL-8 and IL-1, A3AR expression and activation have an anti-inflammatory impact. Therefore, activating the A3 adenosine receptor may serve as a remedy for disorders with mild inflammation (Lee et al., 2006, Ren et al., 2014).![Fig. 6 a and b exhibit the 2D interaction of Asperuloside and 2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol as antagonist sites to human TNF-α in complex with the antagonist. Fig. 6 c and d display the 3D interaction of Asperuloside as an agonist site and the 2D interaction of GalphaCT as an agonist site with rhodopsin mutants bound to galphact peptide.](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102897-fig6.png)
Fig. 6 a and b exhibit the 2D interaction of Asperuloside and 2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol as antagonist sites to human TNF-α in complex with the antagonist. Fig. 6 c and d display the 3D interaction of Asperuloside as an agonist site and the 2D interaction of GalphaCT as an agonist site with rhodopsin mutants bound to galphact peptide.
Previously, results showed that mature adipocytes play a critical role in the altered Kyn production in obese people. Research into the mechanisms by which Kyn exerts its effects has shown that it indirectly affects immune cells through decreasing adipocytes' lipid balance and insulin sensitivity. Specifically, Kyn promotes AHR expression, which activates AHR/Stat3/IL-6 signaling in adipocytes and has a systemic influence on the development of obesity and insulin resistance. As a result, minimizing the harmful effect of AHR in obesity requires focusing on AHR (Huang et al., 2022). In our study, Asperuloside exhibits a high docking score at the antagonistic site of the AHR receptor (-7.607 kcals/mole) in PDB: 7VNH and 2D analysis show H-bond at LEU 281 and VAL 316 415 with a distance of 2.62 Å, and 1.67 Å, respectively (Fig. 5d).
Therapeutic targets for the treatment of obesity have been the focus of a large number of investigations. There is mounting evidence that activating TGR5 can, directly and indirectly, affect glucose and lipid metabolism. It has been discovered that the TGR5 selective agonist INT-777 stimulates the secretion of glucagon-like peptide 1 (GLP-1) from enteroendocrine L cells of the intestinal epithelium. The insulin sensitivity and lipid-loading of obese mice are improved by INT-777 (Kumar et al., 2016). Accordingly, it is generally accepted that TGR5 can be pharmacologically focused on obesity and its related morbidities by establishing its ligands. In our study of the in-silico interaction of asperuloside with the agonist binding site at TGR5, we explored the high docking score of −7.549 kcal/mole with a glide emodel of −65.747 kcal/mole (PDB ID: 7BW0).
Furthermore, 2D analysis exhibits two H-bonds at SER 247 and one H-bond at ASN 93 with a distance of 1.65 /1.86 Å and 2.79 Å, respectively [Fig. 5 e and f]. The orthosteric binding region may therefore be able to recognize many ligands and accommodate receptor activation. This is because the agonist binding structure of TGR5 supports a convergent activation process. Understanding the subtleties in Asperuloside identification can help design more potent and selective medicines. Our research led us to assume that TGR5 is an ideal model for determining how other steroid-sensing GPCRs function mechanistically.
4 Limitation of the study
It relied solely on in-silico biomolecular fractions and computational analysis to examine the anti-obesity effects of Asperuloside. While this approach can provide valuable insights into the mechanisms of action of drugs, further in vivo tests are necessary to confirm the efficacy and safety of Asperuloside in animals and humans. Additionally, the study focused on a limited number of potential targets for enhancing metabolic health and did not investigate other important pathways that may contribute to obesity.
5 Conclusion
This study investigates the affinity of a ligand for the adenosine receptor-mediated therapeutic effect of obesity. Our findings show that asperuloside has therapeutic affinity towards the binding site of adenosine receptors, which reduces inflammation and improves thermogenesis-mediated obesity through a novel binding interaction. This comprehensive work utilized in-silico biomolecular fractions of adenosine receptors and other potential targets, providing a significant step towards understanding the mechanism of action of Aspruloside. Further in vivo tests are necessary to determine the pharmacokinetic characteristics and metabolite production in animals, as well as the effects of chronic daily absorption, to complete the process.
Future Perspective in this field may involve the development of more potent and selective adenosine receptor ligands that can improve the therapeutic efficacy and reduce the side effects associated with current obesity drugs. Moreover, the use of advanced computational techniques, such as machine learning and artificial intelligence, may help identify novel targets and drug candidates for enhancing metabolic health. In vivo studies will be crucial to validate the efficacy and safety of these new drugs and provide insights into their pharmacokinetics and metabolism. Additionally, the integration of personalized medicine approaches, such as genomics and metabolomics, may help identify patient-specific factors that influence the response to obesity therapy and allow for more tailored and effective treatments. Overall, these advancements will pave the way for the development of safer and more effective drugs for the treatment of obesity and related metabolic illnesses.
Funding
This research work was funded by Institutional Fund Projects under grant no. (IFPIP:1250-156-1443). The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Acknowledgments
This research work was funded by Institutional Fund Projects under grant no. (IFPIP:1250-156-1443). The authors gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Structure-based design and synthesis of acyclic and substituted heterocyclic phosphonates linearly linked to thiazolobenzimidazoles as potent hydrophilic antineoplastic agents. Chem. Pap.. 2017;71(10):1961-1973.
- [CrossRef] [Google Scholar]
- Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat. Chem. Biol.. 2021;17(1):20-29.
- [CrossRef] [Google Scholar]
- Evidence for Arrhythmogenic Effects of A2A-Adenosine Receptors. Front. Pharmacol.. 2019;10
- [CrossRef] [Google Scholar]
- Adenosine receptors as drug targets–what are the challenges? Nat. Rev. Drug Discov.. 2013;12(4):265-286.
- [CrossRef] [Google Scholar]
- The Adenosine System at the Crossroads of Intestinal Inflammation and Neoplasia. Int. J. Mol. Sci.. 2020;21(14):5089.
- [CrossRef] [Google Scholar]
- Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology (Baltimore, MD). 2016;64(3):760-773.
- [CrossRef] [Google Scholar]
- Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd.. 2014;50(3):444-457.
- [CrossRef] [Google Scholar]
- Fujikawa, T., Hirata, T., Hosoo, S., Nakajima, K., Wada, A., Yurugi, Y., Soya, H., Matsui, T., Yamaguchi, A., Ogata, M., Nishibe, S., 2012. Asperuloside stimulates metabolic function in rats across several organs under high-fat diet conditions, acting like the major ingredient of Eucommia leaves with anti-obesity activity. Journal of Nutritional Science. 1, e10. https://doi.org/10.1017/jns.2012.12
- Gohlke, H. and G. Klebe, 2002. Approaches to the Description and Prediction of the Binding Affinity of Small-Molecule Ligands to Macromolecular Receptors. Angewandte Chemie International Edition. 41 (15), 2644-2676. https://doi.org/10.1002/1521-3773(20020802)41:15<2644::AID-ANIE2644>3.0.CO;2-O.
- Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol.. 2016;12(1):15-21.
- [CrossRef] [Google Scholar]
- Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des.. 2010;24(6):591-604.
- [CrossRef] [Google Scholar]
- The Restorative Effects of Eucommia ulmoides Oliver Leaf Extract on Vascular Function in Spontaneously Hypertensive Rats. Molecules (Basel, Switzerland). 2015;20(12):21971-21981.
- [CrossRef] [Google Scholar]
- A comprehensive definition for metabolic syndrome. Dis. Model. Mech.. 2009;2(5–6):231-237.
- [CrossRef] [Google Scholar]
- Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun.. 2022;13(1):3489.
- [CrossRef] [Google Scholar]
- Docking and scoring in virtual screening for drug discovery: methods and applications. Nat. Rev. Drug Discov.. 2004;3(11):935-949.
- [CrossRef] [Google Scholar]
- KD-64—A new selective A2A adenosine receptor antagonist has anti-inflammatory activity but contrary to the non-selective antagonist—Caffeine does not reduce diet-induced obesity in mice. PLoS One. 2020;15(6):e0229806
- [CrossRef] [Google Scholar]
- Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet & #x3b1; Cells to Promote Glucose Homeostasis *. J. Biol. Chem.. 2016;291(13):6626-6640.
- [CrossRef] [Google Scholar]
- Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med.. 2018;24(8):1113-1120.
- [CrossRef] [Google Scholar]
- A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;291(4):R959-R969.
- [CrossRef] [Google Scholar]
- Computational methods for biomolecular docking. Curr. Opin. Struct. Biol.. 1996;6(3):402-406.
- [CrossRef] [Google Scholar]
- Phytochemical and pharmacological properties of asperuloside, a systematic review. Eur. J. Pharmacol.. 2020;883:173344.
- [CrossRef] [Google Scholar]
- Identification of membrane-type receptor for bile acids (M-BAR) Biochem. Biophys. Res. Commun.. 2002;298(5):714-719.
- [CrossRef] [Google Scholar]
- Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560(7716):102-106.
- [CrossRef] [Google Scholar]
- Study of CXCR4 chemokine receptor inhibitors using QSPR and molecular docking methodologies. J. Theor. Comput. Chem.. 2019;18(04):1950018.
- [CrossRef] [Google Scholar]
- Asperuloside Improves Obesity and Type 2 Diabetes through Modulation of Gut Microbiota and Metabolic Signaling. iScience. 2020;23(9)
- [CrossRef] [Google Scholar]
- Molecular implications of adenosine in obesity. Mol. Aspects Med.. 2017;55:90-101.
- [CrossRef] [Google Scholar]
- How to Achieve Better Results Using PASS-Based Virtual Screening: Case Study for Kinase Inhibitors. Front. Chem.. 2018;6:133.
- [CrossRef] [Google Scholar]
- Ren, T., Qiu, Y., Wu, W., Feng, X., Ye, S., Wang, Z., Tian, T., He, Y., Yu, C., Zhou, Y., 2014. Activation of adenosine A3 receptor alleviates TNF-α-induced inflammation through inhibition of the NF-κB signaling pathway in human colonic epithelial cells. Mediators Inflamm. 2014, 818251. https://doi.org/10.1155/2014/818251
- Multiple e-pharmacophore modelling pooled with high-throughput virtual screening, docking and molecular dynamics simulations to discover potential inhibitors of Plasmodium falciparum lactate dehydrogenase (PfLDH) J. Biomol. Struct. Dyn.. 2019;37(7):1783-1799.
- [CrossRef] [Google Scholar]
- Epik: a software program for pK(a) prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des.. 2007;21(12):681-691.
- [CrossRef] [Google Scholar]
- Time-restricted feeding for the prevention of cardiometabolic diseases in high-stress occupations: a mechanistic review. Nutr. Rev.. 2019;78(6):459-464.
- [CrossRef] [Google Scholar]
- Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484-489.
- [CrossRef] [Google Scholar]
- Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2012;303(2):R135-R143.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102897.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







