Translate this page into:
Virulence factors analysis and determination of the suitable chemical agent to inhibit Streptococcus mutans growth and biofilm formation
⁎Corresponding author. Aghilan@ksu.edu.sa (Abdul-Kareem Mohammed Ghilan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Streptococcus mutans is one of the most important causes of tooth decay and oral diseases, and it has received wide attention from researchers worldwide. The present work aimed to investigate, evaluation and comparisons effect of the antimicrobial and anti-biofilm property of some commercial compounds (chlorhexidine, hyaluronic acid, sodium fluoride and silver nanoparticles) against oral bacteria pathogens of S. mutans. In this work, S. mutans ATCC 25175 was used and its virulence factors were analyzed using Seed Viewer and The Comprehensive Antibiotic Resistance Database (CARD). The antibacterial susceptibility testing (AST) confirms effects of chlorhexidine mouthwash on S. mutans ATCC 25175 beggar than all components. The minimum inhibitory concentration (MIC) minimum bactericidal concentration (MBC) and Minimum biofilm inhibition concentration (MBIC) of the commercial compounds were determined. The statistical analysis was performed using One-Way ANOVA (Tukey test). The VITEK 2 system was used to confirm the biochemical features of S. mutans ATCC 25175 and susceptibility testing was done to compare the results with Seed Viewer and CARD analysis. The findings showed that The MICs, MBCs, and MBIC means of chlorhexidine against S. mutans ATCC 25175 was 0.0079, 0.0038, and 0.0039 μg/mL respectively, where its biological activity was significantly (P < 0.05) higher compared to other commercial compounds included in this study. The S. mutans strain used in this study showed sensitivity to all tested antibiotics and the Seed Viewer and CARD reported that it has several virulence factors such as adhesion genes (hsp33, Cpa, PrtF, PrtF2, MsmRL, Nra, SOF, LepAL, Spy0135, and RofA) and antibiotic-resistant genes (vanY, vanT, and mdeA). This study concluded that chlorhexidine was the best in inhibiting S. mutans growth as well as preventing biofilm formation, and the study recommends further investigation about antibiotic resistance genes and susceptibility profile.
Keywords
Streptococcus mutans
Anti-biofilm
Chlorhexidine
Hyaluronic acid
Sodium fluoride
Silver nanoparticles
1 Introduction
Microbial infections that can cause several human and animal diseases are considered public health issues worldwide (Carshon-Marsh et al., 2022). According to numerous types of research, one of the most common health problems is dental caries that directly relate to bacterial strains of S. mutans. (Krzyściak et al., 2014; Patel, 2020). Clarke initially described S. mutans as a gram positive facultative anaerobic bacterium that occurs in short chains (Lemos et al., 2019). S. mutans strains are diagnosed in considerably higher quantities in carious lesions compared with caries-free people's teeth. The bacterium S. mutans is a typical part of the mouth's ecology; but, if a person consumes a lot of sugar and their oral hygiene isn't up to par, the bacteria might cause caries. The bacterium metabolizes sucrose and creates dextran, a sticky extracellular polysaccharide that is the primary source of plaque on the gums' surface (Patel, 2020; Jakubovics et al., 2021).
In order for S. mutans to cause disease, it essential first adhere to the tooth surface and procedure a biofilm. Once the biofilm is established, dietary carbohydrates can be utilized to produce acid and initiate caries progression. The main virulence properties related with S. mutans are its adhesion to the tooth surface, biofilm formation, acid production (acidogenicity; discussed above), and acid tolerance. Other bacteria, such as Lactobacillus acidophilus and Actinomyces viscous, attach to the plaque, producing enough acid to destroy tooth enamel and cause caries. On a global scale, S. mutans is the most cariogenic of all of the oral streptococci and is the primary cause of dental caries (Krzyściak et al., 2014). Many of the more than 750 bacterial species found in the mouth cavity can cause various oral related illnesses. Because of their vast range of diseases, members of the Streptococcus genus, have gained international prominence (Parks et al., 2015).
According to an alarming report by the WHO, dental caries is observed in 60–90 % of schoolchildren and nearly 100 % of adults (at least once in their lifetime) worldwide, fourth most expensive diseases to be treated in most of the countries (Singh et al., 2014; Dumitrescu, 2016). A variety of oral resident microbiota and commensal flora influence oral cavity conditions, and their normal equilibrium with the host might shift from one extreme to the other, resulting in the appearance of potentially dangerous bacteria. Important virulence factors in S. mutans are linked to the etiology and pathophysiology of dental carries. The bacterium to live, it must be able to withstand extreme environmental changes as well as antimicrobial agents. Streptococci denote 20% of the oral bacteria and, together with sucrose, are mostly responsible for biofilms formation (Sheiham et al., 2015). The ability of S. mutans to withstand various hazardous chemicals during their development in the oral biofilm has been studied (Aqawi et al., 2021; Heliawati et al., 2022). As a result, it's comprehensible that S. mutans evolved ways to colonize and retain a dominant position in the oral cavity throughout time. To prevent and treat this bacterial infectious disease, antimicrobial therapies have been used for centuries. Earlier reports highlighted some systemic antibiotics, including penicillin, tetracyclines, metronidazole, macrolides, and clindamycin, describing their application, mechanisms, side effects, and resistance. Antibiotics alone were unable to stop the demineralization process in dental biofilm, which could lead to antibiotic resistance as a result of biofilm formation (Kouidhi et al., 2015). Regular use of mouthwashes, it creates adverse effects, and many aren't interested in using chemical mouth rinses for a long time (Mor-Reinoso et al., 2016). Because of their propensity to develop strong and compatible biofilms, removal of such diseases is not constantly easy or effective. Furthermore, the rising occurrence of multidrug resistance (MDR) in microbial infections, as well as poor progress in the discovery of new anti-infective drugs, has forced the development of alternative drugs (Lebeaux et al., 2014; Cheesman et al., 2017).
Tooth decay is damage to a tooth's surface, or enamel by the intervention of the pathogenic microorganisms. Among the pathogenic microorganisms, Streptococcus mutans were crucial in attaching mouth make acids, which triggers the enamel decay. However, it is very important to suppress the growth of the pathogenic S. mutans bacteria by various routes. S. mutans creates dental decay and creates huge mouth associated infections. Therefore, to overcome the spreading of S. mutans combination of antimicrobial substances and various mouthwashes such as chlorhexidine, sodium fluoride, hyaluronic acid, silver nanoparticles developed and evaluated for its inhibition properties towards S. mutans (Abdelkader et al., 2021; Sadony and Abozaid, 2020).
Many researches have tried to combat this bacterial strain by using several natural and synthetic compounds in serious attempts to eradicate and reduce tooth decay. L. japonica, R. officinalis, protamine sulphate, and chlorhexidine digluconate all showed synergistic effects against the S. mutans, which causes dental caries, according to Yoo et al. (2020). The study found that mixing sub-MIC chlorhexidine digluconate or protamine sulphate with sub-MIC L. japonica and R. officinalis extracts demonstrated efficient drug synergistic actions, with the exception of chlorhexidine digluconate and L. japonica (Yoo et al., 2020).
In a 2015 study, Ghapanchi et al. examined the antibacterial effects of different mouthwash doses on oral strains of S. mutans and E. coli. Oral B and Chlorhexidine each inhibited E. coli in a different manner. On the other hand, neither E. coli nor S. mutans were susceptible to the antibacterial effects of persica. (Ghapanchi et al., 2015) Krzyściak et al., 2014 report that, Silver nanoparticles (AgNPs) compounds became the research objective. (AgNPs) is known for its lethal activity against a wide spectrum of bacteria, viruses, and fungi. Its antibacterial activity is due to the ability of cell membrane disintegration, internal penetration, and destruction of intracellular organelles.
Currently, various traditional medicine and their bioactive compounds are safe to use, there has been an increase in interest in researching alternative materials, notably medicinal plants, as an emerging novel agent their growth of pharmaceutical molecules. This is a way of possible treatments for diseases and its pathogens, because it is safe for both humans and animals. Anti-biofilm qualities are found in essential oil molecules, and these properties have been researched (Vaou et al., 2021). Recently, metallic nanoparticles were applied to control the S. mutans more effectively with significant results (SWETHA et al., 2020; Amissah et al., 2021).
Our aim through this study is to determine the cultural characteristics of this strain on different cultural media. Additionally, the determination of the biochemical features and susceptibility testing of S. mutans was confirmed using VITEK 2 system. The analysis of virulence factors using Seed Viewer and CARD was one of the objectives of this work. The evaluation of the biological activity of chlorhexidine, hyaluronic acid, sodium fluoride, and silver nanoparticles as antimicrobial and anti-biofilm agents against S. mutans was investigated for selecting the best agents among them.
2 Materials and method
To determine the antimicrobial and anti-biofilm we use deferent materials the experiment included chlorhexidine (Gluconate) (Avalon Pharma, PS-2035, Riyadh, Saudi Arabia), Hyaluronic acid (Lifecore Biomedical, HA15 M−5, and Chaska, MN, USA), Sodium fluoride (Sigma-Aldrich, 01148-500G, STBG4988V, USA) and silver nanoparticles (10 nm) (Sigma-Aldrich, 730685-25ML, MKCK8345, and USA).
2.1 Bacterial strains and culture media conditions
The culture of S. mutans ATCC 25175 obtained from Microbiology Laboratory, Botany and Microbiology Department, Collage of Science, KSA, Saudi Arabia. This bacterial strain is suitable for investigating the dental caries. The bacterial strain was cultivated on brain heart infusion (BHI) (Merck KGaA, Darmstadt, Germany) for 48 h at 37 °C. The bacterial cultures were maintained at −80 °C for long time storage BHI containing sterile 30% glycerol and for routine work, it was stored at −4°C with BHI slant agars (Hakimiha et al., 2014; Goc et al., 2019; Zayed et al., 2021; Pourhajibagher et al., 2022).
2.2 Culture characteristics and growth of S. mutans on various selective media
To determine the ability of S. mutans to grow on mitis-salivarius medium (MSM) agar (Mitis Salivarius, HiMedia Laboratories,Mumbai, India) (Ezzeldin et al., 2021) and brain heart infusion (BHI) (Merck KGaA, Darmstadt, Germany) (Hakimiha et al., 2014) will be observed and investigated.
2.3 Biochemical analysis and suscebtibilty testing
The biochemical and bio-number of S. mutans ATCC 25175 was confirmed using VITEK 2 system (VITEK® 2 GP ID card for biochemical and bio-number analysis and Vitek® 2 AST Card for antimicrobial susceptibility testing). The peptide mass fingerprint (PMF) of S. mutans ATCC 25175 was determined using VITEK® MS according to (Singhal et al., 2020).
2.4 Bioinformatics analysis
To determine the most important genes (virulence factors, disease, and defense) that are responsible for pathogenicity of S. mutans ATCC 25175 were analysed of using SEED Viewer ( https://rast.nmpdr.org/seedviewer.cgi ). The whole genome shotgun sequencing project (GenBank: AOCB00000000.1) of S. mutans ATCC 25175 was obtained from NCBI Sequence Set Browser, and all contigs (AOCB01000001.1, AOCB01000002.1, AOCB01000003.1, AOCB01000004.1, AOCB01000005.1, AOCB01000006.1, AOCB01000007.1, AOCB01000008.1, AOCB01000009.1, and AOCB01000010.1,(https://www.ncbi.nlm.nih.gov/Traces/wgs/AOCB01?display = contigs) were analysed to predict resistome(s) suing The Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca/).
2.5 Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
Determination and evaluation of the antibacterial susceptibility testing (AST) for all components by 96-well plate to determination of MIC and MBC and examine the effects of various mouthwashes (chlorhexidine, sodium fluoride, hyaluronic acid, and silver nanoparticles) against S. mutans ATCC 25175 were evaluated using the tube broth dilution method according to Clinical and Laboratory Standards Institute (CLSI) (Enciso et al., 2020; Zhang et al., 2021). The MIC and MBC were performed using the microbroth dilution technique. In brief, the S. mutans ATCC 25175 diluted in a series of two-fold dilutions in TSB. Freshly grown bacterial suspensions in TSB adjusted to approximately 106 CFU/mL using standard plate count. For MIC and MBC determination, 100 μL of inoculated TSB with the bacterial strain were added to 8 wells and the first well was received 100 μL of tested agent, then 100 μL were transferred to the next well and the process was repeated giving a two-fold serial dilution. For 24 h, the microplates were kept at 37 °C. The lowest concentration of the agent that showed ability to inhibit growth (no bacterial growth comparing with control groups) of bacterium was calculated as MIC (mg/mL or μg/mL). MBCs were determined using sub-cultivation method (Fig. 3) where the complete absence of bacterial growth refer to lowest concentration of the agent that showed ability to kill the bacterial cells (Mitrakul et al., 2018).
2.6 Biofilm microplate assay
To determine the Biofilm assays performed by using of S. mutans ATCC 25175 grown in BHI medium supplemented with 1% sucrose (BHIS). The two-fold serial micro-dilution method applied using a 96-well tissue culture plate. Each received 100 μL of BHIS inoculated with the bacteria where the total count of bacterial cells was 5 × 106 CFU/ml for each well. The first well received 100 μL of the tested chemical agent solution (the concentration was determined based on the tested agent). After the mixing well, 100 μL was transferred to the adjacent well then the procedure was repeated to the last well where 100 μL was discarded into an ethanol solution (70%) (Ansari et al., 2017). The plates were aerobically incubated for overnight at 37 °C. the biofilm plates were then stained with crystal violet according to standard protocols. Briley, non-adherent bacterial cells washed off by submerging the plate in water, and adherent bacterial cells stained with 0.1% crystal violet reagent. The stained bacterial cells washed twice with water, dried at room temperature, and treated using 33% acetic acid for 10 min to resuspend them. The absorbance of the re-suspended solution measured at 590 nm on a BioTek microplate reader (Mitrakul et al., 2018; He et al., 2019; Zhang et al., 2021). In this investigation concentration of, chlorhexidine (0.02 mg/mL), hyaluronic acid (8 mg/ml), sodium fluoride (16 mg/ml), and silver nanoparticles 10 nm (0.02 mg/ml) tested as antibiofilm formation agents. The antibiofilm testing done in this investigation with tri-replicates for each chemical agent.
2.7 Experimental design and statistical analysis
The present work was designed in a completely randomized design where the samples and treatments were randomly tested and analyzed (it means that each sample and test had the same chance of treatment and analysis). The average responses to different treatments were analyzed using an OriginPro 2018 to evaluate significant differences between the treatments.
3 Results
3.1 Culture characteristics and growth of s. Mutans on various selective media
The ability of S. mutans to grow on mitis salivarius medium (MSM) agar, and brain heart infusion (BHI) medium was investigated. Single colony S. mutans for each medium was characterized depending on their features (shape, size, color, surface appearance, and texture). On mitis salivarius medium, S. mutans colonies were distinct hard raised, convex, undulate, opaque, pale blue colonies and glass appearance whereas on brain heart infusion (BHI) medium the colonies were round colony, convex with, white color, smooth with a small size.
3.2 Biochemical characteristics and susceptibility testing
In this work, the biochemical and susceptibility testing were done using the Vitek system. Table 2 showed that this strain of S. mutans is sensitive to benzylpenicillin, ampicillin, cefotaxime, ceftriaxone, levofloxacin, moxifloxacin, erythromycin, clindamycin, linezolid, vancomycin, tetracyclines, tigecycline, and chloramphenicol. The peptide mass fingerprint (PMF) of S. mutans ATCC 25175 is displayed in Fig. 1. The highest intensity appeared at 2,500 and 3,500 mass m/z (Da). The data (Table 1) reported that the bionumber of this S. mutans strain is 100011164751731, and it has ability to metabolize LeuA, AGAL, ALaA, POLYB, dGAL, LAC, NAG, dMAL, BACI, NOVO, dMAN, dMNE, dRAF, O129R, SAL, SAC, dTRE, and OPTO. *% Identity of Matching Region (%IMR) and % length of Reference Sequence (LRS).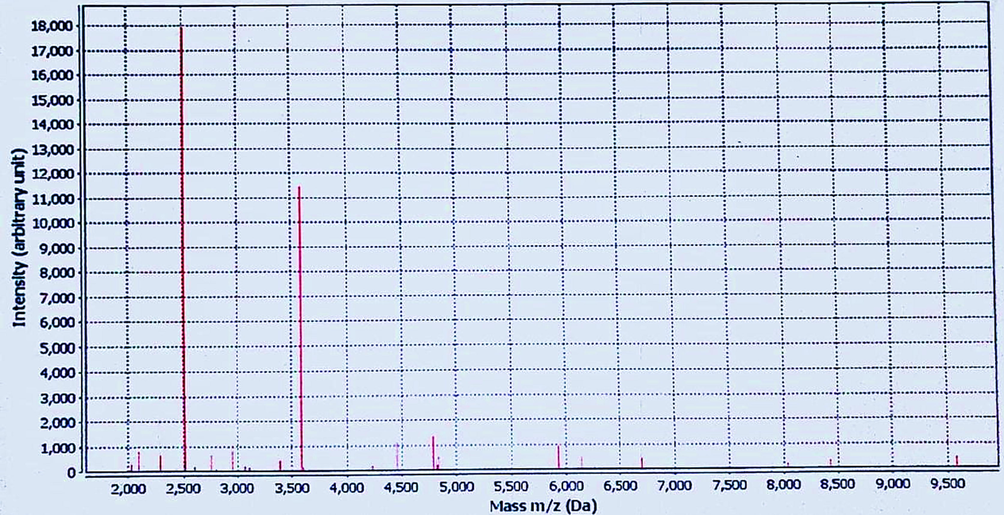
The peptide mass fingerprint (PMF) of S. mutans ATCC 25175 using VITEK® MS.
Biochemical test*
Test
R**
Test
R
Test
R
Test
R
Test
R
Test
R
AMY
+
PIPLC
–
dXYL
–
ADH1
–
BGAL
–
AGLU
–
APPA
–
CDEX
–
AcpA
–
BGAR
–
AMAN
–
PHOS
–
LeuA
+
ProA
–
BGURr
–
AGAL
+
PyrA
–
BGUR
–
ALaA
+
TyrA
–
dSOR
–
URE
–
POLYB
+
dGAL
+
dRIB
–
LLATk
–
LAC
+
NAG
+
dMAL
+
BACI
+
NOVO
+
NC6.5
–
dMAN
+
dMNE
+
MBdG
–
PUL
–
dRAF
+
O129R
+
SAL
+
SAC
+
dTRE
+
ADH2c
–
OPTO
+
*Bionumber = 100011164751731 **R = Result
Susceptibility testing
Antimicrobial***
MIC
Int.
Antimicrobial
MIC
Int.
Antimicrobial
MIC
Int.
Benzylpenicillin
<= 0.06
S
Levofloxacin
1
S
Vancomycin
1
S
Ampicillin
<= 0.25
S
Moxifloxacin
0.25
S
Tetracyclines
0.5
S
Cefotaxime
<= 0.12
S
Erythromycin
<= 0.12
S
Tigecycline
<= 0.06
S
Ceftriaxone
<= 0.12
S
Clindamycin
<= 0.06
S
Chloramphenicol
2
S
Linezolid
<= 0.06
S
Vancomycin
1
S
*** Int. = Interpretation, S = Sensitive
Contig
AMR gene
AMR gene family
Drug class
Resistance mechanism
%IMR*
%LRS*
AOCB01000001.1
vanY gene in vanG cluster
vanY, glycopeptide resistance gene cluster
Glycopeptide antibiotic
Antibiotic target alteration
37.9
89.25
AOCB01000009.1
vanT gene in vanG cluster
glycopeptide resistance gene cluster, vanT
Glycopeptide antibiotic
Antibiotic target alteration
30.4
52.11
mdeA
major facilitator superfamily (MFS) antibiotic efflux pump
Fluoroquinolone antibiotic, aminoglycoside antibiotic, penam, tetracycline antibiotic, disinfecting agents and antiseptics
Antibiotic efflux
99.12
100
3.3 Bioinformatics analysis
The Virulence factors analysis of S. mutans ATCC 25175 using The Sees Viewer (https://rast.nmpdr.org/seedviewer.cgi) (Fig. 2) showed that this S. mutans strain have genome with size 1,999,673 arraigned in ten Contigs, and number of Subsystems, coding sequences, RNAs are 10, 247, 1917 and 79 respectively. Subsystem (A Subsystem is a combination of functional roles, which together perform a particular biological operation or structural complex) category distribution analysis reported that amino acids, protein metabolism, and carbohydrates are the most distributed. Subsystem feature counts showed that there 31 collections of functional roles that have role in virulence, disease and defense. The adhesion genes (S. pyogenes recombinatorial zone) include hsp33, Cpa, PrtF, PrtF2, MsmRL, Nra, SOF, LepAL, Spy0135, and RofA whereas bacteriocins, and ribosomally synthesized antibacterial peptides (Colicin V and bacteriocin production cluster) include nine genes (R1, DedA, R3, R4, R5, DedD, DedE, R8 and PurF). The results confirmed that there 17 genes paly functional roles in resistance to antibiotics and toxic compounds distributed as follows: copper homeostasis (3 genes), cobalt-zinc-cadmium resistance (6 genes), vancomycin tolerance locus (2 genes), resistance to fluoroquinolones (4 genes), beta-lactamase (1 genes), and multidrug resistance efflux pumps (1 genres).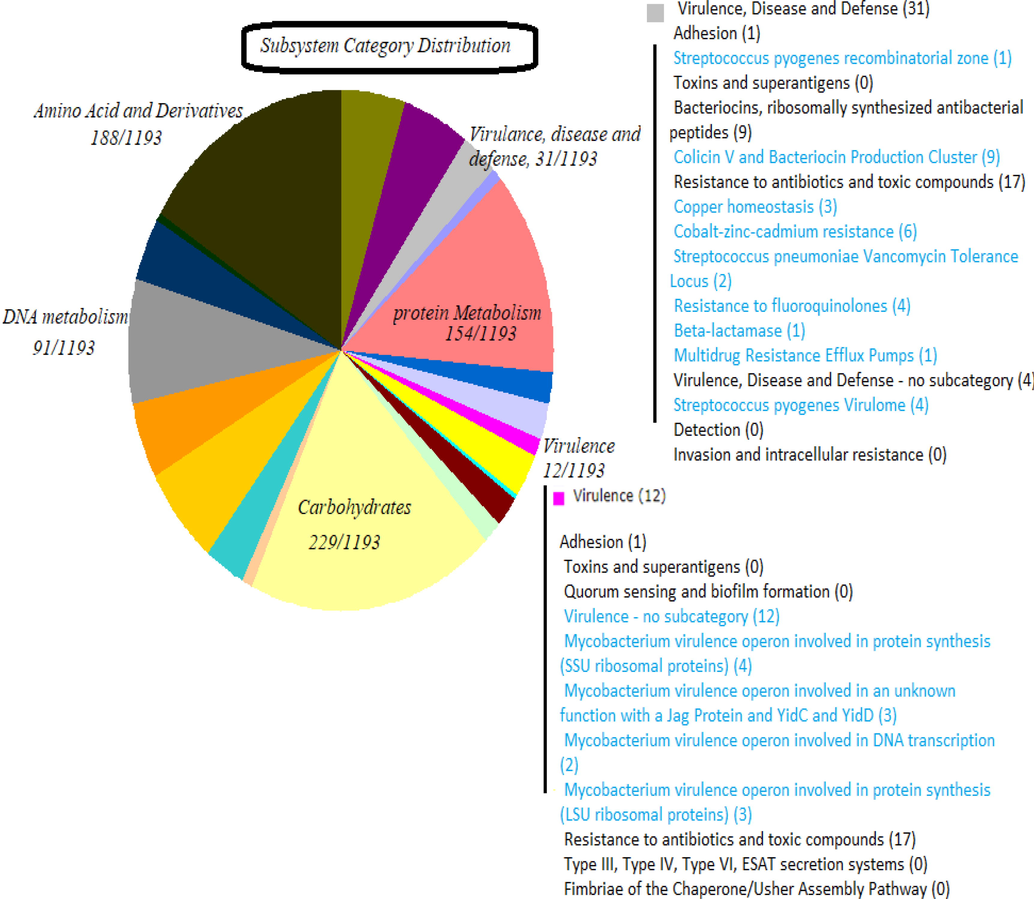
Virulence factors analysis of S. mutans ATCC 25175 using The Sees Viewer (https://rast.nmpdr.org/seedviewer.cgi).
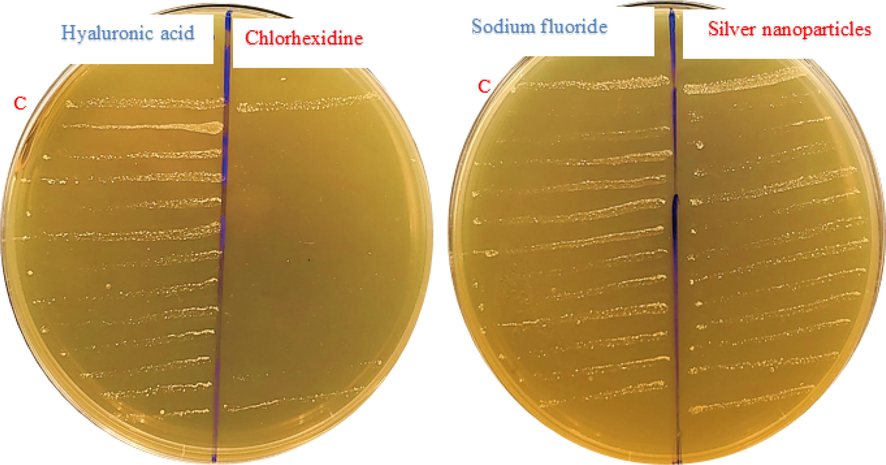
Sub-cultivation method used to determine the minimum bactericidal concentration (MBC) for chlorhexidine, hyaluronic acid, sodium fluoride and silver nanoparticles C- Control.
The prediction of antimicrobial resistance genes (AMR) in the whole genome shotgun sequencing project (GenBank: AOCB00000000.1) of S. mutans ATCC 25175 using Resistance Gene Identifier (RGI) in The Comprehensive Antibiotic Resistance Database (CARD) was performed and the data was summarized in Table 2. The results showed that this strain has many genes that encode to resist glycopeptide, fluoroquinolone, aminoglycoside, penam, tetracycline antibiotics, disinfecting agents and antiseptics. These genes include vanT gene in vanG cluster and mdeA.
3.4 Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) determination
The antibacterial susceptibility testing (AST) confirms effects of chlorhexidine mouthwash on S. mutans ATCC 25175 beggar than all components. The MIC and MBC of tested compounds were demonstrated in Figs. 4 and 5. The mean of MIC for chlorhexidine, hyaluronic acid, sodium fluoride. and silver nanoparticles were 0.0079 μg/mL, 2 mg/mL, 4 and 0.05 mg/mL respectively. The Fig. 5 illustrations the mean of MBC for chlorhexidine (0.039 μg/mL), hyaluronic acid (4 mg/mL), sodium fluoride (8 mg/mL), and silver nanoparticles (0.11 mg/mL). The statistical analysis of MIC and MBC means reported that there are significantly different (P < 0.05) using One Way ANOVA (Tukey test).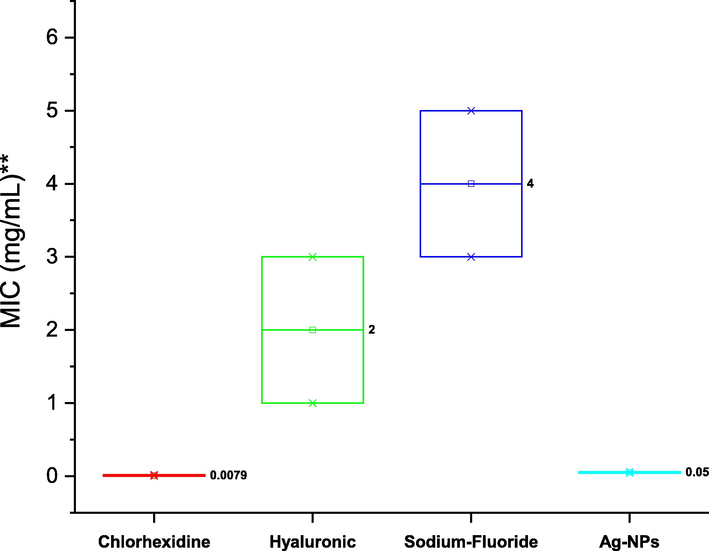
Minimum inhibitory concentration (MIC) of chlorhexidine, hyaluronic acid, sodium fluoride, and silver nanoparticles (Ag-NPS) against S. mutans ATCC 25175, and one-way ANOVA analysis at the 0.05 level using the Tukey test.
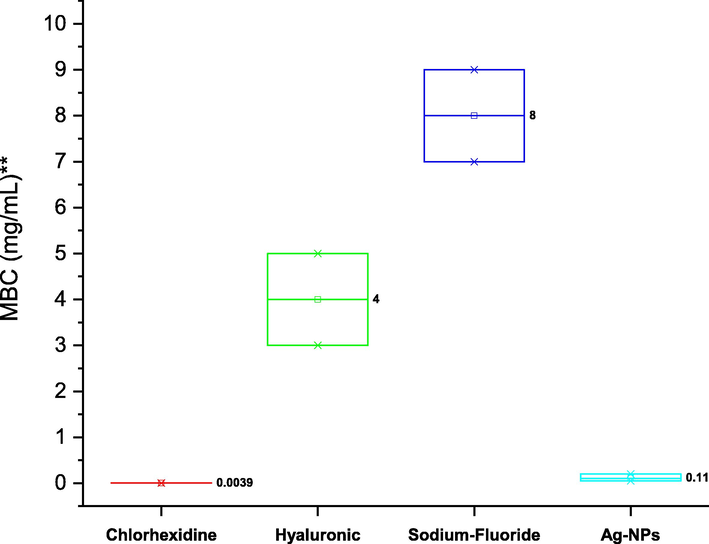
Minimum bactericidal concentration (MBC) of chlorhexidine, hyaluronic acid, sodium fluoride, and silver nanoparticles (Ag-NPS) against S. mutans ATCC 25175, and one-way ANOVA analysis at the 0.05 level using the Tukey test.
3.5 Minimum biofilm inhibitory concentration (MBIC)
Fig. 6 shows results of the MBIC against S. mutans strain used in this study. The MBICs of chlorhexidine, hyaluronic acid, sodium fluoride, silver nanoparticles were 0.0039 μg/mL, 0.5 mg/ml, 0.25 mg/ml, and 0.1 mg/ml respectively. Notwithstanding the three replications of MBIC for each compound have the same result, so the standard deviation is zero and the statistical analysis to test the significant differences were not done in this test, the chlorhexidine showed high activity against biofilm formation.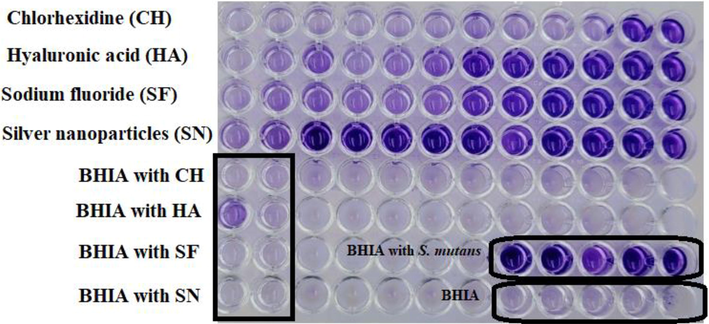
Minimum biofilm inhibition concentration (MBIC) of chlorhexidine, hyaluronic acid, sodium fluoride and silver nanoparticles (Ag-NPS) against S. mutans ATCC 25175.
4 Discussion
Dental caries is among the most prevalent and widespread oral disorders that affects both children and adults globally. It has been reported that the primary causative agent in the development of dental caries is S. mutans (Zayed et al., 2021). One of the most prevalent conditions affecting the mouth is dental caries, which mostly brought on by the facultative anaerobic bacterium S. mutans that plays a significant role in the development of dental caries, which may then progress to the deadly condition of infective endocarditis, due to its capacity to create biofilms and to facilitate the attachment of microorganisms to both one another and the tooth enamel. Therefore, preventing this disease has been and continues to be one of the most difficult issues in oral dental practice. Its ability to become more resistant to various antimicrobial drugs and to be less phagocytized by immune cells is what makes it most pathogenic. (Zayed et al., 2021).
For dental plaque and cavities to occur, oral S. mutans must be able to initiate biofilm growth on the surface of the teeth. In particular, S. mutans sticks to teeth and uses dietary sucrose to make extracellular insoluble glucans that can act as a framework for the colonization of other microorganisms. When S. mutans and other bacteria in the plaque biofilm create acids that erode enamel, tooth decay results. The exopolysaccharides glucan and dextran, as well as the S. mutans biofilm composition, have all described in detail. Micro colonies of S. mutans connected to a substrate reproduce, expand vertically, and interact with one another via extracellular polymers to form the multi structure of the biofilm. Water channels transfer nutrients and waste products across the biofilm matrix. (Ansari et al., 2017).
Due in large part to its ability to adhere to teeth and produce a molecular scaffold of glucan polysaccharides on the tooth surface, S. mutans is the primary cause of dental cavities. Disrupting the structure of S. mutans biofilms could inhibit the formation of biofilm communities that lead to cavities and tooth decay (Ansari et al., 2017).
There are many attempts to use several compounds to combat this microbe, for example, the development of S. mutans biofilm examined by Alshahrani and Gregory (2020) in relation to the effects of nicotine exposure on cinnamon water extract. 2.5 mg/ml cinnamon water extract inhibited nicotine-induced S. mutans biofilm formation by 34 to 98% at various nicotine dosages (0–32 mg/ml) and nicotine-induced S. mutans biofilm development reduced by 34 to 98% when 2.5 mg/ml cinnamon water extract added. This investigation supported the negative effects of nicotine and provided evidence for the biofilm-inhibitory properties of cinnamon extract (Alshahrani and Gregory, 2020).
Determination and understanding of virulence factors of pathogens considered the first and most important stages for investigating the manner of development of a disease and fighting these microbial pathogens. The findings obtained from The Seed Viewer and CARD analysis confirmed that S. mutans ATCC 25175 have several functional genes that enhance its ability to resist the drugs and cause the disease. Additionally, the data reported the functional roles of adhesion genes (hsp33, Cpa, PrtF, PrtF2, MsmRL, Nra, SOF, LepAL, Spy0135, and RofA) include chaperonin (heat shock protein 33), collagen-binding adhesin, fibronectin-binding protein, fibronectin-binding protein 2, multiple sugar metabolism regulator, negative transcriptional regulator, serum opacity factor, signal peptidase I, sortase, and transcriptional regulator. These genes are mostly identical and similar in function to genes identified in S. pyogenes. S. pyogenes and S. mutans adhesions have been studied extensively in a number of scientific studies such as Brouwer and Barnett (2016), Banas (2004), Hu et al., (2021), and other studies (Banas, 2004; Brouwer et al., 2016; Hu et al., 2021).
The morphological fractures of S. mutans ATCC 25175 observed in this work agreement with many previous studies such as Zayed et al (2021) who examined and studied the features of S. mutans strain on mitis salivarius medium, the results showed distinct hard raised, convex, undulate, opaque, pale blue colonies and frosty glass appearance. On brain heart infusion, the UAB90 strain grew as a slightly rough, convex, round colony with an erose margin. It has been confirmed that mutation led to clearly changes in morphological characteristics of S. mutans strains.
Our findings reported that chlorhexidine, sodium fluoride, hyaluronic acid, and silver nanoparticles have ability to inhibit growth of S. mutans ATCC 25175 and they are also able to prevent the formation of biofilms. The chlorhexidine showed the highest efficacy and was able to inhibit and kill S. mutans ATCC 25175 at 0.039 and 0.0079 μg/mL respectively. Malvania et al. (2019) investigated the anti- S. mutans activity of licorice root extract in comparison to fluoride and chlorhexidine mouthwash. They found that the antibacterial effectiveness of licorice ethanolic root extract was comparable with that of fluoride mouthwash. In a 2015 study, Ghapanchi et al. examined the antibacterial effects of different mouthwash doses on oral strains of S. mutans and E. coli. Oral B and Chlorhexidine each inhibited E. coli in a different manner.
Many researches have tried to combat this bacterial strain by using many natural and synthetic compounds in serious attempts to eradicate and reduce tooth decay. The effects of quercetin and kaemferol on the formation of S. mutans biofilms examined by Zeng et al. (2019). Both the quercetin and kaemferol composites demonstrated anti-biofilm action when compared to the negative control. The results of this study showed quercetin and kaemferol have inhibitory effects on S. mutans biofilms, suggesting that these compounds could serve as substitute anti-caries medications in the search for new anti-caries therapeutics. Singhal et al. (2020) looked into the antibacterial and antibiofilm properties of cranberries on S. mutans and L. acidophilus. According to the findings, cranberry extract had MBCs of 25 mg/dL and 12.5 mg/dL and MICs of 12.5 mg/dL and 6.125 mg/dL against S. mutans and L. acidophilus, respectively. Effective reduction in biofilm growth and bacterial cell adherence discovered at MIC (50%) and MBC (70%). Cranberry extract was discovered to have time- and dose-dependent bactericidal, bacteriostatic, and antibiofilm properties against S. mutans and L. acidophilus (Singhal et al., 2020). Zhong et al. (2021) examined the consequence of Ligustrum robustum extort (LRE) towards S. mutans biofilm production, as well as the mode of action and chemical components. HPLC-MS and nuclear magnetic resonance used to identify the compounds, and four phytochemicals were discovered (Ligurobustoside B, Ligurobustoside N, Ligurobustoside J, and Ligurobustoside C). The antimicrobial toxicity of LRE assessed against S. mutans biofilm formation and exopolysaccharide (EPS) synthesis with confocal laser scanning microscopy (CLSM) and crystal violet by dose-dependent (0.5 to 2.0 g/L) method. Scanning electron microscopy (SEM) used to study the biofilms microstructure of S. mutans treated with LRE and ex vivo bovine dental enamel. LRE also suppressed the appearance of the S. mutans glucosyl-transferase genes gtfB, gtfC, and gtfD, as well as the quorum sensing (QS) factors comD and comE. Furthermore, LRE has adequate biocompatibility was CCK-8 test against human oral cells. These findings suggested that this herbal tea, which is drunk daily, could be used to prevent caries (Zhang et al., 2021).
Our work did not discuss the mechanism by which the compounds (chlorhexidine, sodium fluoride, hyaluronic acid, and silver nanoparticles) inhibited biofilm formation, but we speculate that they somehow affected the factors that bacteria depend on for biofilm formation. It is likely that these compounds affected glucosyltransferase (GTF) enzymes. S. mutans adheres and attaches to tooth enamel primarily due to its ability to synthesize glucan, the building block of polysaccharide matrix derived from sucrose that enhances adhesion and attachment efficiency. S. mutans possesses the glucosyltransferase (GTF) enzyme that converts sucrose to fructose and glucose, which are then added to the growing exopolysaccharide matrix composed of glucan polymer. Cariogenic bacteria encode three glucosyltransferase enzyme (gtf) genes (gtfB, gtfC, gtfD). GTFB and GTFC enzymes produce water-insoluble glucans rich in −1, 3-glucosidic linkages, whereas GTFD produces water-soluble glucans rich in −1, 6-glucosidic linkages, enhancing the coherence of microorganisms and adhesion to tooth enamel. The glucan improves the adhesion of S. mutans by forming a hydrogen bond with the salivary pellicle and other bacteria, thereby increasing the biofilm's resistance to various chemotherapeutic agents and the host's defense. In addition, the binding of S. mutans to glucan is mediated by glucan binding protein (Gbp), which consists of the four subtypes A, B, C, and D. GbpC and GbpB are associated with the bacterial cell wall of S. mutans and function as a specific receptor for glucan, which is involved in microbial adhesion and biofilm formation. (Zayed et al., 2021).
In the present work, the finding obtained from CARD analysis reported that this strain (S. mutans ATCC 25175) has some the genes (vanT gene in vanG cluster and mdeA) that play role in resistance to glycopeptide, fluoroquinolone, aminoglycoside, penam, tetracycline antibiotics, disinfecting agents and antiseptics. In contrast to this result, the results of Vitek system showed that this bacterial strain is sensitive to all the antibiotics that were selected in this study. We believe that this phenomenon needs further investigation and research. It is known that gene expression requires the conversion of the genetic code into functions through numerous and complex steps that require specific factors. It has been reported that the bacterial strains need to control the expression of genetic information for achieving antibiotic resistance via certain mechanisms (Depardieu et al., 2007).
Conclusion
There is little question that our knowledge of the makeup and function of the biofilm linked to the majority of oral infections has improved significantly over the past few decades, but there is still more to be discovered. Microorganisms create complex structures called oral biofilms to help them survive, and pathogenicity typically rises in these well-organized groups. Different biofilms may, however, be more or less harmful. Currently, it is acknowledged that complex multispecies biofilms, rather than a single pathogen, are what cause oral illnesses. Furthermore, it makes sense that oral disorders arise from an imbalance in the dynamic interactions between the biofilm, host, and microenvironment. As a result, efforts have been made to increase our understanding of the structure and physiology of health-associated biofilms that are compatible with hosts in order to comprehend the changes that cause disease. It is possible that much of what we currently term therapy continues, albeit with less empiricism and greater specificity. On the other hand, our approach to these infections may undergo significant shifts in the very near future. In fact, ecological therapeutic strategies like chlorhexidine, hyaluronic acid, sodium fluoride, and silver nanoparticles of varying sizes have developed to stop the formation of biofilms, to change the environment that makes oral biofilm pathogenicity more likely, and to boost the immune system of the host in order to restore oral homeostasis. Treatment of biofilm-associated oral diseases will rely on general mechanical methods for preventing biofilm formation and disorganizing the existing oral biofilm until these alternative strategies are scientifically demonstrated to be effective. This study has shown beyond any doubt that this S. mutans ATCC 25175 possesses multiple virulence factors and that it has distinct biochemical and cultural features. In addition, it is sensitive to most standard antibiotics, and there is a range of chemical agents that can inhibit the biofilm formation of this pathogenic bacterial strain, and the best of them, according to the results of this study, was chlorhexidine. In the near future, I will work on improving on mixing these compounds as a new formulation to know the potential effect on the genetic level, as well as their ability as anti-bacterial and anti-biofilms.
Acknowledgement
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2023R70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The antibacterial activity of nanosilver coupled edible plant extracts against streptococcus mutans, the cause of dental caries. J. Pharm. Res. Int. 2021;33:167-186.
- [Google Scholar]
- In vitro cariostatic effects of cinnamon water extract on nicotine-induced streptococcus mutans biofilm. BMC Complementary Medicine and Therapies. 2020;20(1):1-9.
- [Google Scholar]
- Nanotechnology-based therapies for the prevention and treatment of streptococcus mutans-derived dental caries. J. Oral Biosci.. 2021;63(4):327-336.
- [Google Scholar]
- Anti-biofilm activity of a self-aggregating peptide against streptococcus mutans. Front. Microbiol.. 2017;8:488.
- [Google Scholar]
- Anti-biofilm activity of cannabigerol against streptococcus mutans. Microorganisms. 2021;9(10):2031.
- [Google Scholar]
- Virulence properties of streptococcus mutans. Frontiers in Bioscience-Landmark. 2004;9(2):1267-1277.
- [Google Scholar]
- Streptococcus pyogenes adhesion and colonization. FEBS Lett.. 2016;590(21):3739-3757.
- [Google Scholar]
- Child, maternal, and adult mortality in sierra leone: Nationally representative mortality survey 2018–20. Lancet Glob. Health. 2022;10(1):e114-e123.
- [Google Scholar]
- Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev.. 2017;11(22):57.
- [Google Scholar]
- Modes and modulations of antibiotic resistance gene expression. Clin. Microbiol. Rev.. 2007;20(1):79-114.
- [Google Scholar]
- Antibacterial effectiveness of four concentrations of the hydroalcoholic extract of solanum tuberosum (tocosh) against streptococcus mutans atcc 25175tm: A comparative in vitro study. International Journal of Dentistry 2020
- [Google Scholar]
- Molecular assessment of oral streptococcus mutans isolated from patients with different ages and caries activity via selective media and protein pattern. Egypt. Dent. J.. 2021;67(1-January (Conservative Dentistry and Endodontics)):789-800.
- [Google Scholar]
- The antibacterial effect of four mouthwashes against streptococcus mutans and escherichia coli. JPMA. 2015;65(350)
- [Google Scholar]
- 10-undecynoic acid is a new anti-adherent agent killing biofilm of oral streptococcus spp. PLoS One. 2019;14(4):e0214763.
- [Google Scholar]
- The susceptibility of streptococcus mutans to antibacterial photodynamic therapy: A comparison of two different photosensitizers and light sources. J. Appl. Oral Sci.. 2014;22:80-84.
- [Google Scholar]
- Antimicrobial activity of cinnamaldehyde on streptococcus mutans biofilms. Front. Microbiol.. 2019;10:2241.
- [Google Scholar]
- Phytochemical profile of antibacterial agents from red betel leaf (piper crocatum ruiz and pav) against bacteria in dental caries. Molecules. 2022;27(9):2861.
- [Google Scholar]
- Discovery of myricetin as an inhibitor against streptococcus mutans and an anti-adhesion approach to biofilm formation. Int. J. Med. Microbiol.. 2021;311(4):151512
- [Google Scholar]
- Jakubovics, N.S., Goodman, S.D., Mashburn‐Warren, L., Stafford, G.P. and Cieplik, F. 2021. The dental plaque biofilm matrix. Periodontology 2000, 86(1): 32-56.
- Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb. Pathog.. 2015;80:39-49.
- [Google Scholar]
- The virulence of streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis.. 2014;33(4):499-515.
- [Google Scholar]
- Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev.. 2014;78(3):510-543.
- [Google Scholar]
- In vitro analysis of licorice (glycyrrhiza glabra) root extract activity on streptococcus mutans in comparison to chlorhexidine and fluoride mouthwash. J. Contemp. Dent. Pract.. 2019;20(12):1389-1394.
- [Google Scholar]
- Cymbopogon citratus (lemongrass oil) oral sprays as inhibitors of mutans streptococci biofilm formation. J. Clin. Diagn. Res.. 2018;12(12)
- [Google Scholar]
- Inhibition of de novo plaque growth by a new 0.03% chlorhexidine mouth rinse formulation applying a non-brushing model: A randomized, double blind clinical trial. Clin. Oral Invest.. 2016;20(7):1459-1467.
- [Google Scholar]
- Invasive streptococcal disease: A review for clinicians. Br. Med. Bull.. 2015;115(1)
- [Google Scholar]
- Quorum quenching of streptococcus mutans via the nano-quercetin-based antimicrobial photodynamic therapy as a potential target for cariogenic biofilm. BMC Microbiol.. 2022;22(1):1-18.
- [Google Scholar]
- Antibacterial effect of metallic nanoparticles on streptococcus mutans bacterial strain with or without diode laser (970 nm) Bulletin of the National Research Centre. 2020;44(1):1-6.
- [Google Scholar]
- Billions with oral disease: A global health crisis—a call to action. J. Am. Dent. Assoc.. 2015;146(12):861-864.
- [Google Scholar]
- Oral health: How much do you know?–a study on knowledge, attitude and practices of patients visiting a north indian dental school. European Journal of Dentistry. 2014;8(01):063-067.
- [Google Scholar]
- Antimicrobial and antibiofilm effect of cranberry extract on streptococcus mutans and lactobacillus acidophilus: An in vitro study. International Journal of Clinical Pediatric Dentistry. 2020;13(1):11.
- [Google Scholar]
- Controlling of streptococcus mutans and lactobacillus in in-vitro using herbal formulation of ocimum sanctum and justicia adhatoda mediated silver nanoparticles. Plant Cell Biotechnology And Molecular Biology 2020:109-117.
- [Google Scholar]
- Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021;9(10):2041.
- [Google Scholar]
- Synergistic antibacterial efficacies of chlorhexidine digluconate or protamine sulfate combined with laminaria japonica or rosmarinus officinalis extracts against streptococcus mutans. Biocontrol Sci.. 2020;25(1):41-44.
- [Google Scholar]
- Biofilm formation by streptococcus mutans and its inhibition by green tea extracts. AMB Express. 2021;11(1):1-10.
- [Google Scholar]
- Activity of quercetin and kaemferol against streptococcus mutans biofilm. Arch. Oral Biol.. 2019;98:9-16.
- [Google Scholar]
- Synergistic antibacterial effects of reuterin and catechin against streptococcus mutans. LWT. 2021;139:110527
- [Google Scholar]







