Translate this page into:
Probiotic Lactobacillus strains isolated from date waste and wastewater for the kidney stone, intestinal oxalate-degradation and antioxidant activity
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lactic acid bacteria (LAB) are probiotic organisms and are promising alternatives to manage kidney stone problems. Oxalate digesting LAB was screened from date wastes and date wastewater. LAB was isolated using De Man, Rogosa and Sharpe agar (MRS agar) medium and was subjected to oxalate degradation assay in a calcium oxalate plate. The strain was further subjected to assay for its capacity to tolerate acidic pH value and bile salt. Then, anti-urolithiatic activity assay was carried out by in vitro analysis. Based on preliminary characteristics, among 62 oxalate degrading strains, twenty-four strains exhibited a greater than 10 mm zone. The potent bacterial strains were identified as Lactobacillus cassei, L. acidophilus, L. gasseri and L. fermentum. Isolates from date wastes showed better antagonistic properties towards bacterial pathogens. At 50 µg Calcium oxalate concentration, 75.6 ± 0.865 degradation was achieved by L acidophilus. LAB isolated from date waste showed promising anti-urolithiasis activity. Hence, date waste may serve as a potential source for LAB probiotics to treat hyperoxaluria.
Keywords
Kidney stone
Oxalate degradation
Lactobacillus
Probiotics
Hyperoxaluria
1 Introduction
Urinary stone is an expanding health-associated problem in various countries (Ram and Poojamoteriya, 2015). Genetic factors, environmental factors, food habits and metabolic disturbances are the most important causes of urinary stones. More than 10% of the total population will have urinary stones during their lifetime (DeFrances et al., 2010). The development of kidney stones is a complex mechanism. The concentration of urine was achieved by the supersaturation mechanism in which the concentration of oxalate, and calcium were increased in the urine than the saturation limit. Oxalate is one of the highly oxidized substances reported in various foodstuffs, mainly cereals and vegetables contained in large quantities. The unusual rise of oxalate in the food intake leads to the process called hyperoxaluria. This condition is a risk for kidney stone disease in humans (Borghi et al., 2010). The prevalence of kidney stone was high when fluoride content is more than greater than 3.0 ppm in the drinking water (Abeygunasekera, 2011). Effective prophylaxis is required to prevent the development of kidney stone disease using oxalate-degrading bacteria (Gomathi et al., 2014).
Probiotics are widely used interventions for the treatment of several diseases (Al-Dhabi et al., 2020) These are living microbial food supplements enabling the intestinal microbial flora highly stable and make individuals more healthy against various enteric pathogens. The probiotic bacterial strains were used to treat diarrhoea, acute infantile, irritable bowel syndrome and inflammatory bowel disease (Borthakur et al., 2008). Probiotic bacteria in the gut maintain oxalate homeostasis by utilizing oxalate from the intestine, thus considerably decreasing the excretion of oxalate through urine (Campieri et al., 2001). Oxalobacter formigenes is one of the well-known oxalate-degrading microbes that used available oxalate in the gut to maintain oxalate homeostasis. Moreover, its application in the intestine as a probiotic has been very limited use because highly specialized oxalotropohic nature, various nutrient requirements and colonizing ability. In recent years, Lactic acid bacteria (LAB) were widely screened for their probiotic properties (Anbazhagan et al., 2013). Bacteria such as Bifidobacterium sp. and Lactobacillus sp. are involved in the reduction of luminal oxalate and minimized the risk of urinary oxalate excretion in animals and humans (Ito et al., 1996). The application of expressed oxalate degrading gene in probiotic organisms improved oxalate degradation and controlled enteric hyperoxaluria (Heilig et al., 2002). According to WHO, about 80% of the global population are using alternative medicines to cure diseases. Although a number of bacteria were reported to degrade oxalate and there is no report on the oxalate degrading properties of LAB from date wastes. The study aimed to screen the oxalate degrading property of LAB from date wastes and wastewater from dates processing industries.
2 Materials and methods
2.1 Sample collection and characterization of LAB
Totally eight samples (n = 8) were obtained from date processing industry waste and wastewater and brought into the laboratory in ice. To isolate LAB, one gram of collected wasted dates was ground with sterile double distilled water. To the 1.0 ml sample, 99 ml peptone water was added. It was serially diluted and plated on MRS agar medium (Himedia, Mumbai, India). The culture plate was incubated for three days at 37 °C. About 10 – 15 well-isolated bacterial colonies were selected and sub-cultured on MRS medium. Isolated bacteria were characterized using various morphological and biochemical tests (Vijayaraghavan et al., 2016; Al-Dhabi et al., 2020).
2.2 Analysis of oxalate degrading property
The oxalate degrading ability of the bacterial strain was carried out according to the method of Anbazhagan et al. (2013). Calcium oxalate was incorporated with the nutrient agar medium and broth culture was loaded into each well. It was incubated for 12 h at 30 ± 1 °C. The formation of a clear zone around the well indicated the presence of decomposition of oxalate by the isolates. The zone of clearance was analyzed and the bacterial strains showing more than 10 mm of the zone were subjected to the quantitative determination of oxalate degradation (secondary screening). To analyze their capacity to degrade oxalate, the selected bacterial stains were cultured in an MRS medium. In the culture medium, potassium oxalate was supplemented at a 10 mM level and incubated for five days. Then the amount of oxalate concentration was determined as described previously (Federici et al., 2004).
2.3 Probiotic properties of LAB
LAB strain was cultured in MRS broth medium containing 0.3% bile salt concentration. It was incubated for 12 h at 30 ± 1 °C. After the incubation period, the broth culture was diluted appropriately with sterile water. Then it was spread on MRS agar medium and the developed colonies were counted using an automated colony counter. The viability of the bacteria was determined by analyzing the survival rate (Al-Dhabi et al., 2020).
2.4 Antibiotic sensitivity of LAB strains
The antibiotic susceptibility of the selected bacterial strains was analyzed on MRS agar medium with the standard antibiotic disc (Himedia, Mumbai, India). The overnight culture of LAB strains was grown on MRS agar and the antibiotics (Gentamicin, Vancomycin, Ampicillin, Kanamycin, Chloramphenicol, Tetracycline, Erythromycin and Streptomycin) were placed. The plates were incubated for 24 h and the results were observed (Wu et al., 2020).
2.5 Molecular characterization
LAB isolates with excellent oxalate degrading activity was further analysed through 16S rDNA sequence analysis. Bacterial genomic DNA extraction and PCR amplification were carried out by the method of Gomathi et al. (2014) with little modifications. The potent bacterial strain was subjected to 16S rDNA gene sequencing as described previously.
2.6 Antimicrobial activity
The antimicrobial activity was carried out by the method described by Wang et al. (2010). Bacterial strains such as Bacillus subtilis ATCC 6633, Bacillus cereus NCIM 245, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 6538, and Escherichia coli ATCC 25922 were used as the indicator strains.
2.7 Antioxidant activity of LAB
Antioxidant properties of LAB isolates were performed as suggested previously by Arasu et al. (2015). Briefly, phenanthroline was prepared at 0.75 mmol concentration in sodium phosphate buffer. To the 1.0 ml phenanthroline solution, 1 ml ferrous sulphate was added. To the reaction mixture, 0.5 ml of 1010 CFU/ml LAB strain was incorporated and mixed. Then hydrogen peroxide was added and incubated for 90 min. Then the supernatant was subjected for the determination of scavenging activity (Atif et al., 2020).
2.8 Estimation of anti-urolithiatic activity
The cell-free extract of LAB was obtained as described previously. These two samples are comparatively assessed with the standard (cystone). This procedure was performed three times and the average value was considered for data processing. The dissolution percentages of the calcium oxalate crystals were calculated for each sample to evaluate the activity (Pawar and Vyawahare, 2015).
3 Result
3.1 Oxalate degrading property f LAB
A total of 11 samples were subjected to initial screening and 62 bacterial strains were initially identified as LAB. They were catalase-negative and Gram-positive and capable to form a clear zone in 0.5% CaCO3. Based on these characters these were tentatively identified as LAB. From the 62 isolated bacterial strains, 24 bacterial strains showed clear zone above 10 mm (Table 1). Oxalate degradation was significant in four strains, and they showed more than 46% of oxalate degradation activity, and three strains showed more than 50% of activity (Table 1).
Sources
Strains
Oxalate degradation (%)
LS12
46
LS 24
54.6
Date waste
LS 32
36.46
LS34
52.1
LS45
28.11
LS49
33
LS56
42
LS F13
51
LS F21
32
LS F32
41.4
Date wastewater
LS F40
22
LS F46
17.2
LS F 60
18
3.2 Characterization of LAB
Oxalate degrading strains (greater than 45%) were identified and tabulated in Table 2. Among the four strains, 3 strains (Lactobacillus cassei, Lactobacillus acidophilus and Lactobacillus gasseri) were isolated from the date waste whereas Lactobacillus fermentum was isolated from date processing wastewater (Table 2).
Biochemical test
Results
L. cassei
L. acidophilus
L. gasseri
L. fermentum
Gram-staining
Positive
Positive
Positive
Shape
Rod
Rod
Rod
Rod
Starch hydrolysis
Negative
Negative
Negative
Negative
Indole test
Negative
Negative
Negative
Negative
Methyl red
Negative
Negative
Negative
Negative
Voges-Proskaur test
Positive
Positive
Positive
Positive
Citrate utilization test
Negative
Negative
Negative
Negative
Lactose fermentation
Positive
Positive
Positive
Positive
Glucose fermentation
Positive
Positive
Positive
Positive
Spore formation
Negative
Negative
Negative
Negative
Capsule formation
Negative
Negative
Negative
Negative
Motility
Non-motile
Non-motile
Non-motile
Non-motile
Catalase fermentation
Negative
Negative
Negative
Negative
Oxidase
Negative
Negative
Negative
Negative
Urease
Negative
Negative
Negative
Negative
Gas-production
Negative
Negative
Negative
Negative
3.3 Survival rate of LAB in acid and bile
The selected LAB strains survived at various tested pH values (2.5–3.5). This result indicated that these strains were highly acid-tolerant. The highest survival rate was observed in isolates Lactobacillus acidophilus and Lactobacillus fermentum with 81.2%, 76%, 83%, and 75%, respectively. The survival rate was also found to be high in the presence of 3% bile salt (Table 3).
LAB strains
Survival rate of isolates (%)
pH 2.5
pH 3.5
Bile tolerance
L. cassei
72 ± 1.3
64 ± 1.1
62 ± 2.2
L. acidophilus
81.2 ± 2.9
76 ± 2.2
64.5 ± 1.6
L. gasseri
69 ± 1.8
71 ± 1.5
59 ± 2.8
L. fermentum
83 ± 2.4
75 ± 2.2
66 ± 2.1
3.4 Antibacterial activity
Antibiotic activity of LAB strains was carried out against five human bacterial pathogens and observed different inhibitory properties. The selected bacteria exhibited potential antagonistic properties. Among all tested strains, L. fermentum showed significant inhibitory results against E. coli, and P. aeruginosa however, the cell-free extract from S. aureus was found to be resistant against Lactobacillus cassei and the other strains showed moderate inhibition. The results were tabulated in Tables 4 and 5. R–resistant; S – sensitive.
Bacteria strains
Antagonistic activity
E. coli
S. typhi
P. aeruginosa
B. subtilis
S. aureus
L. cassei
16 ± 2
12
13 ± 2
17 ± 2
13 ± 1
L. acidophillus
17 ± 1
13
17 ± 1
17 ± 1
18 ± 2
L. gasseri
16 ± 2
17 ± 2
17 ± 1
13 ± 1
18 ± 1
L. fermentum
23 ± 1
22
23 ± 2
18 ± 2
13 ± 1
Antibiotics
LAB strains
L. cassei
L. acidophilus
L. gasseri
L. fermentum
Gentamicin
R
S
R
S
Vancomycin
S
S
S
S
Ampicillin
R
S
S
R
Kanamycin
R
R
R
R
Chloramphenicol
S
S
R
S
Tetracycline
S
S
S
S
Erythromycin
R
S
R
R
Streptomycin
S
S
S
S
3.5 Antibiotic sensitivity of the LAB strains
The four selected LAB isolates were analyzed for their antibiotic sensitivity effect against various commercial antibiotics. The tested bacterial strains were highly susceptible to streptomycin and tetracycline. Antibiotic sensitivity varied widely based on the strain and the results were described in Table 6.
Cell-free extract
Calcium oxalate degradation (%)
12.5 µg
25 µg
50 µg
L cassei
39.3 ± 0.325
46.5 ± 0.515
61.4 ± 0.573
L acidophilus
45.3 ± 0.632
52.2 ± 0.713
75.6 ± 0.865
L gasseri
43.3 ± 0.525
50.1 ± 0.225
66.7 ± 0.522
L fermentum
49.2 ± 0.262
53.2 ± 0.274
72.4 ± 0.385
3.6 Antioxidant activity
The antioxidant property of LAB strains was studied. The selected bacterial strains showed potent antioxidant activity and it varied widely. L. cassei showed 78.4 ± 13.2%, whereas L. acidophilus showed 87.3 ± 10.4% antioxidant capacity (Fig. 1).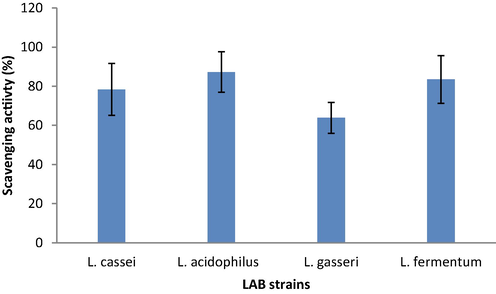
Antioxidant activity of selected bacterial strains.
3.7 Anti-urolithiatic activity of the cell-free extract of LAB isolates
The anti-urolithiatic property of the LAB strain was compared with the standard (cystone). Cell-free extract of LAB showed significant anti-urolithic activity as the standard cystone drug. The highest dissolution percentage was yielded by the extract of L. acidophilus at 50 µg concentration (Table 6).
4 Discussion
Probiotic is one of the leading health issues in the world. It is generally formed due to an increasing absorption of oxalate in the intestine. This increased absorption rate increased the formation of urinary stones. It has been a highly common chronic disease in the genitourinary tract system; however, there is no preventive measure or safety treatment (Su et al., 2016). Absorption of ammonium or calcium oxalate in the urinary tract is capable of causing pelvic inflammation. It is usually developed with the symptoms of pain and bleeding (Whittamore and Hatch 2017). Kidney stone formation leads to develop kidney tissue damage, renal dysfunction and acute or chronic renal failure. Kidney transplantation is the ultimate solution to overcome this problem. To treat kidney stones, Transurethral Uretero-Lithotripsy, Percutaneous Nephrolithotomy and Extracorporeal Shock Wave Lithotripsy are recommended, however, these were highly expensive (Nikpay et al., 2016). Hence the researchers paid more attention towards the use of LAB probiotics and herbal plants as an alternative and safe for the past two decades in prevent kidney stone formation. Many studies reported oxalate-degrading bacteria from various sources. In the present study, oxalate-degrading Gram-positive bacteria such as L. cassei, L. acidophilus, L. gasseri and L. fermentum were isolated from date wastes and date processing wastewater, respectively. The selected bacteria were able to survive at pH 2.5 and 3.5. This result indicated that the stains were acid-tolerant. However, the highest survival rate was observed in strains L. acidophilus and L. fermentum with 81.2% and 76%, respectively. Previous studies have documented that the Lactobacillus sp. showed good survival at pH 3.0, but lowering viability resulted, when exposed to pH 2.0 (Wang et al., 2010; Al-Dhabi et al., 2020). Earlier reports have proven that the L. fermentum strains could survive at a low pH value (pH 2), however, the survival rate was reportedly less than that L. fermentum (96%) strains grown at pH 3 (Likotrafiti et al., 2013). In this study, the maximum survival rate of L. fermentum (83%) strains was observed at pH 2.5. In the oxalate degradation efficiency, cell-free extract of L. acidophilus found to have strong anti-urolithiatic activity (75.6 ± 0.865) however it could be possible because of the presence of oxalyl-CoA decarboxylase and formyl-CoA transferase (Azcarate-Peril et al., 2009) and these are mainly determined by the presence of frc and oxc genes, respectively (Amini et al., 2016). Oxalate oxidases and oxalate decarboxylases are belonging to the members of the cupin superfamily of proteins (Burrell et al., 2007). The enzymes formyl-CoA transferase and Oxalyl-CoA decarboxylase are involved in oxalate degradation within the gastrointestinal tract. The strain, L. gasseri showed 66.7 ± 0.522% of anti-urolithiatic activity and this result was similar to a previous study (Turroni et al., 2007). Human intestinal bacterial strains exhibited pragmatic oxalate degradation ability. L. fermentum and its effectiveness on oxalate degradation are found to be similar to the study of Gomathi et al. (2014). The cell-free extract of LAB was similar to the standard drug, cystone (75.6 ± 0.865). L. fermentum exhibited strong antagonistic activity against E. coli and P. aeruginosa. However, the antagonistic activity of lactobacilli strains varied based on pathogenic strains (Srigopalram et al., 2017; Arasu et al., 2014; Zhang et al., 2020; Wang et al., 2020). LAB showed variations in the production of lactic acid, H2O2, and bacteriocin (Kučerová et al., 2007). More than 13% of urinary stones resulted due to formation of COM papillary calculi and this deposition in renal cavities, comprises 16% of renal stones (Arasu et al., 2015).
5 Conclusions
The cell-free extracts of LAB probiotics isolated from date waste and wastewater showed promising anti-urolithiatic activity. Hence date waste and Lactobacillus sp. may be used as potential sources of LAB probiotics for treating hyperoxaluria. LAB strains are coded with oxalate-degrading enzymes, of which p170-ODC-LAB and ODC- LAB showed potential oxalate degradation, inhibition of calcium oxalate stone formation, and hyperoxaluria reduction. The variation among the isolated individual LAB species revealed that probiotics formulated with the combination of bacteria in the LAB group may be useful for reducing oxalate degradation. Further clinical research is required on the preparation of multiple probiotic strains of Lactobacillus.
Acknowledgement
The author would like to thank Deanship of Scientific Research at Majmaah University for supporting this work under Project Number No. R-2023-589.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Co-Fermentation of Food Waste and Municipal Sludge from the Saudi Arabian Environment to Improve Lactic Acid Production by Lactobacillus rhamnosus AW3 Isolated from Date Processing Waste. Sustainability. 2020;12(17):6899.
- [Google Scholar]
- Probiotic and Antioxidant Potential of Lactobacillus reuteri LR12 and Lactobacillus lactis LL10 Isolated from Pineapple Puree and Quality Analysis of Pineapple-Flavored Goat Milk Yoghurt during Storage. Microorganisms. 2020;8(10):1461.
- [Google Scholar]
- Evaluation of oxalate-degrading activity and molecular recognition of Oxc, Frc Genes in lactic acid bacterium of inhabit in human colon. Int. J. Pharma. Technol.. 2016;8(3):16055-16066.
- [Google Scholar]
- In vitro degradation of oxalate by recombinant Lactobacillus plantarum expressing heterologous oxalate decarboxylase. J. Appl. Microbiol.. 2013;115(3):880-887.
- [Google Scholar]
- In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol.. 2014;64(3):1333-1346.
- [Google Scholar]
- Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann. Microbiol.. 2015;65(1):15-25.
- [Google Scholar]
- Essential oils of two medicinal plants and protective properties of jack fruits against the spoilage bacteria and fungi. Ind. Crop Prod.. 2020;147:112239
- [Google Scholar]
- Temporal gene expression and probiotic attributes of Lactobacillus acidophilus during growth in milk. J. Dairy Sci.. 2009;92(3):870-886.
- [Google Scholar]
- Probiotics and dietary manipulations in calcium oxalate nephrolithiasis: two sides of the same coin? Kidney Int.. 2010;78(11):1063-1065.
- [Google Scholar]
- The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J. Nut.. 2008;138(7):1355-1359.
- [Google Scholar]
- Oxalate decarboxylase and oxalate oxidase activities can be interchanged with a specificity switch of up to 282 000 by mutating an active site lid. Biochemistry. 2007;46(43):12327-12336.
- [Google Scholar]
- Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int.. 2001;60(3):1097-1105.
- [Google Scholar]
- DeFrances, C.J., Golosinskiy, A., Hall, M.J., Schwartzman, A., Williams, S.N., 2010. National hospital discharge survey; 2007 summary. (385), 1-19.
- Characterization and heterologous expression of the oxalyl coenzyme A decarboxylase gene from Bifidobacterium lactis. Appl. Env. Microbiol.. 2004;70(9):5066-5073.
- [Google Scholar]
- Gomathi, S., Sasikumar, P., Anbazhagan, K., Sasikumar, S., Kavitha, M., Selvi, M.S., Selvam, G.S., 2014. Screening of indigenous oxalate degrading lactic acid bacteria from human faeces and South Indian fermented foods: assessment of probiotic potential. Sci. World J. 2014.
- Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol.. 2002;68(1):114-123.
- [Google Scholar]
- In vitro degradation of oxalic acid by human feces. Int. J. Urol.. 1996;3(3):207-211.
- [Google Scholar]
- Screening of lactic acid bacteria for antimicrobial properties from mayonnaise-based products and raw materials. Eur. Food Res. Technol.. 2007;226(1–2):265-272.
- [Google Scholar]
- Development of antimicrobial synbiotics using potentially-probiotic faecal isolates of Lactobacillus fermentum and Bifidobacterium longum. Anaerobe. 2013;20:5-13.
- [Google Scholar]
- Frequency of kidney stone different compositions in patients referred to a Lithotripsy Center in Ilam. West of Iran. J. Pediat. Nephrol.. 2016;4(3):102-107.
- [Google Scholar]
- Anti-urolithiatic activity of standardized extract of Biophytum sensitivum against zinc disc implantation induced urolithiasis in rats. J. Adv. Pharm. Technol. Res.. 2015;6(4):176.
- [Google Scholar]
- An Overview of some promising medicinal plants with in vitro anti-urolithaitic activity. IOSR J. Pharma.. 2015;5(5):23-28.
- [Google Scholar]
- Isolation, in vitro probiotic characterization of Lactobacillus plantarum and its role on italian ryegrass silage quality enhancement. Int. J. Agric. Biol.. 2017;19(1):164-170.
- [Google Scholar]
- Effect of statins on kidney disease outcomes: a systematic review and meta-analysis. Am. J. Kidney Dis.. 2016;67(6):881-892.
- [Google Scholar]
- Oxalate consumption by lactobacilli: evaluation of oxalyl-CoA decarboxylase and formyl-CoA transferase activity in Lactobacillus acidophilus. J. Appl. Microbiol.. 2007;103(5):1600-1609.
- [Google Scholar]
- Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in in vitro clot lysis. Front. Microbiol.. 2016;7:361.
- [Google Scholar]
- Host associated mixed probiotic bacteria induced digestive enzymes in the gut of tiger shrimp Penaeus monodon. Saud. J. Biol. Sci.. 2020;27(9):2479-2484.
- [Google Scholar]
- Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe. 2010;16(6):578-585.
- [Google Scholar]
- The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis. 2017;45(1):89-108.
- [Google Scholar]
- Characterization of biofilm formed by multidrug resistant Pseudomonas aeruginosa DC-17 isolated from dental caries. Saudi Journal of Biological Sciences. 2020;27(11):2955-2960.
- [Google Scholar]
- Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saud. J. Biol. Sci.. 2020;27(12):3505-3513.
- [Google Scholar]







