Translate this page into:
Evaluation of sub-acute and sub-chronic toxicity of aqueous extract of Coptosapelta flavescens Korth. in a Swiss albino mice model
⁎Corresponding author. ptnhut@ntt.edu.vn (Tri Nhut Pham)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Coptosapelta flavescens Korth. is one of the medicinal plants used as a traditional medicine in Vietnam for a long time. However, few documents proved its safety for a long time. Therefore, the study aimed to determine the toxicity effects of the aqueous extract of C. flavescens by determining its effects after subchronic oral administration in Swiss albino mice. The toxicity of C. flavescens extract was tested at doses of 400 and 1200 mg/kg, respectively, over 30 and 60 days on an in vivo model. Animal weight, behavior, and condition changes were monitored throughout the process. At the end of the observation periods, hematological indices and histopathological features of the animal specimens were analyzed. The administration of C. flavescens extract at doses of 400 and 1200 mg/kg caused a weight difference in male mice after 30 days and in both sexes after 60 days of administration compared to the control group. There were no significant changes in hematological parameters and biochemical indexes of the liver and kidney compared with the control group. The histological structure of the liver showed changes when treated with C. flavescens at both doses after 60 days, whereas the histological structure of the kidneys exhibited differences only at the dose of 400 mg/kg. Overall, the findings of this study indicate that Coptosapelta flavescens Korth. is unlikely to have subacute and subchronic oral toxicity. However, there are some minor effects on the liver and kidneys.
Keywords
Coptosapelta flavescens Korth.
Swiss albino mice
Subacute toxicity
Subchronic toxicity
Hematological parameters
Histopathological parameters
1 Introduction
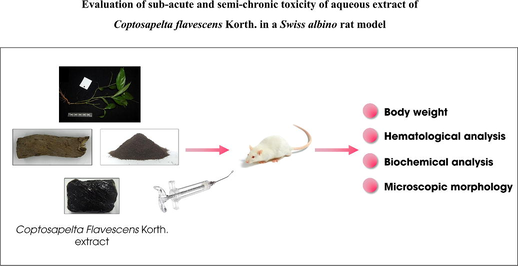
Plant-based products have been used for medicinal purposes for a long time. Until now, it is estimated that>80% of the world's population is dependent on medicinal herbs or products of plant origin for their health protection needs (James T. Mukinda & Eagles, 2010). In developing countries, most people believe in traditional medicines because of their availability, accessibility, and effectiveness, which is a part of their culture. Herbal remedies are also considered an alternative to orthodox pharmaceutical therapies in these countries. These traditional remedies are recognized to potentially treat diseases such as arthritis, osteoporosis, kidney and liver, cardiovascular disorders, and diabetes diseases (Kunle O.F. et al., 2012). In addition, it has been suggested that herbal remedies are safer and less harmful to the human body than synthetic drugs because they are of natural origin (Unuofin et al., 2018). For these reasons, the World Health Organization (WHO) has recognized that traditional medicine is essential in treating diseases and encourages member states to develop appropriate policies for control and development (WHO, 2005). However, although drugs from plants are widely used, their effectiveness is rarely scientifically evaluated. In addition, patients often cannot distinguish the similarities and differences between herbal and approved drugs. Many people think herbs are natural alternatives to synthetic drugs but do not realize they are biologically active compounds. In particular, some compounds can be toxic to humans if used in excess. Many studies have reported the toxic effects on the human body of medicinal herbs (El Hilaly et al., 2004; Taziebou et al., 2007). Therefore, evaluating and developing standards for each remedy to detect and control undesirable effects when treating human diseases is necessary (see Fig. 1).
Schematic representation of subacute and subchronic toxicity of C. flavescens extract in Swiss albino mice model.
Coptosapelta flavescens Korth. is a medicinal herb found and used for a long time by many ethnic groups in Southeast Asian countries as traditional medicine. There have been several reports documenting the effectiveness of using this medicinal herb in the treatment of diseases and health care, such as used as a skin cream, in the treatment of helminths, abdominal pain, and nasal ulcers, antipyretic and anti-rheumatic (Bremer & Eriksson, 2009; Lin, 2005). In addition, the extract from C. flavescens was reported to have the ability to inhibit bacteria more effectively than metronidazole, treat dysentery, lower blood pressure, anti-inflammatory, treatment of malaria (Hounkong et al., 2015; Kongyen et al., 2014; Kosala et al., 2017, 2018; Sutomo, 2017). Some studies analyzing the chemical composition of C. flavescens have recorded the presence of anthraquinones, saponins, and diverse phytochemical compounds (Nguyen et al., 2020). Thereby basically verifying that the effectiveness of this medicinal herb is closely related to the biological compounds available in the plant.
According to the traditional folk experience of the Hre ethnic group in central Vietnam, C. flavescens root extract is used to disinfect wounds, promote skin recovery, treat colds and flu, and support disease treatment, Gout. Furthermore, this medicinal herb is processed into a product known as “Cao Khai,” which is used as a traditional medicine to treat bone and joint diseases, anti-inflammatory, antibacterial, and diarrhea cure.
A primary concern regarding the therapeutic use of natural products is the lack of scientific evidence to support reported uses based solely on traditional knowledge. To our knowledge, several studies have been conducted on the acute toxicity of C. flavescens extract (Pham et al., 2022). However, these studies only performed the contents individually. In particular, the toxicity reports of this plant have only published results that are limited to the acute level, without detailing immediate changes in the test animals such as weight change, behavioral manifestations, hematological profile, and histological analysis. Moreover, the studies only stopped at the acute level but were not conducted at the subacute, chronic, or later levels. This is a flaw in previous studies, making it difficult to make an overall assessment of the safety of this medicinal herb.
Therefore, a detailed scientific data set on the subacute and subchronic toxicity of the aqueous extract of C. flavescens collected in Vietnam will be provided in this study. Accordingly, this study evaluates toxicity in an in vivo model of Swiss albino mice in a two-stage subacute and subchronic (30 days and 60 days) formulation. The study provides data on the changes in test subjects after using C. flavescens extracts, including body weight, changes in hematological parameters, biochemical parameters, and microscopic morphology. These results will be the basis for evaluating the effects of C. flavescens when used for a long time on the human body in the future.
2 Materials and methods
2.1 Plant material collection and extraction
Roots of C. flavescens were collected in September 2020 in Ninh Son district, Ninh Thuan Province, Vietnam. Specimens were identified and authenticated by the Oriental Medicine Association of Ninh Thuan Province, Vietnam. A sample form has been kept at the Faculty of Pharmacy, Nguyen Tat Thanh University, Vietnam. Raw materials are dried in the shade for one week, and then preliminary treatment by removing leaves and cutting them into small pieces (d = 0.5 mm). The ingredients are then ground into a fine powder in a blender and stored in sealed polyester bags. C. flavescens root powder was mixed with water (1:3 ratio) and then boiled at 100 °C for 48 h. The extraction process was repeated three times to obtain the liquid solution. The solution was then concentrated until the moisture content reached below 10%. Finally, the resulting dried extract was vacuum-sealed and stored in sealed polyester bags at room temperature.
2.2 Animals
Mice, male and female (50% each), Swiss albino strain, six weeks old, weighing 18–24 g were used in the study, provided by the Institute of Vaccines and Medical Biologicals Nha Trang, Khanh Hoa Province, Vietnam. Animals were kept in polypropylene cages (separately raised for males and females) with 25 × 35 × 15 cm dimensions containing sterilized rice husks as a cage lining material. The animals were healthy and showed no abnormalities, and they were provided adequate drinking water and food with rice flour, soybeans, corn, and vitamins. The study was conducted according to the “Guidelines for pre-clinical and Clinical Trials of Traditional Medicines and Herbal Medicines” issued by the Ministry of Health (Vietnam) under Decision No. 141/QD-K2ĐT dated October 27th/ 2015. The study was conducted as part of a scientific research project under the authority of the Department of Science and Technology of Ninh Thuan province, Vietnam (Code: 12/2020/HĐ-SKHCN) and was approved for ethical research to implement animal assessment.
2.3 Experimental design for subacute toxicity study
Group I: Control
Group II: Control after 30 days: distilled water, dose 10 ml/kg/day for 30 consecutive days
Group III: C. flavescens extract, the dose of 400 mg/kg for 30 consecutive days
Group IV: C. flavescens extract, the dose of 1200 mg/kg for 30 consecutive days
Group V: Distilled water, dose 10 ml/kg/day for 60 consecutive days
Group VI: C. flavescens extract, the dose of 400 mg/kg for 60 consecutive days
Group VII: C. flavescens extract, the dose of 1200 mg/kg for 60 consecutive days
Mice were continuously treated with distilled water or sample daily (volume 10 ml/kg/time) in the morning (8–––10 am) for 30 or 60 days, depending on the experimental layout. The animals fasted for two hours before drinking. Mice in group I played the role of control and were slaughtered after 05 days to investigate the parameters of the mice before the test. Similarly, mice in groups II and V were used to control for fluctuations in control indices after 30 and 60 days, respectively. The remaining mice were divided into two groups to receive C. flavescens extract (dose 400 and 1200 mg/kg) for 30 days (groups III and IV) and 60 days (groups VI and VII). Changes in the body weight of mice were recorded by weighing the mice once a week. The general behavior and responses of the mice were observed continuously.
2.4 Hematological and biochemical analysis
After 30 or 60 days, mice were anesthetized with CO2 stone and quickly operated to take blood from the heart to perform tests on hematological indicators such as red blood cell count (RBC), white blood cell count (WBC), and white blood cell formula, platelet count (PLT), hemoglobin (HGB), erythrocyte hematocrit (HCT), mean red blood cell volume (MCV) (hematopoietic function test) using ABX MICROS hematology 60 (Horiba Medical, France). Check liver function (aspartate aminotransferase (AST), alanine amino-transferase (ALT), total bilirubin, albumin, and total cholesterol); renal function (urea, creatinine) was checked by Cobas C501 automatic biochemical analyzer and corresponding test kit (Roche, Germany). Analytical parameters were performed on the same blood sample. The collected blood samples were transferred into specialized tubes and kept at a temperature between 2 and 8 °C until analysis, which was performed within a maximum of 2 h after collection. (Serfilippi et al., 2003; Danneman et al., 2000).
2.5 Histopathological examination
On day 60, observe and record the changes in the macroscopic and internal organs. Then, the liver and kidneys were washed with cold control saline and dried. Next, the liver and kidney were immersed in 10% formalin. Six liver and kidney samples in one group were randomly collected for microscopic analysis after hematoxylin-eosin (HE) staining by optical microscopy (Labomed, USA). The degree of inflammation and liver damage was assessed according to the HAI-Knodell scale (HAI - hepatitis activity index) with an 18-point scale based on the degree of necrosis around the portal space (0–4 points); necrosis around the central vein (0–6 points); necrosis in the lobules (0–4 points); periportal inflammation (0–4 points) (Brunt, E.M., 2000). If, during the test, a mouse dies, consider the condition of the internal organs through anatomy.
2.6 Statistical analysis
The results are presented as mean ± SEM (standard error of the mean). The differences between the lots were analyzed by Kruskal-Wallis and Mann-Whitney tests with SPSS 22.0 software. The difference was statistically significant when p < 0.05.
3 Results
3.1 Sub-acute and sub-chronic toxicity study
A subacute toxicity study of C. flavescens extract was conducted according to “Guidelines for pre-clinical and clinical trials of oriental medicines and herbal medicines” issued by the Ministry of Health (Vietnam) under Decision No. 141/QD-K2ĐT dated October 27, 2015. All study animals were treated daily with C. flavescens extract at study doses (400 and 1200 mg/kg), and they all survived the entire experiment. The group of mice treated with the extract at 30 and 60 days did not observe any toxicity when compared to the initial control group of mice.
3.2 General condition and weight change
All mice in the lots had smooth hair and bright eyes; they performed normal activities, eating and cleaning. Two groups of mice treated with high oral doses of C. flavescens extract, 400 mg/kg and 1200 mg/kg, had 2/10 mice with lymphadenopathy in the neck region. The body weights of mice during the test are presented in Table 1.
Period
Average weight ± SEM (g)
Group
Sex
Control
400 mg/kg
1200 mg/kg
Day 0
Both
23.5 ± 0.2
23.0 ± 0.4
23.0 ± 0.4
Male
23.9 ± 0.3
23.7 ± 0.4
22.5 ± 0.5
Female
23.1 ± 0.4
22.2 ± 0.6
23.5 ± 0.5
Day 7
Both
28.2 ± 0.5
25.6 ± 0.6**
25.8 ± 0.4**
Male
28.0 ± 0.7
27.4 ± 0.6##
26.2 ± 0.4*
Female
28.4 ± 0.8
23.9 ± 0.6**
25.4 ± 0.8*
Day 14
Both
31.3 ± 0.6
28.7 ± 0.4**
28.5 ± 0.5**
Male
32.5 ± 0.7
29.2 ± 0.5**
28.6 ± 0.6**
Female
30.2 ± 0.7
28.2 ± 0.7
28.5 ± 0.7
Day 21
Both
31.7 ± 0.6
30.6 ± 0.5
31.0 ± 0.6
Male
36 ± 0.6
31.7 ± 0.7
31.4 ± 0.6
Female
30.8 ± 0.9
29.5 ± 0.7
30.6 ± 1.1
Day 28
Both
34.2 ± 0.6
32.5 ± 0.6
32.9 ± 0.6
Male
35.8 ± 0.5##
34.0 ± 0.6##
34.5 ± 0.8##
Female
32.6 ± 0.8
31.1 ± 0.7
31.3 ± 0.7
Day 35
Both
39.3 ± 1.1
36.8 ± 0.7
37.8 ± 1.0
Male
40.3 ± 0.9
38.0 ± 0.6
39.9 ± 0.6#
Female
38.2 ± 2.1
35.6 ± 1.0
35.7 ± 1.4
Day 42
Both
41.8 ± 1.4
38.1 ± 0.8*
39.2 ± 1.1
Male
44.2 ± 0.7
40.2 ± 0.5**##
41.3 ± 0.8*#
Female
39.4 ± 2.2
36.1 ± 0.7
37.1 ± 1.5
Day 49
Both
44.1 ± 1.5
39.8 ± 0.8*
41.4 ± 1.2
Male
46.6 ± 0.8
41.5 ± 0.9**#
44.2 ± 0.7
Female
41.6 ± 2.4
38.0 ± 0.9
38.7 ± 1.7
Day 56
Both
46.4 ± 1.4
40.4 ± 0.7**
42.1 ± 1.3*
Male
48.6 ± 1.1
41.0 ± 0.8**
45.0 ± 0.7*
Female
44.2 ± 2.3
39.8 ± 1.1
39.1 ± 1.7*
The results show that control mice gain weight steadily from 2 to 5 g after each week. The group treated with C. flavescens extract at doses of 400 mg/kg and 1200 mg/kg had an increase in body weight of about 2–3 g per week, a lower increase than the control group. The mean weight of the group treated with the 400 mg/kg dose was statistically significantly lower than that of the control mice at the same time on days 7, 14, 42, 49, and 56 (p < 0.05). In the group treated with 1200 mg/kg, the body weight of mice was significantly lower than that of the control group on days 7, 14, and 56 (p < 0.05). When compared in each group, control male and female mice had no significant difference in weight (p > 0.05). In contrast, the body weight of female mice treated with the extract was lower than that of male mice of the same batch, with statistically significant differences at day 28 and day 42 (p < 0.05).
3.3 Hematological analysis
The hematological parameters are recorded and presented in Table 2. First, the parameters related to leukocytes: after 30 days, the mice group treated with C. flavescens extract at a 400 mg/kg dose had no significant changes in leukocyte parameters compared to the control group (p > 0.05). In contrast, the group treated with the 1200 mg/kg dose had a statistically significant lower total white blood cell count and lymphocyte count (p < 0.05). After 60 days, the white blood cell counts in two groups of mice treated with C. flavescens extract were higher than those in the control group at the same time. The number of neutrophils in the group treated with the 400 mg/kg dose and the total white blood cells, neutrophils, and lymphocytes in the group treated with the 1200 mg/kg dose increased statistically significantly (p < 0.05). However, the leukocyte parameters of the tested mice were within the normal range of the mice. When comparing the two sexes in the same group, the results showed that the control group and the group treated with the 400 mg/kg extract had the most leukocyte parameters, not significantly different between male and female mice in the same group (p > 0.05). In the group treated with a dose of 1200 mg/kg, after 30 days, female mice had lower total white blood cells and lymphocytes than male mice (p < 0.05). However, after 60 days, the female mouse group had more total white blood cells, lymphocytes, and monocytes than male mice (p < 0.05). WBC: White Blood Cell; RBC: Red Blood Cell; Hgb: Hemoglobin; HCT: Hematocrit; MCV: Mean Corpuscle Volume; PLT: Platelet Count.
Parameter
Group
Sex
After 30 days
After 60 days
Control
400 mg/kg
1200 mg/kg
Control
400 mg/kg
1200 mg/kg
WBC (109/L)
Both
12.9
± 0.811.7
± 1.09.9
± 0.8*
8.8
± 1.111.0
± 1.212.7
± 1.3*
Male
13.5
± 1.013.0
± 1.511.9
± 1.1#
6.0
± 0.4#
9.3
± 1.7*
9.5
± 1.0**##
Female
12.3
± 1.210.4
± 1.17.9
± 0.4*
11.6
± 1.312.6
± 1.516.0
± 1.1**
Neutrophil count (109/L)
Both
0.6
± 0.10.5
± 0.10.5
± 0.10.3
± 0.00.7
± 0.1**
0.7
± 0.1**
Male
0.4
± 0.0#
0.6
± 0.20.6
± 0.20.2
± 0.00.7
± 0.1**
0.8
± 0.1**
Female
0.7
± 0.10.4
± 0.00.3
± 0.1*
0.4
± 0.10.6
± 0.10.7
± 0.1
Lymphocyte count (109/L)
Both
11.0
± 0.79.7
± 0.88.1
± 0.7*
7.5
± 0.98.7
± 1.110.6
± 1.2*
Male
12.0
± 0.910.6
± 1.29.6
± 0.9#
5.2
± 0.4#
6.8
± 1.27.4
± 0.8*##
Female
9.9
± 1.08.8
± 1.16.6
± 0.4*
9.8
± 1.210.5
± 1.413.7
± 1.0*
Monocyte count (109/L)
Both
1.4
± 0.21.5
± 0.21.3
± 0.31.0
± 0.21.7
± 0.21.4
± 0.1
Male
1.1
± 0.11.7
± 0.51.6
± 0.50.6
± 0.0#
1.8
± 0.4**
1.3
± 0.1**#
Female
1.6
± 0.41.2
± 0.11.0
± 0.31.4
± 0.21.5
± 0.31.6
± 0.1
RBC (1012/L)
Both
10.05
± 0.159.23
± 0.25*
8.94
± 0.29**
10.04
± 0.1110.45
± 0.219.98
± 0.13
Male
9.94
± 0.189.13
± 0.378.77
± 0.53*
10.00
± 0.2110.27
± 0.2810.02
± 0.18
Female
10.15
± 0.249.33
± 0.379.10
± 0.26*
10.08
± 0.1010.63
± 0.319.94
± 0.22
Hgb (g/dL)
Both
14.0
± 0.313.4
± 0.412.8
± 0.5*
13.9
± 0.114.3
± 0.314.0
± 0.2
Male
13.8
± 0.413.3
± 0.512.5
± 1.013.9
± 0.114.0
± 0.413.9
± 0.2
Female
14.1
± 0.513.4
± 0.613.1
± 0.314.0
± 0.214.5
± 0.414.0
± 0.4
HCT
(%)
Both
52.2
± 1.149.9
± 1.347.1
± 2.0*
53.7
± 0.752.7
± 1.250.6
± 1.0*
Male
51.8
± 1.049.7
± 1.347.5
± 3.854.8
± 0.852.3
± 1.551.4
± 0.8*
Female
52.6
± 2.050.2
± 2.446.7
± 1.8*
52.6
± 0.853.0
± 1.749.9
± 1.8
MCV (fL)
Both
52 ± 1
54 ± 1
53 ± 1
53 ± 1
51 ± 1**
51 ± 1*
Male
52 ± 1
55 ± 1
54 ± 2
55 ± 1#
51 ± 0**
51 ± 1*
Female
52 ± 2
54 ± 1
51 ± 2
52 ± 1
50 ± 1
50 ± 1
PLT (109/L)
Both
1198
± 781035
± 591203
± 55990
± 431133
± 791060
± 57
Male
1052
± 1111071
± 881202
± 106995
± 611107
± 103979
± 42
Female
1344
± 65999
± 84*
1205
± 48986
± 721160
± 1251140
± 97
About erythrocyte-related parameters after 30 days of testing, the RBC index in the group treated with the extract dose of 400 mg/kg and the RBC, HgB, and HCT indexes in the group treated with the 1200 mg/kg dose were statistically significantly lower than that of control mice at the same time (p < 0.05). After 60 days, the parameters of red blood cells in mice treated with 400 mg/kg dose did not change significantly compared with control mice. The 1200 mg/kg dose group decreased HCT and MCV compared to the control group (p < 0.05). However, these parameters are all within the normal range of mice (HCT: 40 – 55 %; MCV: 48 – 56 fL) (Serfilippi et al., 2003; Suckow M.A., Danneman P., 2001). Red blood cell flow parameters between the two sexes in the same experimental group did not significantly differ from one another (p > 0.05).
Regarding platelet count, the group of mice that received 400 mg/kg and 1200 mg/kg extracts for 30 days and 60 days had no significant difference in platelet count (PLT) compared with the control group at the same time survey (p > 0.05). The platelet counts of the two groups of male and female mice did not significantly differ when compared within the same experimental group in any group (p > 0.05).
3.4 Liver function analysis
The liver parameters are recorded and presented in Table 3. The results showed that after 30 and 60 days of testing, the group of mice treated with extracts at doses of 400 mg/kg and 1200 mg/kg had no significant changes in liver function parameters (AST, ALT, total bilirubin, albumin, and cholesterol levels) compared with the control group (p > 0.05). Notably, the index of albumin and total cholesterol in the group of mice taking the extract at a dose of 400 mg/kg for 60 days had a statistically significant decrease compared with control mice at the same time (p < 0.05). Most liver function indexes were not significantly different between the sexes when compared within the same experimental group. Although there is a difference between male and female mice in some indicators, this difference is random, does not follow a particular trend, and occurs in the whole control group. Physiological differences between individual mice can explain this difference. ALT: Alamin amino transferase; AST: Aspartat amino transferase;
Parameter
Group
Sex
After 30 days
After 60 days
Control
400 mg/kg
1200 mg/kg
Control
400 mg/kg
1200 mg/kg
AST
(U/L)
Both
76.37
± 6.2077.68
± 5.3076.56
± 6.5090.29
± 5.72106.77
± 6.8091.11
± 3.02
Male
64.31
± 2.70#
69.58
± 6.3169.71
± 12.2480.62
± 7.4896.15
± 9.2393.42
± 6.04
Female
88.42
± 9.6585.79
± 7.3383.40
± 4.1499.97
± 6.88117.39
± 10.3188.79
± 1.39
ALT
(U/L)
Both
38.11
± 3.4837.24
± 2.6633.50
± 2.1345.58
± 4.6446.14
± 7.3338.61
± 3.45
Male
38.83
± 6.1933.97
± 3.8834.25
± 2.9851.54
± 7.5042.46
± 2.0546.23
± 3.92#
Female
37.39
± 3.9840.51
± 3.3832.76
± 3.3539.62
± 4.6549.82
± 13.9331.00
± 3.03
Bilirubin
(µmol/L)
Both
1.62
± 0.211.62
± 0.191.71
± 0.161.90
± 0.261.52
± 0.291.54
± 0.22
Male
1.88
± 0.341.64
± 0.302.04
± 0.14#
2.53
± 0.21#
1.97
± 0.511.87
± 0.33
Female
1.36
± 0.211.59
± 0.281.37
± 0.211.27
± 0.511.06
± 0.361.20
± 0.22
Albumin
(g/L)
Both
41.90
± 1.3938.82
± 1.4838.00
± 2.1149.27
± 1.4044.97
± 0.80*
45.66
± 1.16
Male
45.19
± 0.74##
38.22
± 2.52**
37.16
± 3.33**
52.21
± 1.16#
45.18
± 1.52*
46.48
± 1.49*
Female
38.60
± 1.6539.43
± 1.8238.84
± 2.9446.34
± 1.7444.76
± 0.8544.84
± 1.89
Cholesterol
(mmol/L)
Both
2.34
± 0.162.34
± 0.172.59
± 0.202.93
± 0.102.56
± 0.13*
3.02
± 0.14
Male
2.61
± 0.212.59
± 0.262.61
± 0.333.12
± 0.102.66
± 0.233.08
± 0.07
Female
2.07
± 0.192.09
± 0.182.57
± 0.252.74
± 0.152.46
± 0.182.96 ± 0.28
3.5 Kidney function analysis
The results of the parameters to evaluate kidney function are presented in Table 4. After 30 days of testing, the urea and creatinine indexes of the group of mice treated with C. flavescens extract at doses of 400 mg/kg and 1200 mg/kg had no significant difference compared with the control group (p > 0.05). After 60 days, the group of mice taking the extract at a dose of 400 mg/kg had statistically significant reductions in urea and creatinine parameters (p < 0.05). The group taking an extract dose of 1200 mg/kg had a high urea index (p < 0.05), while the creatinine index was similar compared with the control group at the same time. However, the urea and creatinine results of the mice in the experimental groups were within the normal range. The renal function indexes between the sexes in the same experimental group were not significantly different (p > 0.05).
Parameter
Group
Sex
After 30 days
After 60 days
Control
400 mg/kg
1200 mg/kg
Control
400 mg/kg
1200 mg/kg
Urea
(mmol/L)
Both
5.43
± 0.594.34
± 0.435.36
± 0.518.12
± 0.515.17
± 0.52**
9.72
± 0.39*
Male
4.93
± 0.854.25
± 0.625.01
± 0.697.10
± 0.53#
5.66
± 0.599.45
± 0.57*
Female
5.93
± 0.854.43
± 0.655.70
± 0.819.15
± 0.764.69
± 1.39**
9.99
± 0.56
Creatinin (µmol/L)
Both
28.74
± 1.1227.76
± 1.6230.04
± 1.3834.82
± 1.3330.97
± 1.42*
34.15
± 1.90
Male
29.04
± 1.1628.07
± 2.6229.15
± 2.4634.44
± 1.4730.18
± 1.29*
35.70
± 3.80
Female
28.43
± 2.0627.45
± 2.2230.94
± 1.4635.20
± 2.6831.75
± 2.4132.59
± 0.75
3.6 Microscopic liver morphology analysis
The results of the microscopic analysis of mice liver cells are presented in Table 5 and Figs. 2, 3, and 4. The control group had 2/6 normal liver samples, 4/6 minimal hepatitis samples with an HAI score of 1/18, and 2/18 with intralobular necrosis. This may be because, after 60 days, the mice's hepatocyte structure changed over time. In the group that treated C. flavescens extract 400 mg/kg for 60 days, 4/6 samples had minimal hepatitis with an HAI score between 1/18–––4/18, and 2/6 samples with moderate hepatitis with a score of HAI were 5/18 and 7/18, respectively. Samples showed periportal necrosis, periportal necrosis, intralobular necrosis, and periportal inflammation. The group that treated the extract at a 1200 mg/kg dose had 6/6 samples of minimal hepatitis with a score of 1/18–––2/18.
Group (n = 6)
Liver microscopy
Control
400 mg/kg
1200 mg/kg
Normal
2/6
0/6
0/6
Minimal hepatitis
4/6
4/6
6/6
Moderate hepatitis
0/6
2/6
0/6
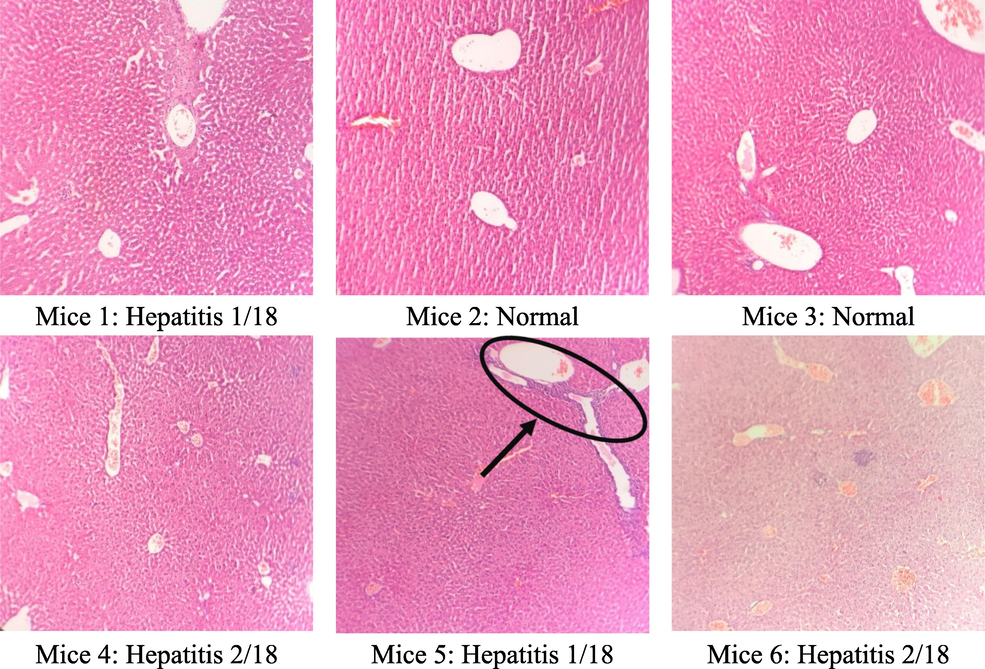
Microstructure of mice hepatocytes in the control group.
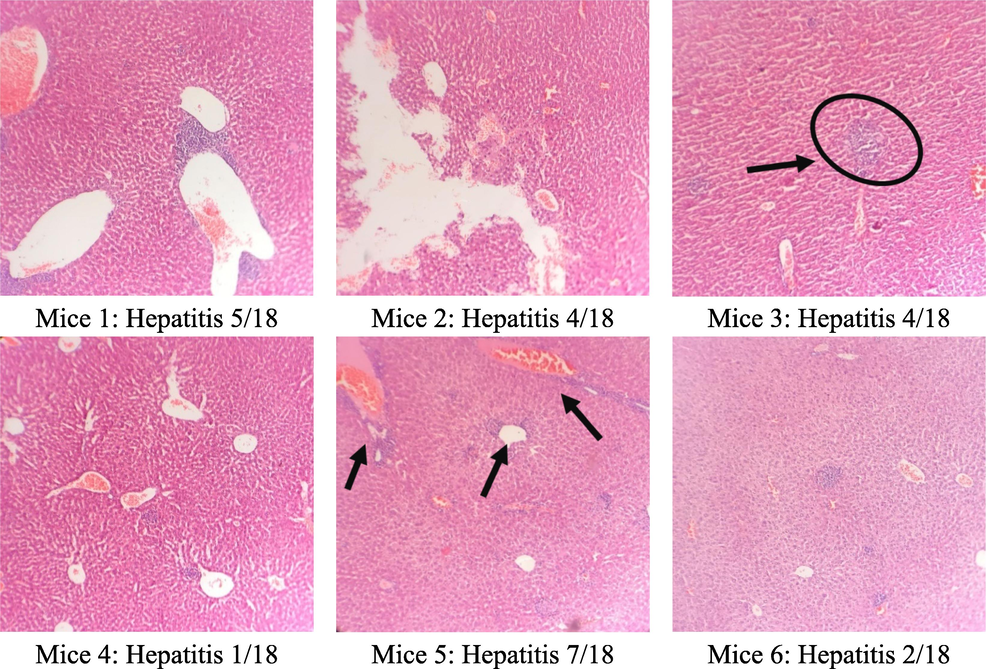
The microstructure of mice liver cells in the group taking C. flavescens extract dose of 400 mg/kg.
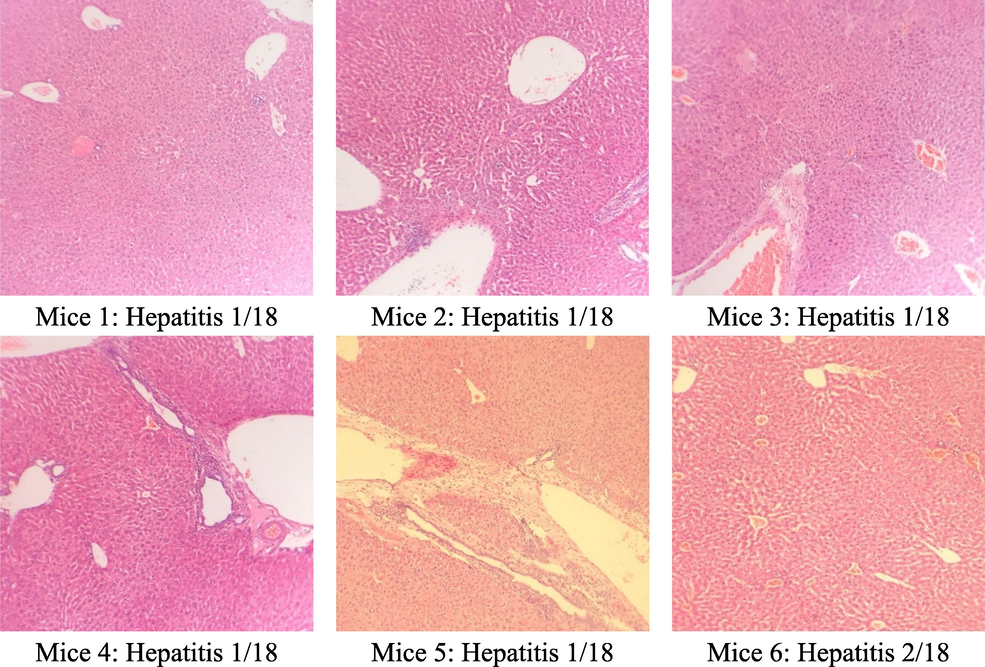
The microscopic structure of mice liver cells in high oral C. flavescens dose 1200 mg/kg.
3.7 Effect of plant extracts on microscopic kidney morphology
The results of the microscopic analysis of the kidney cells of the tested mice after 60 days are presented in Table 6 and Figs. 5–7. After 60 days of testing, the control group had 5/6 normal kidney samples and 1/6 mild pyelonephritis - pyelonephritis. The mice group treated with the extract at a dose of 400 mg/kg had 6/6 samples of mild nephritis - pyelonephritis with the phenomenon of infiltration of many lymphocytes and plasma cells in the interstitial peritubular and renal tubules. In addition, a small number of renal tubules were reported to be destroyed. All renal samples in the 1200 mg/kg dose group showed normal renal microstructure.
Group (n = 6)
Renal microstructure
Control
400 mg/kg
1200 mg/kg
Normal
5/6
0/6
6/6
Nephritis - mild pyelonephritis
1/6
6/6
0/6
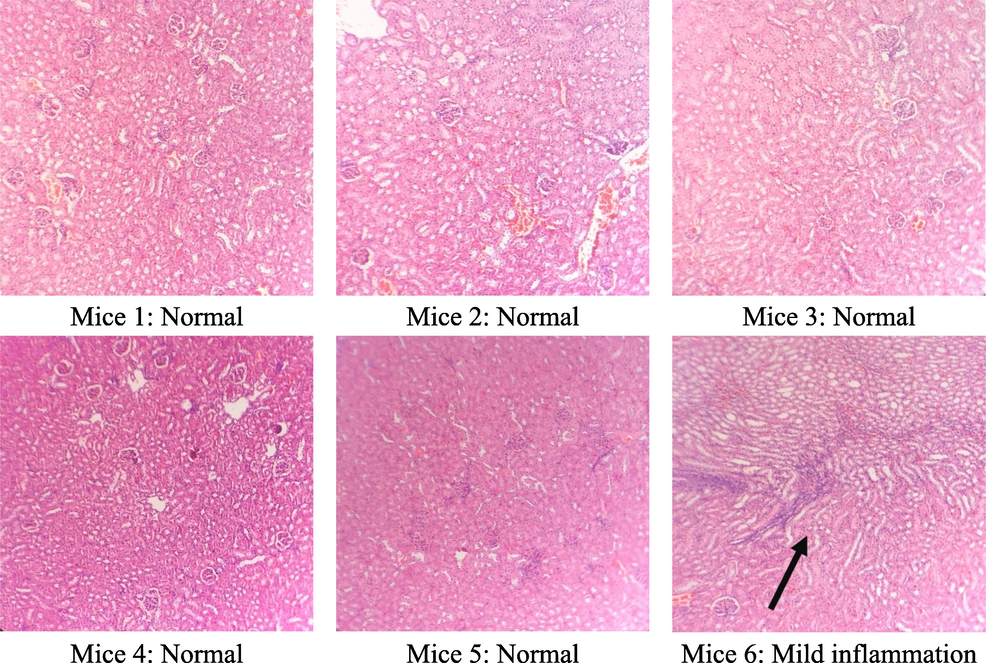
Microstructure of mice kidney cells in the control group.
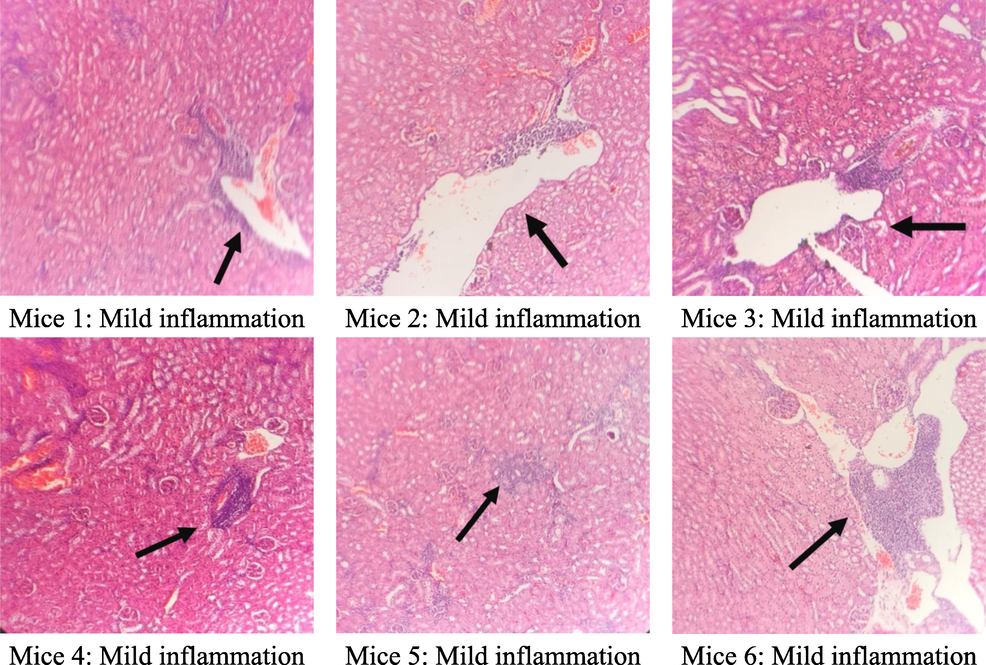
Microstructure of mice kidney cells in the group treated with C. flavescens extract dose of 400 mg/kg.
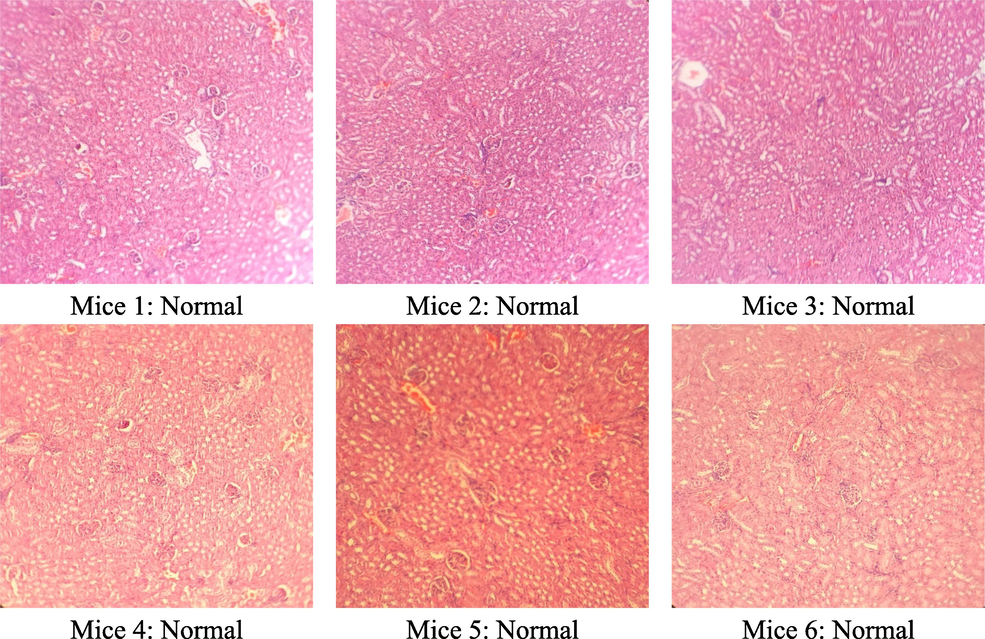
Microstructure of mice kidney cells in the group treated with C. flavescens extract dose of 1200 mg/kg.
4 Discussion
Extract from C. flavescen is a traditional alternative medicine used to treat various illnesses. The vast majority of rural communities believe herbal preparations and remedies are secure and efficient, which encourages their indiscriminate use. At the same time, the lack of scientific data will trigger the long-term use of these remedies without controlling the dose and concentration. From there, it can cause side effects or harm the patient's body. Therefore, an analysis of oral toxicity is needed to observe the effects on the body and will help determine a safe dose range for further studies.
The present study evaluated the subacute toxicity of C. flavescens extract in animal models. No deaths were reported after 400 or 1200 mg/kg oral administration for 30 days and 60 days. The animals did not change their behaviour during the test at 30 days and 60 days. In addition, there were no notable modifications in food or water intake in the tested mice during 30 and 60 days, which indicates that the animals can tolerate food and water.
Body weight changes indicate an animal's control and pathological health status. Weight change is a toxicity marker in laboratory animals, as determined by toxicity assays (Unuofin et al., 2018). In this trial, the use of C. flavescens extracts at doses of 400 and 1200 mg/kg induced a slight effect on the body weight of mice, reducing the degree of weight gain compared with the control group. The difference in body weight between the two sexes of animals can be observed, which may be attributed to a combination of factors, including the absorption of the plant extract and inherent physiological differences among mice, resulting in slight changes in the animals' eating habits.
Because of their susceptibility to toxic compounds, hematological parameters must be evaluated when determining health status. In addition, blood profiling will often provide meaningful information about the body's response to injury and stress (Ezeja et al., 2014). It has been observed that increased white blood cell release is a biomarker of stress. However, it also aids in protecting the body against certain inflammatory conditions, infections, leukemias, and hemorrhages. In general, oral administration of C. flavescens extract to mice at doses of 400 mg/kg and 1200 mg/kg for 30 or 60 consecutive days did not cause any significant effects on hematopoietic function compared with the control mice. Thereby, it was shown that plant extracts did not cause toxic effects on the hematopoietic system.
The liver and kidney serve as source organs for xenobiotic activity, with the liver acting as the primary organ for xenobiotic biotransformation and the kidney acting as an excretory organ for xenobiotics (Unuofin et al., 2018). The serum concentration of bioidentical enzymes is a commonly used biochemical parameter to observe the toxic effects on the liver (J. T. Mukinda & Syce, 2007). Among them, AST and ALT, also known as transaminases, are sensitive indicators of hepatocellular injury, and their elevation is thought to occur due to hepatocellular necrosis, leading to their leakage into the blood during toxic liver injury (Ezhilarasan et al., 2012). The biochemical indicators related to the liver, such as AST, ALT, bilirubin, albumin, and cholesterol, showed no abnormal changes compared with the control group. Accumulation of cholesterol is one cause of insulin-mediated impairment of metabolism, which can contribute to type 2 diabetes (Ezeja et al., 2014). For the kidney, dysfunction in this part is usually determined through the change of urea and creatinine (James T. Mukinda & Eagles, 2010). The serum transaminases (ALT and AST), bilirubin, albumin, cholesterol in the liver, and the values of urea and creatinine in the kidney were not affected by the C. flavescens extract, which is a definite sign that it does not harm these organs (Tables 3 and 4). The extract from C. flavescens is expected to have significant potential in aiding the control of cardiovascular issues and providing support for patients with diabetes. This outcome is in line with earlier research showing that C. flavescens extract is a source that can be used to obtain polyphenolic compounds, which have been shown to help prevent type 2 diabetes (Nguyen et al., 2022; Pham et al., 2022; Fernandes et al., 2022). Histological evaluation was used as a benchmark to identify pathological changes in tissues and organs in test animals. The histological analysis of the liver and kidney in this study showed that the use of C. flavescens plant extract at doses of 400 and 1200 mg/kg affected the microstructure of the liver after 60 days of use. At the same time, the microstructure of the kidney was observed to have a slight effect when using the plant extract at a dose of 400 mg/kg for 60 days. In general, the change of microstructure in these organs is insignificant. These also support the confirmation of previous results of slight variations in hematological parameters and biochemical parameters of the liver and kidneys. Previous studies have reported that damage and increased liver and kidney biochemical parameters are related to oxidative stress (Ezhilarasan et al., 2012; Thangavelu et al., 2020). Oxidative stress is an imbalanced state caused by excessive free radicals that overwhelm endogenous antioxidant capacity, resulting in the oxidation of a biological macromolecule, such as enzymes, proteins, DNA, and lipids. Oxidative stress is essential to chronic degenerative diseases, including coronary heart disease, cancer, and ageing. Much evidence has been demonstrated for the close association of oxidative stress with the onset of various diseases in humans and laboratory animals (Ezhilarasan & Karthikeyan, 2016). The C. flavescens extract has been reported to have a potent antioxidant capacity, which has also been confirmed by ensuring biochemical parameters and minimizing abnormal changes in the body of experimental animals in this study (Nguyen et al., 2020). Based on the results obtained, it can be seen that the use of C. flavescens has the potential to support the treatment of some health issues and does not significantly cause adverse changes to the quality of life of the experimental animals when used for an extended period.
5 Conclusion
The study on subacute and subchronic toxicity of C. flavescens extract revealed that repeated administration of this extract for 60 days, even at high doses (1200 mg/kg), did not result in any appreciable change in hematological parameters. There were no abnormal changes observed in the liver and kidney function indices. The histological structure of the liver changed after 60 days of treatment with C. flavescens at both doses of 400 mg/kg and 1200 mg/kg, whereas the histological structure of the kidneys changed at the 400 mg/kg dose compared to the control group. Overall, this study suggests that C. flavescens extract can be considered safe to use, but further studies are needed on renal effects with long-term use.
Acknowledgment
We appreciate the funding provided by the Ninh Thuan Department of Science and Technology and the facilities provided by Nguyen Tat Thanh University in Ho Chi Minh City, Vietnam, which allowed us to complete this work. [Pham Tri Nhut] was funded by Vingroup Joint Stock Company and supported by the Domestic Master/ PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Vingroup Big Data Institute (VINBIGDATA), code [VINIF.2020.ThS.91].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Time tree of rubiaceae: Phylogeny and dating the family, subfamilies, and tribes. Int. J. Plant Sci.. 2009;170(6):766-793.
- [CrossRef] [Google Scholar]
- Grading and staging the histopathological lesions of chronic hepatitis: The Knodell histology activity index and beyond. Hepatology. 2000;3:241-246.
- [CrossRef] [Google Scholar]
- Danneman, P.J., Suckow, M.A., Brayton, C., 2000. The Laboratory Mouse, 0 ed. CRC Press. https://doi.org/10.1201/9780849376276.
- Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol.. 2004;91(1):43-50.
- [CrossRef] [Google Scholar]
- Acute and sub-chronic toxicity profile of methanol leaf extract of Gouania longipetala in rats. J. Ethnopharmacol.. 2014;151(3):1155-1164.
- [CrossRef] [Google Scholar]
- Silibinin alleviates N-nitrosodimethylamine-induced glutathione dysregulation and hepatotoxicity in rats. Chin. J. Nat. Med.. 2016;14(1):40-47.
- [CrossRef] [Google Scholar]
- Ameliorative effect of silibinin against N-nitrosodimethylamine-induced hepatic fibrosis in rats. Environ. Toxicol. Pharmacol.. 2012;34(3):1004-1013.
- [CrossRef] [Google Scholar]
- The Role of Nutraceutical Containing Polyphenols in Diabetes Prevention. Metabolites. 2022;12:184.
- [CrossRef] [Google Scholar]
- Mechanisms of 1-hydroxy-2-hydroxymethylanthraquinone from Coptosapelta flavescens as an anti-giardial activity. Acta Trop.. 2015;146:11-16.
- [CrossRef] [Google Scholar]
- Anthraquinone and naphthoquinone derivatives from the roots of Coptosapelta flavescens. Nat. Prod. Commun.. 2014;9(2):219-220.
- [CrossRef] [Google Scholar]
- In vitro Exploration of Vasodilation Activity of the Methanol Extract of the Coptosapelta flavescens Korth. stem. Journal of Islamic Medicine Research. 2017;1(2):10-14.
- [Google Scholar]
- In vitro and in vivo anti-inflammatory activities of Coptosapelta flavescens Korth. Root’s methanol extract. J. Appl. Pharm. Sci.. 2018;8(9):42-48.
- [CrossRef] [Google Scholar]
- Standardization of herbal medicines - A review. Int. J. Biodivers. Conserv.. 2012;4(3):101-112.
- [CrossRef] [Google Scholar]
- Ethnobotanical study of medicinal plants used by the Jah Hut peoples in Malaysia. Indian J. Med. Sci.. 2005;59(4):156-161.
- [CrossRef] [Google Scholar]
- Acute and sub-chronic oral toxicity profiles of the aqueous extract of Polygala fruticosa in female mice and rats. J. Ethnopharmacol.. 2010;128(1):236-240.
- [CrossRef] [Google Scholar]
- Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol.. 2007;112(1):138-144.
- [CrossRef] [Google Scholar]
- Phytochemical screening and antioxidant potential of crude drug “Cao Khai” in Ninh Thuan Province. Vietnam. IOP Conf. Ser.: Mater. Sci. Eng.. 2020;991(1)
- [CrossRef] [Google Scholar]
- Anti-arthritic activity and phytochemical composition of “Cao Khai” (Aqueous extracts of Coptosapelta flavescens Korth.) Heliyon. 2022;8:e08933.
- [Google Scholar]
- Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemp. Top. Lab. Anim. Sci.. 2003;42(3):46-52.
- [Google Scholar]
- Heme Polymeritation Inhibition Activity Of Ethanol Extract Of Manuran (Coptosapelta tomentosa Valeton Ex K. Heyne) Leaf From Kotabaru South Kalimantan; 2017.
- Acute and subacute toxicity of Aspilia africana leaves. Afr. J. Tradit. Complement. Altern. Med.. 2007;4(2):127-134.
- [Google Scholar]
- Evaluation of the sub-acute toxicity of Acacia catechu Willd seed extract in a Wistar albino rat model. Regul. Toxicol. Pharm.. 2020;113(September 2019):104640
- [CrossRef] [Google Scholar]
- Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia Less. in Wistar rats. J. Integr. Med.. 2018;16(5):335-341.
- [CrossRef] [Google Scholar]
- National policy on traditional medicine and regulation of herbal medicines: report of a WHO global survey. Geneva, Switzerland: World Health Organization; 2005.







