Translate this page into:
Distribution of mosquito species in various agro-ecological zones of Punjab
⁎Corresponding author. mirzashahbazkfueit@outlook.com (Shahbaz Ali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The presence and behavior of mosquitoes in various agro-ecological zones of Punjab are influenced by a combination of seasonal and topographical patterns, cropping schemes, and human settlement. These modifications have a significant impact on the occurrence of diseases. Therefore, determining the distribution of these species could help in halting their spread and disease outbreaks.
Methods
Under these prevailing conditions, surveillance studies were carried out at six zones of Punjab, to know the distribution of mosquitoes. Samples were collected in various seasons (once in winter and rainy seasons, and twice in summer), from all possible habitats, having stagnant water.
Results
Out of twenty-four (24) recorded species, three were included in Anophelinae and Culicinae, eleven from tribe Aedini, eight from Culicini and one each from Ficulbiini and Mansoniini. Sampling was done from 91 m (Ahmed Pur East) elevation to 1759 m (Fort Monroe). High and diversified population was recorded during rainy season. The Anopheline mosquitoes were found in rural as well as urban habitats, including rainfed, wet mountains and irrigated plains whereas Aedine species were confined to northern irrigated plains and mostly recorded from Changa Manga National Forest. Culicine species were more diversified and abundant, in all seasons. However, M. chamberlaini was collected from urban (rainfed lands) and rural (northern irrigated plains) settings. C. crassipes was recorded only from rainfed lands.
Conclusion
Twenty-four mosquito species from the Anophelinae and Culicinae subfamilies were observed in the current investigation. Anophelinae subfamily contained three species. Eleven species of Culicinae belonged to the tribe Aedini, eight to the Culicini, and one each to the Mansoniini and Ficalbiini.
Keywords
GPS
Mosquito species
Punjab
Zone
1 Introduction
The association between wetlands and malaria has been acknowledged by humans since the eras of the Romans and Greeks (Andrade Jr, 1995). The association between wetlands and malaria has been acknowledged for an extended period. The ancient civilizations of Greece and Rome understood the correlation between malaria and marsh environments, and this knowledge has been transmitted over generations. Currently, this comprehension remains pertinent in the context of disease preventive and control endeavors inside wetland regions (Demetrioff, 2020; Sallares, 2002).
Malaria is an infectious disease, mostly transferred to human hosts through the bites of female mosquitoes belonging to the Anopheles genus. Malaria affects a global population of over 500 million individuals and results in an annual mortality rate of 1 million individuals (Holt et al., 2002; Sangbakembi-Ngounou et al., 2022). Presently, malaria is prevalent in > 80 countries, where it is spread by anopheline mosquitoes (Dahmana & Mediannikov, 2020). The effective maturation of the malaria parasite inside the mosquito is contingent upon several variables (Janson et al., 2023). There are 3,500 distinct mosquito species classified into 41 genera (Kain et al., 2022). However, it is important to note that just a subset of 30–40 species belonging to the Anopheles genus are responsible for the transmission of malaria within natural ecosystems. The knowledge on the distribution of mosquito species has significant value in the formulation and implementation of efficient approaches aimed at mitigating the spread and impact of diseases.
Historical origins of vector-borne diseases in Pakistan may be traced back to ancient times like other regions around the globe (Jabeen et al., 2022). Nevertheless, with the passage of time and changes of environmental conditions, there has been a persistent increase in the diversity of vector species and the severity of diseases caused by these vectors. Mosquito-borne diseases like Dengue Fever and Dengue Hemorrhagic Fever (Aedes aegypti, Ae.albopictus and other Aedes spp.), West Nile Fever (Culex tritaeniorhynchus, Cx. quinquefasciatus), Sindbis Fever (Cx. pseudovishnui, Cx. tritaeniorhynchus), Japanese Encephalitis (Cx. tritaeniorhynchus, Cx. pseudovishnui and other species of Culex and Anopheles), Chikungunya Fever (Ae. aegypti, Cx. tritaeniorhynchus), Malaria (An. culicifacies, An. stephensi, An. subpictus and other species), Brugian Filariasis (Mansonia spp.) and Bancroftian Filariasis (Cx. quinquefasciatus and other species of Culex and Aedes) have been recorded from Pakistan (Ashfaq et al., 2014; Attaullah et al., 2023; Khan et al., 2015; Manzoor et al., 2020; Qasim et al., 2014).
Knowledge about the distribution patterns of mosquito species has significant importance in the development of effective measures aimed at preventing and controlling diseases (Becker et al., 2020; Ferraguti et al., 2023). The transmission of vector-borne diseases is impacted in varying ways by human-induced landscape modification, particularly in the context of overall biodiversity decline resulting from increased human pressures on the environment (Glidden et al., 2021). Determination of mosquito species’ diversity, distribution, and relative abundance might provide valuable insights about the ecological characteristics of various locations (Dahmana & Mediannikov, 2020; Moreno-Madriñán & Turell, 2018). The data on the composition and genetic diversity of mosquito species might provide valuable insights for further research and the development of effective management approaches.
The implementation of mosquito control measures is often conducted by regulatory bodies, who mostly depend on their knowledge rather than a systematic approach backed by spatio-temporal data (DeGroote et al., 2007). Understanding the determinants of spatio-temporal distribution patterns in animal or plant species is a fundamental aspect of ecological study. This assessment includes temporal abundance as well as the climatic factors and spatio-temporal study of the landscape in relation to mosquito populations (DeGroote et al., 2007). The breeding of several mosquito species is known to be enhanced by recent changes resulting from agricultural operations and urbanization (Adeleke et al., 2008). Moreover, complex, and interconnected nature of ecological variables has a significant role in influencing population growth over time, as shown by the observed changes in vector behavior (Barik et al., 2009). The effectiveness of mosquito control efforts can be improved by expanding our understanding of both environmental factors and biological processes that impact mosquito populations.
The Punjab province in Pakistan was divided into six distinct agro-ecological zones (Ahmad et al., 2019). The province has a wide range of terrain types, including arid deserts as well as verdant woods, accompanied by a diversified array of agricultural practices. The cropping patterns in the region include wheat-cotton, rice–wheat, vegetable, and fodder crops (Mujtaba et al., 2022). The province hosts a variety of seasons throughout its varied locations. These include intense heat during May and June, monsoon rains occurring between July and September, and a hard winter characterized by both dry and rainy conditions, with snowfall occurring in some places. The seasonal diversity and topography offer a range of environments that support the growth and development of both juvenile and adult stages of vector mosquitoes in the province. This, in turn, helps to control the numbers of these insects across different locations and periods. Understanding population patterns may be crucial in effectively controlling population growth and implementing measures to reduce mosquito-borne diseases such as malaria and dengue.
Therefore, the current study was conducted to map the distribution of mosquito species in different agro-ecological zones of Punjab province. It was hypothesized that the regions would differ for the distribution of the species. The results would provide valuable insights for the management of these species in the province.
2 Materials and methods
2.1 Study area
The current study was conducted in different agro-ecological zones of Punjab. The zones included in the study were sandy desert A (Cholistan), sandy desert B (Thal), rainfed (Barani) Lands, wet mountains, northern irrigated plains, and Sulaiman piedmont. The climatic features and major uses of soil for these zones are presented in Table 1. In the seasons column, 1 = winter, 2 = summer and 3 = monsoon (rainy). Agro-Ecological Zones: 1. Sandy Desert A (Cholistan) 28° 45.991–29° 25.251; 071° 20.040–071° 40.479; 91–140, 2. Sandy Desert B (Thal) 32° 04.443–32° 34.400; 071° 29.554–072° 23.471; 179–213, 3. Rain-fed Lands (Barani) 32° 06.580–33° 47.995; 072° 21.659–074° 52.266; 219–720, 4. Wet Mountains 33° 46.746–33° 50.598; 073° 06.641–073° 18.821; 640–1222, 5. Northern Irrigated Plains 29° 30.770–32° 20.959; 070° 53.343–074° 28.753; 100 – 238, 6 Suleiman Piedmont 29° 55.218–30° 28.668; 069° 59.279–070° 43.455; 107–1759.
Species
Seasons
Agro-Ecological Zones
1
2
3
χ2
p-value
1
2
3
4
5
6
χ2
p-value
An. nigerrimus
0
0
1
1.943
0.379
0
0
1
0
0
0
2.728
0.742
An. stephensi
4
11
6
1.134
0.567
1
1
4
0
10
5
11.69
0.039
An. subpictus
3
12
19
8.936
0.011
4
5
0
0
23
2
15.978
0.007
Ae. pseudotaeniatus
0
2
2
1.376
0.503
0
0
0
4
0
0
164.385
0.000
Ae. vittatus
1
26
38
27.801
0.000
2
4
54
5
0
0
131.634
0.000
Ae. lineatopennis
0
0
4
7.799
0.02
0
0
0
0
4
0
5.084
0.406
Ae. caspius
0
1
6
8.54
0.014
0
0
4
0
3
0
4.462
0.485
Ae. aegypti
1
7
10
5.285
0.071
0
5
1
0
12
0
15.582
0.008
Ae. albopictus
1
21
40
33.717
0.000
0
0
30
11
21
0
97.133
0.000
Ae. unilineatus
0
0
4
7.799
0.02
0
0
0
0
4
0
5.084
0.406
Ae. w-albus
0
0
1
1.943
0.379
0
0
0
0
1
0
1.266
0.938
Ar. subalbatus
0
3
8
8.203
0.017
0
0
0
5
6
0
92.858
0.000
Ve. yusafi
0
0
3
5.842
0.054
0
0
0
0
3
0
3.808
0.577
Ve. indica
1
0
5
6.973
0.031
0
0
0
0
6
0
7.644
0.177
Cx. fuscocephala
2
5
3
0.334
0.846
0
0
2
0
8
0
6.057
0.301
Cx. pseudovishnui
13
48
61
22.39
0.000
4
17
21
0
66
14
26.984
0.000
Cx. quinquefasciatus
66
121
64
12.257
0.002
34
25
44
11
121
16
22.008
0.001
Cx. tritaeniorhynchus
5
49
52
28.592
0.000
4
8
41
0
53
0
24.031
0.000
Cx. malayi
3
23
19
8.399
0.015
0
0
31
0
10
4
47.439
0.000
Cx. minutissimus
1
1
4
2.938
0.23
0
0
1
0
5
0
4.087
0.537
Cx. bitaeniorhynchus
0
1
4
4.924
0.085
0
0
5
0
0
0
13.704
0.018
Lt. fuscanus
4
0
0
12.173
0.002
0
0
0
0
4
0
5.084
0.406
Mi. chamberlaini
2
1
5
3.54
0.17
0
0
4
0
4
0
4.017
0.547
Cq. crassipes
0
2
4
3.485
0.175
2
0
4
0
0
0
10.283
0.068
2.2 Sampling time
The samples of immature mosquitoes were collected from October to February (winter), March to June (hot summer), and July to September (monsoon season). There are significant differences in the beginning of various seasons throughout the nation and during our study. The start of different seasons varies significantly across the country. The samples were collected once during winter, twice during summer, and once in the monsoon season.
2.3 Sampling procedure and data collection
Samples were collected from the Punjab between the X coordinates of 2935380.53 m (Fort Monroe, Suleiman Piedmont) and 3390600.51 m (Narowal, Rain fed Areas) and Y coordinates of 504329.4 m (Derawar Fort, Sandy Desert A) and 1076189.5 m (Murree Road, Wet Mountains). The sampling locations varied from Ahmed Pur East (91 m) in Sandy Desert A (Cholistan) to Fort Monroe (1759 m) in Suleiman Piedmont. Suleiman Piedmont has considerably higher heights and steeper slopes. However, although covering a sizable portion of the province, the Northern Irrigated Plains have a height that ranges from 100 m to 238 m above sea level, which implies less slope, a feature of these alluvial plains of the Punjab.
To determine the importance of the interactions between the various species of collected mosquito larvae found in samples, seasons, and agro-ecological zones, the chi-square test of association was used. Regarding the occurrence of larvae of different species, the obtained p-values for samples with Seasons and Agro-Ecological Zones reveal diverse degrees of significance, ranging from non-significant to highly significant.
Elevation above sea level and geographic coordinates of sampling sites were recorded with the help of Magellan GPS (Global Positioning System) (Explorist 660). The distribution maps of the most frequently observed were created in ArcGIS 9.10 using ArcMap (ESRI (Environmental Systems Resource Institute), 2012). The values of longitudes and latitudes were converted into X coordinates (m) and Y coordinates (m), respectively for preparing the map. Table 1 shows the distribution of mosquito species.
The samples were collected from a variety of habitats, including sewage near houses, catch basins (rain or tap), irrigation channels, rice fields, roadside pools, seepage pools, rock pools, irrigated fields, tree holes, wetlands, ground waters, fish farms, parks, tires, sewerage drains, streams, etc., were used to collect immature mosquitoes (Attaullah et al., 2023; Khan et al., 2015; Manzoor et al., 2020; Qasim et al., 2014). Characteristics of different habitats, their associations with collected immature and method of collection in detail are discussed elsewhere (Naeem-Ullah et al., 2010). Collected samples were brought to the laboratory of Department of Entomology, University of Agriculture, Faisalabad, Pakistan for an identification.
The Swift microscope was used to identify the species using the existing taxonomic keys (Cockerell, 1925; Hoare, 1940; Seth-Smith, 1942). This manuscript's nomenclature, hierarchy, and abbreviations have been followed the Systematic Catalogue of Culicidae 2001, the Walter Reed Biosystematics Unit (WRBU), and Herbach (2004).
Since summer season is long enough, mosquito larvae were collected twice a year. The number of samples collected from Wet Mountains as the zone is small. Northern irrigated plains provided more samples, which cover a large region in the Punjab owing to the existence of the Indus River and its four tributaries, as well as a complex canal system developed from them. These irrigated plains contain a wide range of cropping patterns and cropping methods. This zone contained some of Pakistan's most populated urban areas along a wide farmed area. As a result, the necessity to examine these zones more completely and comprehensively was realized.
3 Results
Twenty-four mosquito species from the Anophelinae and Culicinae subfamilies were observed in the current investigation. Anophelinae subfamily contained three species. Eleven species of Culicinae belonged to the tribe Aedini, eight to the Culicini, and one each to the Mansoniini and Ficalbiini. The distribution maps of the most abundantly recorded species are presented in Figs. 1-8.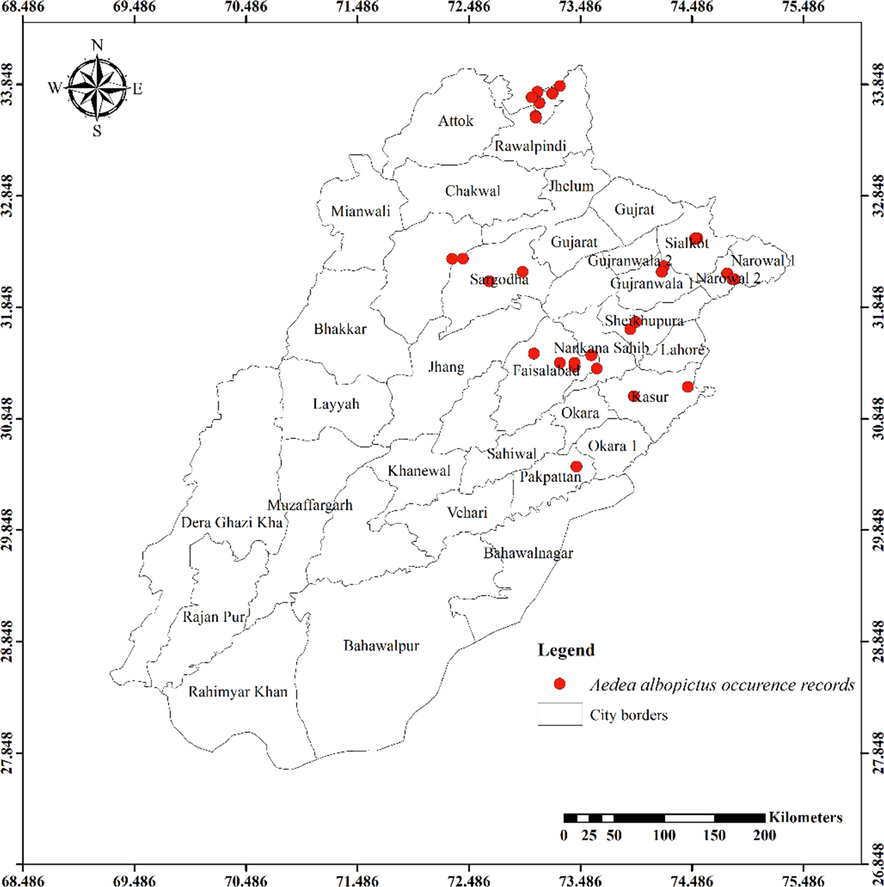
Distribution of Aedes albopictus in different districts of Punjab province.
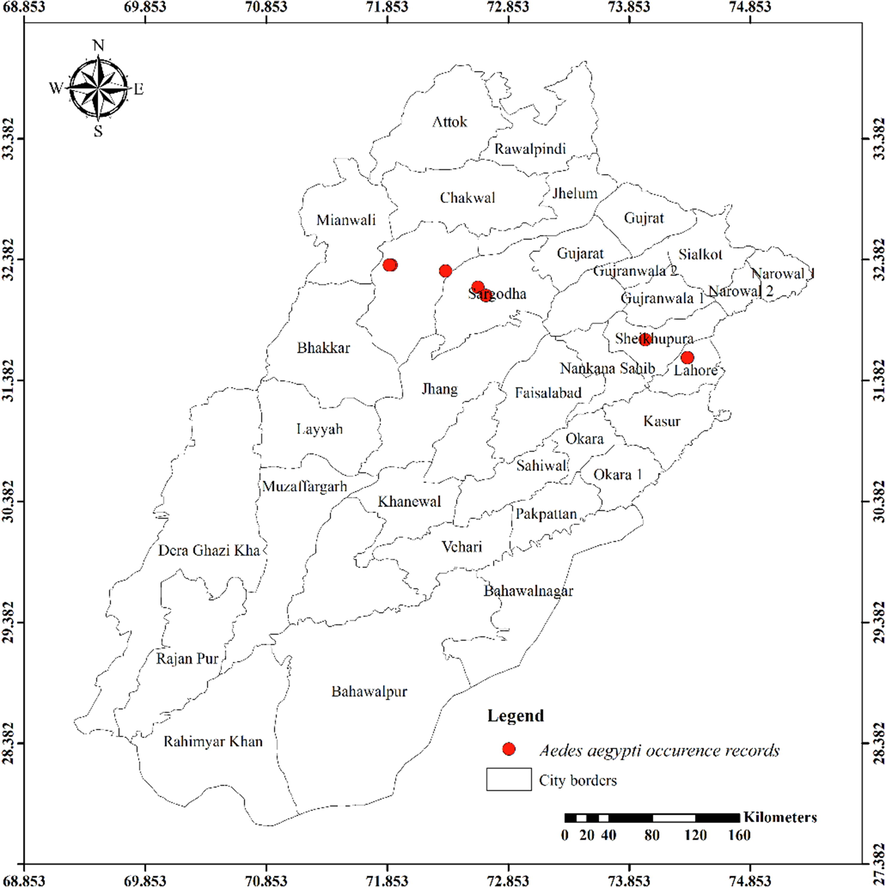
Distribution of Aedes saegypti in different districts of Punjab province.
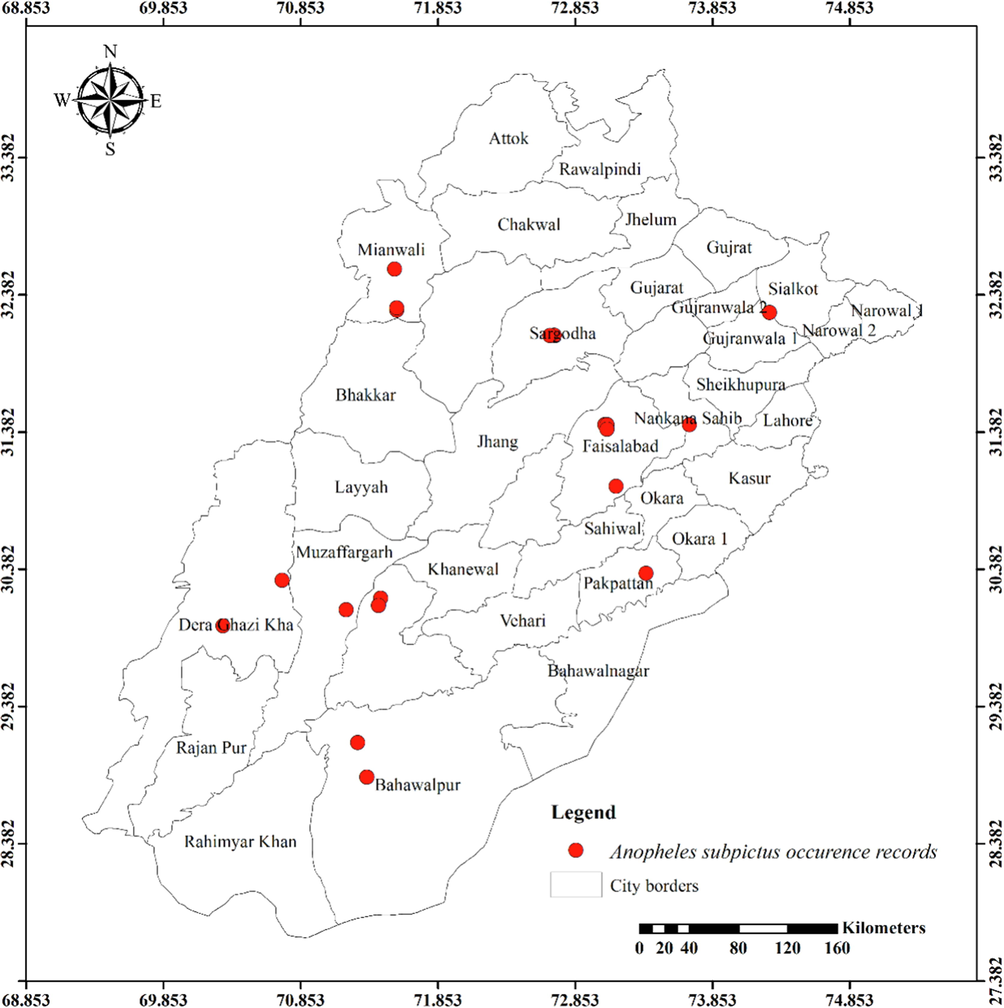
Distribution of Anopheles subpictus in different districts of Punjab province.
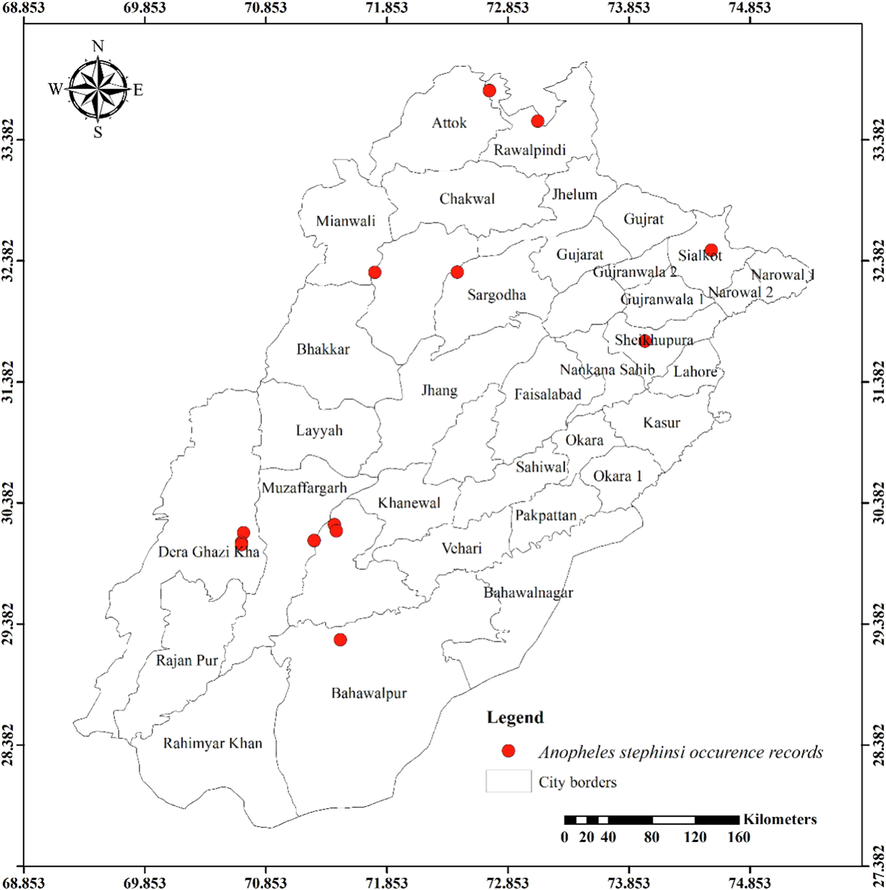
Distribution of Anopheles stephensi in different districts of Punjab province.
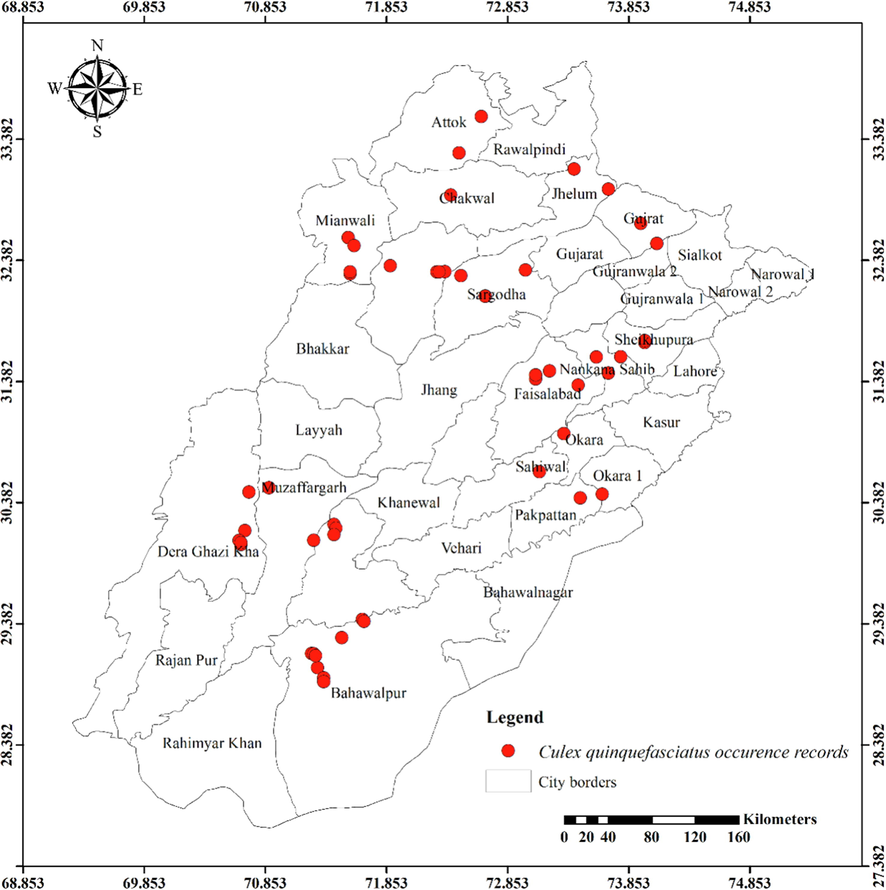
Distribution of Culex quinquefasciatus in different districts of Punjab province.
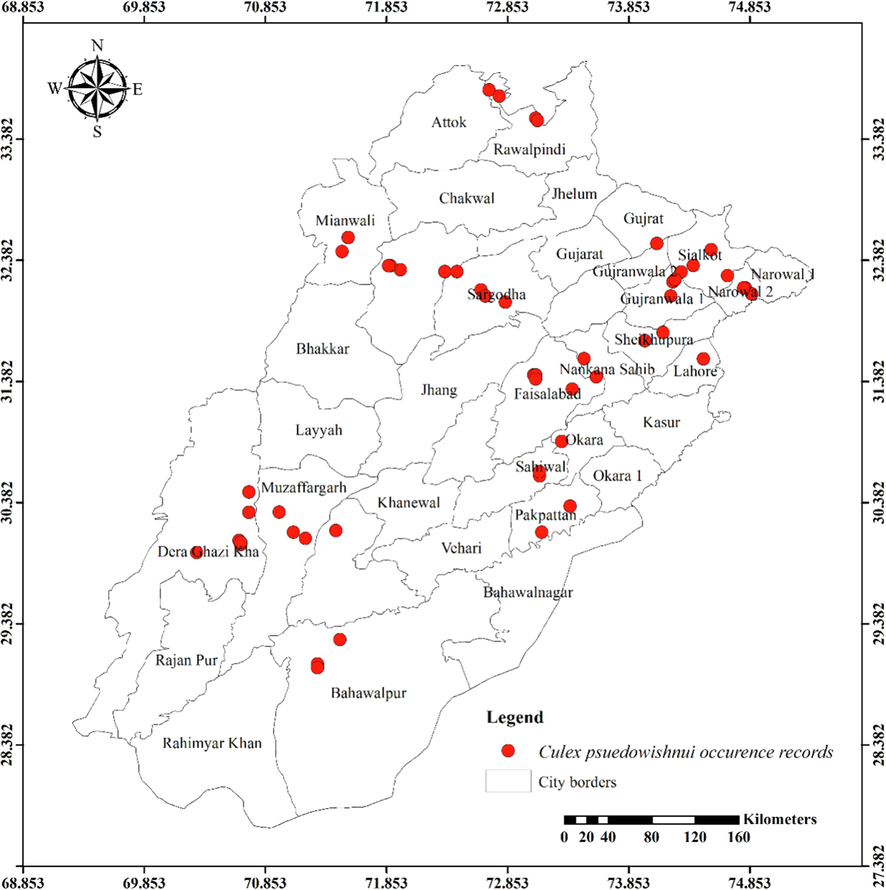
Distribution of Culex pseudovishnui in different districts of Punjab province.
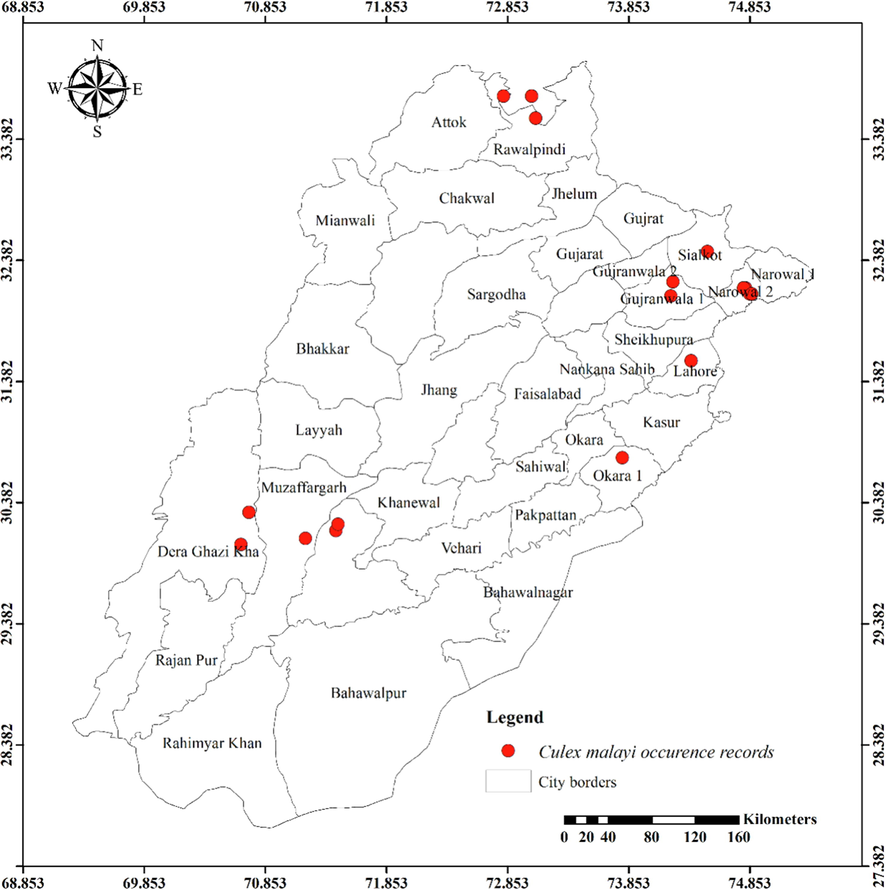
Distribution of Culex malayi in different districts of Punjab province.
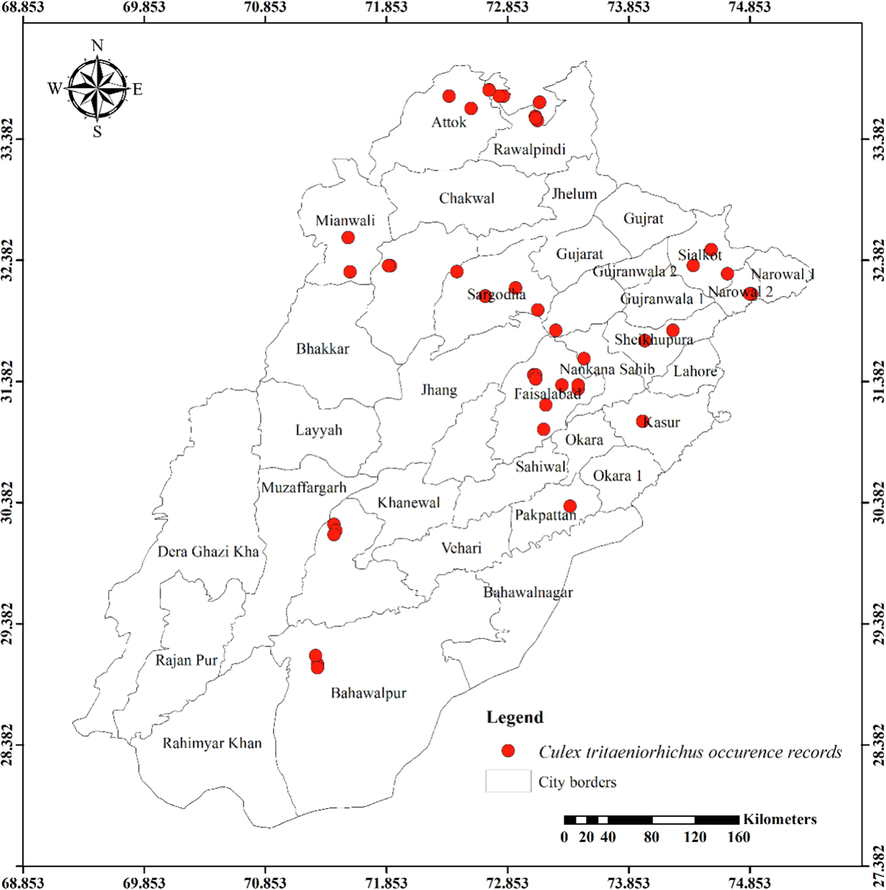
Distribution of Culex tritaeniorhynchus in different districts of Punjab province.
Only An. subpictus, an anopheles mosquito, exhibits a substantial correlation with seasons (0.011) and agro-ecological zones (0.007). Ae. albopictus, Ar. subalbatus, and Ae. vittatus are members of the tribe Aedini and exhibit a significant relationship with both Seasons (p-values of 0.000, 0.017, and 0.000, respectively) and Agro-Ecological Zones (having p-values of 0.000, 0.000 and 0.000, respectively). The seasons are significantly associated with Ae. caspius (0.014), Ae. unilineatus (0.020), and Ae. lineatopennis (0.020). Additionally, Ae. aegypti and Ae. pseudotaeniatus are demonstrating a strong correlation with agro-ecological zones (p-values are 0.000 and 0.008, respectively). Cx. tritaeniorhynchus, Cx. pseudovishnui, Cx. malayi, and Cx. quinquefasciatus are culicine mosquitoes that are demonstrating very significant association with both the seasons (p-values of 0.000, 0.000, 0.015, and 0.002, respectively) and agro-ecological zones (having p-values of 0.000, 0.000, 0.000, and 0.001, respectively). With a p-value of 0.002, Lutzia fuscanus has a strong correlation with the seasons, while Cx. bitaeniorhynchus has a considerable amount of interaction with agro-ecological zones (having 0.018p-value). On the other hand, Mi. chamberlaini and Cq. crassipes representing tribes Ficalbiini and Mansoniini do not exhibit any appreciable correlations with the seasons or agro-ecological zones (Table 1).
Anopheline mosquito larvae, An. nigerrimus, were found in urban areas during the rainy season, but An. stephensi and An. subpictus were found in both rural and urban environments throughout the year. Even though An. subpictus was more frequently observed than An. stephensi, it was not found in wet mountains or rainfed lands. So, compared to the earlier, it occupied a wider variety of agro-ecological zones. Species found only in a few agro-ecological zones and classified under aedine mosquitoes. Ae. lineatopennis, Ae. unilineatus, Ve. indica, Ve. yusafi, and Ae. w-albus were documented only in rural environments of the Northern Irrigated Plains, mostly in Changa Manga National Forest. Despite Ve. indica being recorded in the winter as well, high populations were only observed during the monsoon season. It is the only species of aedine mosquito of those described above that has been discovered in an urban area. Ae. pseudotaneatus is another illustration of an animal that lives in a small space. It was only taken from the Wet Mountains at an elevation of 1222 m above sea level from Pir Sohawa, even though Ae. pseudotaneatus captured during the summer and monsoon seasons.
Except for the northern irrigated plains and Suleiman Piedmont, Ae. vittatus is the only aedine species that has been discovered in all four agro-ecological zones. Rarely sampled, but it was found on rainfed lands in both rural and urban areas, including sandy desert A (Fort Derawar) and Sandy Desert B. The majority of Ae. aegypti specimens were collected from urban habitats, however few were also found in rural Khushab and Quidabad. Urban habitats in Lahore (northern irrigated plains), Gujrat (rainfed lands), and Sheikhupura were sampled throughout the summer and during the rainy season. It wasn't discovered from south of Khushab and Quidabad and was also not found in the Wet Mountains. However, Ae. albopictus was not seen in the south of Faisalabad. It was discovered in rural areas, with city parks and recreational places being the most likely locations. Except for Cx. bitaeniorhynchus, which was exclusively urban dweller and found during the summer and rainy seasons from rainfed lands, whereas other culicine mosquito larvae were discovered during all three seasons of the year. Only winter observations of Lu. Fuscanus was made in Faisalabad (northern irrigated plains). Only two culicine species, Cx. fuscanus and Cx. quinquefasciatus was discovered in the Wet Mountains. All agro-ecological zones recorded Cx.quinquefasciatus population, followed by Cx. pseudovishnui and Cx. tritaeniorhynchus, while the second last was absent from wet mountains and the last from Suleiman Piedmont. They were discovered in a variety of habitats during all three seasons, from both rural and urban areas. In all seasons, Cx. malayi was primarily discovered in urban areas from Suleiman Piedmonts, northern irrigated plains, and rainfed lands, while it was occasionally discovered in rural areas as well. Throughout the year, Mmomyia chamberlaini (Tribe Ficalbiini) was found in both rural (northern irrigated plains) and urban areas (rainfed lands). In both rural and urban settings, Coquillettidia crassipes (Tribe Mansoniini) was found from rural as well as urban areas of Rain Fed Lands in the summer and monsoon seasons.
Table 1 presents the findings for several mosquito species in relation to latitudes (Y coordinates), longitudes (X coordinates), and altitudinal ranges. The range of latitude and longitude for some species’ existence in the Punjab is limited because they were hardly sampled. An. nigerrimus, Ae. pseudotaeniatus, Ae. lineatopennis, and Ae. w-albus, for instance, were all discovered at the same location. An. subpictus and An. stephensi have nearly identical locations, with the former occupying somewhat more northern coordinates and being discovered in some elevated regions (437 m) than the latter (290 m). Ae. unilineatus, Ve. indica, and Ve. yusafi likewise had nearly identical positions and elevations. Whereas Ae. caspius had a moderate range. The largest range of Y coordinates among the aedine species belongs to Ae. vittatus (505868.83–1069193.90) followed by Ae. albopictus (699720.37–1076189.50), Ar. subalbatus (771811.36–1076189.50) and Ae. aegypti (831167.55–904583.31). But as far as X coordinates are concerned, Ae. aegypti (3108922.47––3344090.94) has the widest range followed by Ae. albopictus (3160652.63–3389771.21), Ae. vittatus (3069393.74–3228562.68) and Ar. subalbatus (3184486.76––3313329.16). These four species are listed in descending order according to their range of elevation: Ar. subalbatus, Ae. vittatus, Ae. aegypti, and Ae. albopictus (Table 1). The only mosquito species (Cx. quinquefasciatus) recorded from 1222 m with the broadest range of Y coordinate (520976.32–1069193.90), X coordinate (2936816.47–3390234.75), and altitude was (94 m – 1222 m). Cx. bitaeniorhynchus, in comparison, had the smallest range of coordinates and altitude. Almost similar altitude and coordinates have been shared by Cx. tritaeniorhynchus and Cx. pseudovishnui (Table 1). Cx. malayi has a very extensive range, whereas Lt. fuscanus has a relatively narrow range followed by Cx. fuscocephala, and Cx. minutissimus. Compared to Mi. chamberlaini, Cq. crassipes has a greater range of latitude.
4 Discussion
4.1 Anopheline species
The results for Anopheles nigerrimus are consistent with previous study reporting its presence in agricultural areas, particularly in rice fields and around cities under an open sunlight to moderate shade up to 300 m elevation above sea level (Harrison & Scanlon, 1975). An. stephensi was found in rural areas throughout the summer in Noorpur (sandy desert 2, Thal). An earlier investigation from South Punjab, Pakistan did not discover An. stephensi from such areas (Herrel et al., 2001). The findings addressing the existence of An. subpictus larvae differ as the earlier study sampled waterways and fishponds, but these habitats were not used for the current assessment (Herrel et al., 2001).
4.2 Aedine species
Aedes pseudotaeniatus was documented during the current investigation from an altitude of 1222 m, which is consistent with earlier studies reporting these species from altitudes of 420–3200 m and 340–3230 m, respectively (Rao et al., 1973). The major vector of dengue hemorrhagic fever (DHF) and dengue fever is Ae. aegypti (Ashfaq et al., 2014; Becker et al., 2020; Manzoor et al., 2020). Previously this species has not been reported from Punjab. However, it is widely spread throughout the Indian subcontinent. It was captured by Qutubuddin in the Kohat-Hangu valley in KPK, Pakistan, in 1960. As a result, it was initially reported from the Punjab about specific places covered in our study. (Chareonviriyaphap et al., 2003) reported this species from southern latitudes in Thailand; however, it was observed at northern latitudes in our study. Globally, Ae. albopictus is the most invasive mosquito species and a carrier of several viral diseases, also known as the Asian tiger mosquito (Benedict et al., 2007). In the present study, Ae. aegypti reported from Changa Manga National Forest, Punjab, Pakistan. It was also found in northern Punjab, including Sargodha, Sialkot, Gujrat, Mianwali, Lahore, Gujranwala, Faisalabad, Rawalpindi, and Pakpattan. A somewhat large species of mosquito is Ar. subalbatus, observations regarding its biting habits are in line with earlier studies (Aslamkhan & Salman, 1969; Suleman et al., 1993). During the rainy season, immatures were collected from the water present in old tires located in the center of Sargodha. Even though Ae. caspius was frequently reported from Pakistan, earlier researchers did not record it from the Punjab (Mukhtar et al., 2003; Reisen & Boreham, 1979). It was documented in our study from south to north from cities in Punjab such as Bahawalpur and Rawalpindi.
4.3 Culicine species
The larvae of Culex fuscocephala and Cx. pseudovishnui were recorded in all seasons under present study but larvae of former species were not recorded from winter and of later species from winter and summer under a previous study conducted from Changa Manga National Forest, Punjab (Reisen & Boreham, 1979). This species was classified as a subspecies of Cx. pipiens as fatigans (Reisen et al., 1981). It is now considered a distinct species from pipiens, and quinquefasciatus is now regarded as an acceptable and legitimate name in place of fatigans. In our survey, Cx. tritaeniorhynchus was recovered from a variety of ground pools. Only Faisalabad was the source of Lt. fuscanus in the current survey. The observations made in relation to its relationship are adjacent to those made with Cx. quinquefasciatus by (Reisen et al., 1981).
5 Conclusion
In the present study, a total of twenty-four species of mosquitoes belonging to the Anophelinae and Culicinae subfamilies were recorded. The subfamily Anophelinae had a total of three species. There were a total of eleven species of Culicinae that were classified under the tribe Aedini, while eight species were categorized under the tribe Culicini. Additionally, there was one species each that belonged to the tribes Mansoniini and Ficalbiini. The distribution maps of the species provide valuable insights for their site-specific management.
Acknowledgement
The authors extend their appreciation to the Researchers supporting project number (RSPD2023R691), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mosquito larval habitats and public health implications in Abeokuta, Ogun State, Nigeria. Tanzan. J. Health Res.. 2008;10(2)
- [CrossRef] [Google Scholar]
- Ahmad, A., Khan, M., Shah, S. H. H., Kamran, M., Wajid, S. A., Amin, M., Khan, A., Arshad, M. N., Cheema, M. J. M., & Saqid, Z. A. 2019. Agro-ecological zones of Punjab, Pakistan.
- Bruce-Chwatt’s essential malariology. Rev. Inst. Med. Trop. Sao Paulo. 1995;37(1)
- [CrossRef] [Google Scholar]
- Analyzing mosquito (Diptera: Culicidae) diversity in Pakistan by DNA barcoding. PLoS One. 2014;9(5)
- [CrossRef] [Google Scholar]
- Aslamkhan, M., & Salman, C. 1969. the Bionomics of the Mosquitoes of the Changa Manga National Forest, West Pakistan. J&V, July 1969.
- Diversity, distribution and relative abundance of the mosquito fauna (Diptera: Culicidae) of Malakand and Dir Lower, Pakistan. Braz. J. Biol.. 2023;83
- [CrossRef] [Google Scholar]
- Barik, T. K., Sahu, B., & Swain, V. 2009. A review on Anopheles culicifacies: From bionomics to control with special reference to Indian subcontinent. In Acta Tropica (Vol. 109, Issue 2). https://doi.org/10.1016/j.actatropica.2008.09.017.
- Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector-Borne and Zoonotic Diseases. 2007;7(1)
- [CrossRef] [Google Scholar]
- Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health. 2003;34(3)
- [Google Scholar]
- The Fauna of British India, including Ceylon and Burma. Oligochaeta. Science. 1925
- [CrossRef] [Google Scholar]
- Dahmana, H., & Mediannikov, O. 2020. Mosquito-borne diseases emergence/resurgence and how to effectively control it biologically. In Pathogens (Vol. 9, Issue 4). https://doi.org/10.3390/pathogens9040310.
- DeGroote, J., Mercer, D. R., Fisher, J., & Sugumaran, R. 2007. Spatiotemporal investigation of adult mosquito (diptera: Culicidae) populations in an Eastern Iowa County, USA. Journal of Medical Entomology, 44(6). https://doi.org/10.1603/0022-2585(2007)44[1139:SIOAMD]2.0.CO;2.
- Miasma: Malaria’s Breeding Grounds and Effects on Ancient Rome. Past Imperfect. 2020;22(1):3-32.
- [Google Scholar]
- ESRI (Environmental Systems Resource Institute). 2012. ArcMap 10.1. In ESRI.
- Spatial distribution and temporal dynamics of invasive and native mosquitoes in a large Mediterranean city. Sci. Total Environ.. 2023;896
- [CrossRef] [Google Scholar]
- Glidden, C. K., Nova, N., Kain, M. P., Lagerstrom, K. M., Skinner, E. B., Mandle, L., Sokolow, S. H., Plowright, R. K., Dirzo, R., De Leo, G. A., & Mordecai, E. A. 2021. Human-mediated impacts on biodiversity and the consequences for zoonotic disease spillover. In Current Biology (Vol. 31, Issue 19). https://doi.org/10.1016/j.cub.2021.08.070.
- Medical entomology studies - II. The subgenus Anopheles in Thailand (Diptera: Culicidae) Contribution of the American Entomology Institute. 1975;12(1334)
- [Google Scholar]
- Breeding of Anopheles mosquitoes in irrigated areas of South Punjab, Pakistan. Med. Vet. Entomol.. 2001;15(3)
- [CrossRef] [Google Scholar]
- The Fauna of British India, including Ceylon and Burma. Nature. 1940;146(3699)
- [CrossRef] [Google Scholar]
- The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298(5591)
- [CrossRef] [Google Scholar]
- Jabeen, A., Ansari, J. A., Ikram, A., Khan, M. A., & Safdar, M. 2022. Impact of climate change on the epidemiology of vector-borne diseases in Pakistan. In Global Biosecurity (Vol. 4). https://doi.org/10.31646/gbio.163.
- Development of an automated biomaterial platform to study mosquito feeding behavior. Front. Bioeng. Biotechnol.. 2023;11
- [CrossRef] [Google Scholar]
- Not all mosquitoes are created equal: A synthesis of vector competence experiments reinforces virus associations of Australian mosquitoes. PLoS Negl. Trop. Dis.. 2022;16(10):e0010768.
- [Google Scholar]
- A survey of adult and larval mosquito fauna in Tehsil Daggar and Gagra of District Buner, Khyber Pakhtunkhwa, Pakistan. International Journal of Mosquito Research IJMR. 2015;170(23)
- [Google Scholar]
- Determination of species composition of mosquitoes in Lahore, Pakistan. J. Arthropod. Borne Dis.. 2020;14(1)
- [Google Scholar]
- History of mosquitoborne diseases in the United States and implications for new pathogens. Emerg. Infect. Dis.. 2018;24(5)
- [CrossRef] [Google Scholar]
- Impact of Cropping Pattern and Climatic Parameters in Lower Chenab Canal System—Case Study from Punjab Pakistan. Agriculture (Switzerland). 2022;12(5)
- [CrossRef] [Google Scholar]
- Role of wastewater irrigation in mosquito breeding in south Punjab, Pakistan. Southeast Asian J. Trop. Med. Public Health. 2003;34(1)
- [Google Scholar]
- Grouping of different mosquito species on the bases of larval habitats. Pak. J. Agric. Sci.. 2010;47(2)
- [Google Scholar]
- Mosquito (Diptera: Culicidae) of murree hills, Punjab. Pakistan. Pakistan Journal of Zoology. 2014;46(2)
- [Google Scholar]
- A survey of haematophagous arthropods in Western Himalayas, Sikkim and hill districts of West Bengal. A general account. Indian J. Med. Res.. 1973;61(10)
- [Google Scholar]
- Host selection patterns of some Pakistan mosquitoes. Am. J. Trop. Med. Hyg.. 1979;28(2)
- [CrossRef] [Google Scholar]
- Larval interspecific associations and physico-chemical relationships of the ground-water breeding mosquitoes of Lahore [Includes Ficalbia chamberlaini clavipalpus] Pak. J. Sci. Res. 1981
- [Google Scholar]
- 6 The Pontine Marshes. In: Malaria and Rome. Oxford University PressOxford; 2002. p. :168-191.
- [CrossRef] [Google Scholar]
- Sangbakembi-Ngounou, C., Costantini, C., Longo-Pendy, N. M., Ngoagouni, C., Akone-Ella, O., Rahola, N., Cornelie, S., Kengne, P., Nakouné, E. R., Komas, N. P., & Ayala, D. 2022. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control.” Proceedings of the National Academy of Sciences of the United States of America, 119(21). https://doi.org/10.1073/pnas.2104282119.
- The Fauna of British India: including Ceylon and Burma. Nature. 1942;149(3770)
- [CrossRef] [Google Scholar]
- Ecology of mosquitoes in Peshawar Valley and adjoining areas: Species composition and relative abundance. Pak. J. Zool.. 1993;25(4)
- [Google Scholar]







