Translate this page into:
A multidirectional phytochemical profiling, antimicrobial, antioxidant and toxicity studies of Neurada procumbens L.: A desert medicinal plant
⁎Corresponding authors. mirza.imran@iub.edu.pk; mussarat.ramzan@iub.edu.pk mirza.imran@iub.edu.pk (Mirza Imran Shahzad), mussarat.ramzan@iub.edu.pk (Mussarat Ramzan),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Neurada procumbens L. (Neuradaceae) is a Cholistani desert plant traditionally employed in fever, inflammations, diabetes and hepatitis. The purpose of this study was to determine the chemical composition, anti-bacterial, antibiofilm and antioxidant activities of various extracts of this important medicinal plant; separately for its aerial and floral parts.
Methods
The phytochemical screening (by HPLC analysis), antioxidant (by DPPH assay), antibacterial (by disc diffusion test and MIC) and anti-biofilm potential against seven bacterial strains (E. coli, K. pneumoniae, S. aureus, S. aureus MDR, P. aeruginosa, P. aeruginosa MDR and P. vulgaris) were determined by aqueous, methanol, n-butanol, ethyl acetate, n-hexane and dichloromethane extracts of floral and aerial parts of N. procumbens.
Results
The floral MetOH and DCM extracts were found to contain higher polyphenolic contents including 2,3-di methoxy benzoic acid (38.21 µg/mg), chlorogenic acid (26.59 µg/mg) and catechin (14.24 µg/mg) and exhibited a comparatively higher antioxidant (IC50 < 100 μg/ml) potential in DPPH assay. Moreover, these floral MetOH and DCM extracts were found to be most active (>13.5 mm zone of inhibition and MICs 62.5–125 µg/ml) against P. aeruginosa MDR and S. aureus in anti-bacterial activity. In an anti-biofilm assay, MetOH and DCM floral extracts showed a promising potential (>85 % biofilm inhibition) with IC50 < 150 μg/ml against P. aeruginosa MDR and S. aureus MDR better than moxifloxacin and was further confirmed by light and scanning electron microscopy. Moreover, the floral-MetOH extract of N. procumbens has been found to be safe up to 2 g/kg BW with its lethal dose (LD50) as 3872.98 mg/kg BW in rats.
Conclusion
Hence, due to presence of essential medicinal compounds with low toxicity effects, the plant is recommended to be safely employed in various pharmaceutical preparations.
Keywords
Anti-oxidant
Anti-bacterial
Anti-biofilm
HPLC-PDA
Neurada procumbens L.
Phytochemicals
1 Introduction
Plant based antimicrobial agents are crucial in reducing the global impact of infectious diseases. A rise in antimicrobial resistance demands alternative approaches to address this modern health challenge (Ashraf et al., 2020; Zuo et al., 2018). Antibiotic resistance is mainly linked with improper or over-use of antimicrobials and the formation of complex extracellular matrix called biofilm. The development of biofilm ability involves various mechanisms like quorum sensing, antibiotic resistant cell walls, enzyme production and gene expression (Gomes et al., 2019; Tesfahuneygn and Gebreegziabher 2019). Pathogens like Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa are causative agents of severe infections in humans and livestock (Brambilla et al., 2017; Kim et al., 2020; Maheshwari et al., 2019). The biofilm development by multi drug resistance strains on the surfaces of medical devices have become a major source of infection that is difficult to remove by standard antimicrobial agents (Wijesundara and Rupasinghe 2019).
Medicinal plants have always been recognized as a potent therapeutic source and have pharmacological potential due to existence of secondary metabolites (Bhandari et al., 2017). Among these metabolites, polyphenolics are considered the most active natural compounds since they exhibit numerous biological properties like anti-allergic, antioxidant, anti-microbial, anti-inflammatory, anti-carcinogenic, cardio- and gastro-protective activities (Achakzai et al., 2021, Ahmed et al., 2021, Khan and Khan 2021, Sheikh 2021). The anti-oxidant potential of these compounds is linked with the redox possession that leads them to scavenge the free radicals. Hence, the consumption of anti-oxidant rich plants has potential health benefits. Moreover, it is also well-known that polyphenolic compounds demonstrate antimicrobial potential against acute and chronic infections suggesting their effectiveness in wound healing (Farias et al., 2021).
Neurada procumbens L. (Chipri booti) is a desert plant, distributed in North Africa, Sudan, Saudi Arabia, Ethiopia, Indian Desert and Rohi region of the Cholistan desert (Khurshid et al., 2019; Zareen et al., 2018). This is an edible plant utilized by Cholistani Bedouin, its dried spiny fruit along with rose water is used as a cooling agent (Thaadal) in the desert and also used as a nerve tonic by males (Qureshi et al., 2010). Previously, phytochemical conformation, anti-oxidant and enzyme inhibition assays of the whole plant were investigated and it was reported that n-butanol and methanol extracts of the plant have a huge concentration of phenolic and flavonoid contents that tend to associate with its significant anti-oxidant and enzyme inhibition abilities (Khurshid et al., 2019). Since, Cholistani local healers use only the floral part of the plant in Thaadal and panjeeri, hence it was quite interesting to explore the potential health benefits separately for both floral and aerial parts. Moreover, no data are available until now, regarding its antibacterial and anti-biofilm potentials separately from both these parts. As part of endless efforts to search the alternative sources of natural products with potent biological applications, this study was focused to evaluate the composition, free radical scavenging ability, anti-bacterial potential as well as toxicity studies separately for N. procumbens’s aerial and floral part.
2 Material and Methods
2.1 Plant collection and extraction
N. procumbens plant (floral and aerial parts) were collected from the Cholistan desert near Bahawalpur, then identified by a taxonomist of The Islamia University of Bahawalpur (voucher: Np-707). After washing and shade-drying, they were separately ground into fine powder. Each 50 mg portion was soaked in n-butanol, ethyl acetate, water, methanol, n-hexane and dichloromethane (500 ml each) separately for floral and aerial parts, followed by 72-hour soaking with occasional shaking. The mixtures were filtered, concentrated via rotary evaporation and yields were calculated (Altemimi et al., 2017).
2.2 Phytochemical assessment by HPLC quantification
HPLC-PDA analysis quantified 22 essential polyphenolic compounds (syringic acid, naringenin, 3-hydroxy-4-methoxybenzaldehyde, carvacrol, chlorogenic acid, t-ferulic acid, catechin, gallic acid, benzoic acid, naringin, 2,3-dimethoxybenzoic acid, harpagoside, o-coumaric acid, p-coumaric acid, epicatechin, rutin, sinapinic acid, t-cinnamic acid, vanillic acid, quercetin, 3-hydroxybenzoic acid and 4-hydroxybenzoic acid) in MetOH and DCM of N. procumbens floral and aerial parts by using standard procedure (Locatelli et al., 2017). Briefly, the plant extract was analyzed via HPLC using a Waters system with a 600 solvent pump and 2996 photodiode array detector. A C18 reversed-phase column was employed at 30 ± 1 °C for compound separation. UV/V wavelength ranged from 200 to 500 nm with quantitative analysis at respective maximum wavelengths. The injection volume was 20 μl; the mobile phase, degassed by DEGAS Biotech, was water-acetonitrile (93:7 v:v) with 3% acetic acid. Compounds were quantified using calibration curves.
2.3 Determination of DPPH radical scavenging activity (RSA)
The antioxidant capacity of a plant extract was assessed by its ability to neutralize 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals. In a 96-well microplate, varying concentrations of the extract were mixed with DPPH and incubated in the dark at 37 °C for 30 min. This interaction led to the change of DPPH's purple color to yellow, indicating the antioxidative effect of plant extract. The optical density was measured at 517 nm and radical scavenging potential was determined using a specific equation (Ahmed et al., 2021).
2.4 Determination of anti-bacterial potential
The plant extracts were screened for their anti-bacterial potential against seven microbial strains i.e. gram positive (Staphylococcus aureus, Staphylococcus aureus MDR) and gram negative (Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Pseudomonas aeruginosa MDR and Proteus vulgaris). The cultures were prepared using 200 µl of glycerol stock in 125 ml of sterile nutrient broth medium and kept at 37 °C under continuous shaking till OD600 reached 0.4 (mid-log phase). The anti-bacterial potential of each plant extract was assessed by disc diffusion protocol (Jahan et al., 2018) and minimum inhibitory concentration (MIC) was determined (Bazzaza et al., 2011). Briefly, a 48-h fold inoculum having 0.4 OD of the respective bacterial strain was spread on solidified soy agar media and incubated at 37 °C for 40 min. Pre-soaked GF-1 grade filter paper discs of control or respective plant extract (200 μg of extract/disc) were placed at the agar plates and again incubated for overnight at 37 °C. The zone of inhibition (ZoI) was recorded in millimeter (mm) and MIC was determined for the most active extracts.
2.5 Determination of anti-biofilm activity
An anti-biofilm potential of plant extracts was accomplished according to the standard protocol (Schlafer and Meyer 2017). Briefly, equal amount of the extract and bacterial culture were dispensed into the microtiter plate, incubated for 24 h at 37 °C, cells were then washed with PBS and immobilized with 99 % methanol. Then, staining was done with crystal violet dye, re-solubilized in 33 % glacial acetic acid, OD630 was documented in Uv/Vis spectrophotometer and biofilm inhibition (%) was calculated. The biofilm was formed on a microscopic slide using the standard procedure. Initially, it was observed under an Olympus light microscope and subsequently, the surface structure of the biofilm was examined using scanning electron microscopy (JSM-IT-100) (Gomes and Mergulhão 2017).
2.6 In vivo acute toxicity assay
Albino rats (n = 5 / group), weighing 170–250 g and both sexes were housed at zone-2 of animal house facility at the Department of Pharmacy, The Islamia University of Bahawalpur. The animals were maintained under standard conditions with 12-hour light/dark cycle and at temperature of 22 ± 2 °C and humidity of 35–60 %. The rats had unrestricted access to water and standard diet. All procedures were ethically approved by the Pharmacy of Animal Ethics Committee at The Islamia University of Bahawalpur (Approval: PAEC/20/18, Dated: 15–09-2020).
For acute toxicity assessment of floral-MetOH extract, animals were divided into six groups. Prior to the trial, they were starved overnight but had access to water. Each group received oral doses of 0.5, 1, 2, 3, 4 and 5 g/kg body weight of the extract by following OECD guidelines. The control group received only drinking water. The parameters like behavior, allergic reactions and mortality were monitored during 24 h to determine the lethal dose (LD50) of the extract (Aslam et al., 2023).
2.7 Statistical analysis
Data were presented as mean ± SEM (standard deviation of the mean) of three independent measurements. For comparison between different interventions, one-way analysis of variance (ANOVA) was performed using SPSS 20. IC50 was determined using GraphPad Prism (Aslam et al., 2021).
3 Results and discussion
3.1 Phytochemical assessment by HPLC-PDA quantification
The various extracts of the same plant having diverse level of potentials are due to existence and richness of numerous phytochemical compounds (Alam et al., 2020). In our recent study, phytochemical analysis of N. procumbens aerial and floral parts confirmed the presence of diverse metabolites in Aqu, MetOH, EtAc, n-But and DCM extracts (Aslam et al., 2023). The extracts showed total phenolic content (TPC) ranging from 28.19 to 127.13 mg GAE/g and total flavonoid content (TFC) ranging from 33.2 to 78.23 mg RE/g. The floral MetOH and DCM extracts exhibited the highest TPC (78.15 ± 0.89 mg GAE/g) and TFC (68.31 ± 0.78 mg RE/g) among all extracts (Aslam et al., 2023). The maximum phenolic contents were also observed in MetOH extracts by others (Gomes et al., 2019).
The MetOH and DCM extracts were subjected to HPLC-PDA polyphenolic quantification (Table 1). In the current study, gallic acid (2.77 ± 0.14 μg/mg), catechin (2.34 ± 0.12 μg/mg), naringin (BLQ; below limit of quantification), 2,3 di-MeO benzoic acid (5.24 ± 0.022 μg/mg), quercetin dihydrate (1.58 ± 0.08 μg/mg), harpagoside (BLQ) were observed in MetOH aerial extract. While, MetOH floral extract revealed the presence of gallic acid (2.77 ± 0.14 μg/mg), catechin (14.24 ± 0.70 μg/mg), chlorogenic acid (BLQ), syringic acid (0.3 ± 0.02 μg/mg), rutin (6.78 ± 0.34 μg/mg), naringin (0.51 ± 0.021 μg/mg), 2,3-di-methoxy benzoic acid (38.21 ± 1.91 μg/mg), quercetin dihydrate (0.69 ± 0.03 μg/mg) and harpagoside (BLQ). The syrengic acid (BLQ), t-ferrulic acid (BLQ), 2,3 di-Meo benzoic acid (0.28 ± 0.021 μg/mg) and quercetin dihydrate (BLQ) were observed in DCM aerial extract. The p-hydroxy benzoic acid (BLQ), p-coumaric acid (BLQ), vanillic acid (0.22 ± 0.01 μg/mg), quercetin dihydrate (0.42 ± 0.03 μg/mg) and harpagoside (BLQ) were observed in DCM floral extract. Catechin is a flavonoid compound extracted and identified from Combretum albiflorum extract which has shown significant reduction in biofilm formation of Pseudomonas aeruginosa through interference of quorum-sensing signals in biofilm matrix (Vandeputte et al., 2010). While catechin, coumaric acid, epicatechin, gallic acids, ferulic acid and benzoic acid were also found to be potent anti-microbial agents (Bazargani and Rohloff 2016, Brambilla et al., 2017). According to HPLC quantification, the significant amount of polyphenols were quantified in floral extracts as compared to aerial extracts. Among the floral extracts, maximum number of polyphenols have been quantified in MetOH floral extract, with highest amount of catechin and 2,3-dimethoxybenzoic acid. The vanillic acid and quercetin dihydrate were majorly quantified in DCM floral extract. In case of aerial extracts, 2,3-di methoxybenzoic acid was maximally quantified in MetOH extract. In DCM aerial extract, only 4 polyphenols were identified. RT = retention time, BLQ = below limit of quantification.
N. procumbens
Extract
RT (min)
Compounds
IdentifiedConcentration (µg/mL)
Compound class
Aerial
MetOH
3.940
Gallic acid
2.77 ± 0.14
Phenolic acid
12.622
Catechin
2.34 ± 0.12
Flavonoid
30.010
Naringin
BLQ
Flavonoid
31.254
2,3 di MeOBenzoic acid
5.24 ± 0.02
Phenolic acid
35.257
Quercetin dihydrate
1.58 ± 0.08
Flavonoid
Harpagoside
BLQ
Flavonoid
DCM
17.850
Syrengic acid
BLQ
Phenolic acid
24.851
t-ferrulic acid
BLQ
Phenolic acid
29.104
2,3 di MeoBenzoic acid
0.28 ± 0.02
Phenolic acid
37.718
Quercetin dihydrate
BLQ
Flavonoid
Floral
MetOH
3.990
Gallic acid
3.95 ± 0.20
Phenolic acid
12.352
Catechin
14.24 ± 0.70
Flavonoid
12.772
Chlorogenic acid
BLQ
Phenolic acid
18.140
Syringic acid
0.3 ± 0.02
Phenolic acid
23.682
Rutin
6.78 ± 0.34
Flavonoid
29.797
Naringin
0.51 ± 0.02
Flavonoid
31.113
2,3 di MeOBenzoic acid
38.21 ± 1.91
Phenolic acid
35.42
Quercetin dihydrate
0.69 ± 0.03
Flavonoid
41.226
Harpagoside
BLQ
Flavonoid
DCM
12.719
p-hydroxy benzoic acid
BLQ
Phenolic acid
15.197
p-coumaric acid
BLQ
Phenolic acid
21.150
Vanillic acid
0.22 ± 0.01
Phenolic acid
37.921
Quercetin dihydrate
0.42 ± 0.03
Flavonoid
42.964
Harpagoside
BLQ
Flavonoid
3.2 Determination of DPPH radical scavenging potential
In case of antioxidant potential of N. procumbens extracts, maximum potential was shown by floral extracts in-contrast to aerial extract. Among floral extracts, the significant RSA was observed by MetOH (IC50 = 86 μg/ml) and DCM (IC50 = 97 μg/ml) extracts followed by aqueous extract (IC50 = 149 μg/ml). The present results revealed the presence of abundant concentrations of polyphenolic metabolites in DCM and MetOH floral extracts (Table 2). Among the aerial extracts, highest anti-oxidant potential was found in DCM, MetOH and n-But extracts with IC50 ranging between 110 μg/ml to 137 μg/ml (Table 2). Hence, it could be that the presence of terpenoids and flavonoids like xanthonoids (xanthone), flavones and luteolin in n-But, MetOH and DCM extracts gives anti-oxidant potential to the plant by hydrogen donation and chelation of metal ion abilities. These outcomes are in accordance with a former study wherein the highest phenolic contents in MetOH extract of Launaea procumbens gave significant antioxidant potential (Khan et al., 2012). Therefore, we suggest that TPC has a great contribution towards anti-oxidant potential of the plant in its active extracts. Hence, these results indicate that N. procumbens has a great potential to treat oxidative stress related chronic disorders. Floral extracts showed better scavenging potential compared to aerial extracts. While among the floral extracts, maximum scavenging potential was observed by DCM extract with least IC50, followed by n-But and MetOH extracts. Aqu = aqueous, MetOH = methanol, n-But = n-butanol, EtAc = ethyl acetate, n-Hex = n-hexane, DCM = dichloromethane.
Sr. No.
N. procumbens
(Aerial part)
IC50
(μg/ml)
Sr. No.
N. procumbens
(Floral part)
IC50
(μg/ml)
1
Aqu
331.30 ± 1.21
1
Aqu
149.30 ± 1.61
2
MetOH
131.20 ± 2.34
2
MetOH
86.10 ± 2.01
3
n-But
137.20 ± 2.76
3
n-But
199.10 ± 0.91
4
EtAc
237.10 ± 2.29
4
EtAc
223.20 ± 0.63
5
n-Hex
–
5
n-Hex
643.40 ± 2.19
6
DCM
110.00 ± 2.89
6
DCM
97.10 ± 0.21
7
Ascorbic acid
17.32 ± 0.187
7
Ascorbic acid
17.32 ± 0.19
3.3 Determination of anti-bacterial activity
The anti-bacterial potential from aerial (Fig. 1) and floral (Fig. 2) extracts of N. procumbens was determined by disc diffusion test (Table 3) and MICs assays (Table 4). The maximum phenolic compounds like catechin, gallic acid, rutin and naringin have been quantified by HPLC analysis in floral MetOH and DCM extracts that give antibacterial potential to these extracts. All floral extracts have shown moderate to high anti-bacterial potential with a ZoI ranging from 6.5 to 14 mm. The MetOH extract was found the best among all floral extracts against all selected bacterial strains, especially against P. aeruginosa MDR and S. aureus with a ZoI of 14 mm and MIC 62.5 µg/ml. The DCM extract exhibited maximum anti-bacterial potential with MIC 125–250 µg/ml against S. aureus MDR, E. coli, K. pneumoniae, P. aeruginosa, P. aeruginosa MDR and P. vulgaris. The Aqu extract was found to be effective against K. pneumoniae with ZoI of 10 mm and MIC 250 µg/ml. These findings are also supported by previous work, wherein the methanol extract of Gynura procumbens was reported to have strong antibacterial potential (Ashraf et al., 2020). Almost all our floral extracts were found to be least effective against E. coli strain with least ZoI and highest MIC values. Since, it has also been previously found that E. coli is resistant to anti-bacterial activity of medicinal plants (Tesfahuneygn and Gebreegziabher 2019). The overall anti-bacterial trend of floral extracts was P. aeruginosa MDR > S. aureus > S. aureus MDR > K. pneumoniae > P. vulgaris > P. aeruginosa > E. coli. Among aerial extracts, maximum anti-bacterial potential was exhibited against K. pneumoniae, S. aureus and S. aureus MDR. The MetOH aerial extract exhibited promising potential against K. pneumoniae, S. aureus and S. aureus MDR with ZoI > 9.0 mm. The DCM aerial extracts also showed promising anti-bacterial potential against S. aureus MDR with ZoI of 15.5 mm and MIC of 62.5 µg/ml, while EtAc and n-But aerial extracts showed significant anti-bacterial potential against K. pneumoniae with ZoI of 13 mm and MIC = 125 µg/ml. Similarly, significant anti-bacterial potential of MetOH and EtAc extracts of Scutellaria litwinowii herb was found against S. aureus (Bazzaza et al., 2011). The order of anti-bacterial potential of aerial extracts was S. aureus MDR > K. pneumoniae > S. aureus > P. vulgaris > E. coli > P. aeruginosa MDR > P. aeruginosa. Similar to current observation, Morus alba root bark also showed significant anti-bacterial potential against clinical methicillin-resistant S. aureus (MRSA) isolates compared to aerial extracts (Zuo et al., 2018). The highest anti-bacterial potential of MetOH and DCM floral extracts is due to presence of high quantity of polyphenolic substituents (data obtained by HPLC), since there observed a significant anti-bacterial potential of piper nigrum, piper betle and gnetum due to presence of high TPC/TFC and poly phenolic compounds in these plants. Similarly, many plant-based active metabolites of traditional medicinal plants have also been reported to confer strong anti-microbial and antioxidant activities (Stanković et al., 2016). The results are analyzed using one way ANOVA and are represented as Mean ± SEM of triplicate readings in each group. *=p < 0.05, **=p < 0.01, ***=p < 0.001. Positive control = Ampicllin was used for drug sensitive and Moxifloxacin for MDR strains. Positive control = Ampicllin was used for drug sensitive and Moxifloxacin for MDR strains.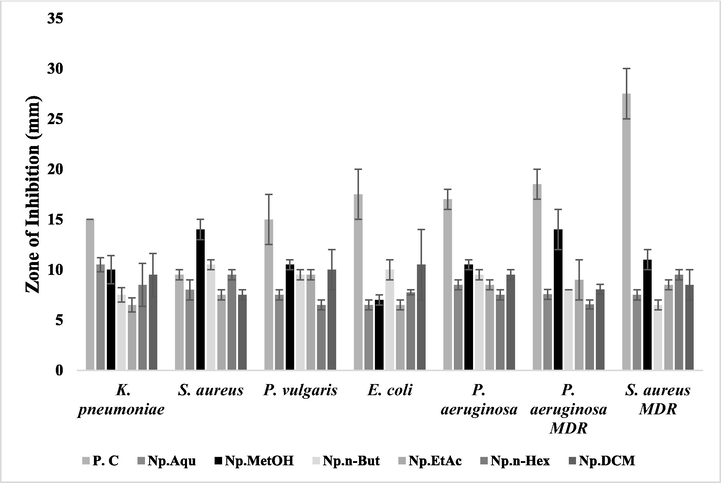
Antibacterial potential of N. procumbens (aerial part) extracts evaluated by disc diffusion method. The results were compared with the standard drug (moxifloxacin). The signifcant zone of inhibition by these extracts was observed even larger than the standad drug in some cases. P.C: Positive control (ampicillin was used for drug sensitive and moxifloxacin for drug resistant) was used as a standard. Np.Aqu = N. procumbens aqueous extract, Np.MetOH = N. procumbens methanol extract, Np.n-But = N. procumbens n-butanol extract, Np.EtAc = N. procumbens ethyl acetate extract, Np.n-Hex = N. procumbens n-hexane extract, Np.DCM = N. procumbens dichloromethane extract.
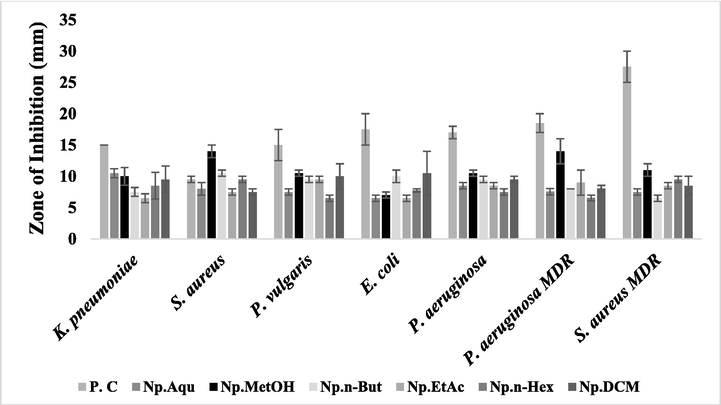
Antibacterial potential of N. procumbens (floral part) extracts evaluated by disc diffusion method. The results were compared with the standard drugs (moxifloxacin). The signifcant zone of inhibition was observed by these extracts, even larger than the standard drug in some cases. P.C: Positive control (ampicillin was used for drug sensitive and moxifloxacin for drug resistant) was used as a standard. Np.Aqu = N. procumbens aqueous extract, Np.MetOH = N. procumbens methanol extract, Np.n-But = N. procumbens n-butanol extract, Np.EtAc = N. procumbens ethyl acetate extract, Np.n-Hex = N. procumbens n-hexane extract, Np.DCM = N. procumbens dichloromethane extract.
N. procumbens
Extracts
K. pneumoniae
S. aureus
P. vulgaris
E. coli
P.aeruginosa
P.aeruginosa MDR
S. aureus MDR
Aerial
Aqu
7.5 ± 0.71
6.5 ± 0.50
8.0 ± 1.00*
9.0 ± 0.50
0.00
6.5 ± 0.50
7.5 ± 1.50
MetOH
10.0 ± 2.83**
11.0 ± 3.0***
7.5 ± 0.50
0.00
6.5 ± 0.71
7.5 ± 0.50
9.5 ± 0.50**
n-But
13.0 ± 1.41***
7.5 ± 0.50
8.0 ± 0.00*
7.5 ± 0.50
0.00
7.0 ± 0.00
9.0 ± 1.00*
EtAc
13.0 ± 1.41***
8.6 ± 0.40*
7.5 ± 0.50
6.5 ± 0.50
0.00
8.5 ± 1.50*
9.0 ± 1.00*
n-Hex
10.5 ± 0.71**
13.5 ± 0.50***
6.5 ± 0.50
0.00
6.5 ± 0.50
7.0 ± 0.00
11.0 ± 3.00**
DCM
10.0 ± 0.00***
7.5 ± 0.50
0.00
0.00
6.5 ± 0.50
7.15 ± 0.50
15.5 ± 0.50***
Floral
Aqu
10.5 ± 0.71**
8.0 ± 1.00**
7.5 ± 0.50
6.5 ± 0.50
8.5 ± 0.50
7.55 ± 0.55
7.5 ± 0.50
MetOH
10.0 ± 1.41**
14.0 ± 1.00***
10.5 ± 0.50***
7.0 ± 0.50
10.5 ± 0.50*
14.0 ± 2.00***
11.0 ± 1.00**
n-But
7.5 ± 0.71
10.5 ± 0.50***
9.5 ± 0.50**
10.0 ± 1.0**
9.5 ± 0.50*
8.0 ± 0.00
6.5 ± 0.50
EtAc
6.5 ± 0.71
7.5 ± 0.50
9.5 ± 0.50**
6.5 ± 0.50
8.5 ± 0.50
9.0 ± 2.00*
8.5 ± 0.50*
n-Hex
8.5 ± 2.13
9.5 ± 0.50***
6.5 ± 0.50
7.75 ± 0.20
7.5 ± 0.50
6.55 ± 0.45
9.5 ± 0.50*
DCM
9.5 ± 2.13*
7.5 ± 0.50
10.0 ± 2.00**
10.5 ± 3.50**
9.5 ± 0.50**
8.05 ± 0.50*
8.5 ± 1.50**
Control
15.0 ± 0.01
9.5 ± 0.50
15 ± 0.50
17.5 ± 2.50
17.0 ± 1.00
18.5 ± 1.50
17.5 ± 2.50
N. procumbens
MIC (µg/mL)
Extracts
K. pneumoniae
S. aureus
P. vulgaris
E. coli
P. aeruginosa
P. aeruginosa MDR
S. aureus MDR
Aerial
Aqu
1000
1000
500
500
–
1000
1000
MetOH
125
250
1000
–
500
1000
125
n-But
125
1000
1000
500
–
1000
1000
EtAc
125
250
500
1000
–
250
125
n-Hex
500
250
1000
–
1000
1000
250
DCM
250
250
–
–
1000
1000
62.5
Floral
Aqu
250
1000
1000
1000
500
1000
1000
MetOH
250
62.5
250
500
250
62.5
250
n-But
1000
500
500
250
500
1000
1000
EtAc
1000
1000
500
1000
1000
125
500
n-Hex
1000
500
1000
500
1000
1000
500
DCM
250
1000
250
125
125
125
250
Control
250
500
125
500
500
250
125
3.4 Determination of biofilm inhibition activity
According to anti-biofilm results, the floral extracts have shown better reduction in biofilm formation than those of aerial part (Table 5). Among floral extracts, MetOH extract exhibited maximum bio-film inhibition > 80 % with IC50 < 140 µg/ml against P. vulgaris, S. aureus, E. coli, K. pneumoniae, P. aeruginosa MDR and S. aureus MDR. The DCM floral extract showed promising biofilm inhibitory activity with IC50 < 140 µg/ml against P. vulgaris, E. coli, P. aeruginosa, K. pneumoniae and P. aeruginosa MDR. Additionally, DCM and MetOH floral extracts exhibited the anti-biofilm activity even better than Moxifloxacin (standard drug) against P. aeruginosa MDR and S. aureus MDR, respectively as like previous studies wherein DCM extract of leaves of Piper regnellii plant exhibited potent anti-microbial and anti-biofilm activities against different MDR strains (Brambilla et al., 2017). Moreover, MetOH extract of Eucalyptus globulus was observed significantly active against S. aureus biofilm, since the abundance of tannins in MetOH extract was found as potent biofilm inhibitors (Gomes et al., 2019). The order of our floral extracts for anti-biofilm assay was MetOH > DCM > Aqu > n-But > n-Hex > EtAc. The sequence of anti-biofilm activity among the bacteria was E. coli > P. aeruginosa MDR > S. aureus MDR > S. aureus > P. vulgaris > P. aeruginosa > K. pneumoniae. The ethanolic extract of Gynura procumbens leaves was found effective in hydrolyzing P. aeruginosa biofilm by disruption of quorum sensing signals (Nain et al., 2022). In case of our aerial extracts, promising anti-biofilm potential was found by MetOH extract (>83 %) with IC50 < 150 µg/ml against P. aeruginosa, S. aureus, P. aeruginosa MDR and S. aureus MDR. Likewise the MetOH extract of H. tiliaceus bark was found to possess potent anti-biofilm activity against S. aureus and S. epidermis due to alkaloids and tannins present in the bark (Daneshfar et al., 2008). The EtAc, n-Hex and DCM aerial extracts have shown bio-film inhibition > 80 % (IC50 < 150 µg/ml) against S. aureus MDR. Likewise, EtAc extract of Orostachys japonicas plant was documented as an effective in the inhibition of S. aureus MDR bio-film (Kim et al., 2020). Aqu extract of aerial N. procumbens significantly reduced E. coli biofilm formation with IC50 > 140 µg/ml, as like previous observation of significant anti-biofilm potential of Aqu plant extract of Clematis viticella against P. aeruginosa (Alam et al., 2020). The trend of anti-biofilm assay by aerial extracts was MetOH > DCM > EtAc > Aqu > n-But > n-Hex. In terms of bacterial strains, the order of biofilm inhibition was S. aureus MDR > E. coli > S. aureus > P. aeruginosa MDR > K. pneumoniae > P. aeruginosa > P. vulgaris. According to previous studies, metabolites like steroids alkaloids, phenolics, tannins, flavonoids and terpenoids are reported as potent anti-biofilm and anti-bacterial agents (Vandeputte et al., 2010, Cock et al., 2018). The catechin and other polyphenol fractions, previously documented as strong bio-film inhibitors (Zacchino et al., 2017) were observed in our MetOH and DCM extracts and showed maximum inhibitory effects in the formation of biofilm (Table 3). MetOH and DCM extracts of both floral and aerial parts exhibited significant bio-film inhibition better than standard drug (Moxifloxacin). The values are represented as Mean ± SEM of triplicate observations in each group. The results are analyzed using one-way ANOVA and IC50 was calculated. *=p < 0.05, **=p < 0.01, ***=p < 0.001. Positive control = ampicillin was used for drug-sensitive and moxifloxacin for MDR strains.
N. procumbens
IC50(µg/ml)
Extracts
K. pneumoniae
S. aureus
P. vulgaris
E. coli
P. aeruginosa
P. aeruginosa MDR
S. aureus MDR
Aerial
Aqu
422.31 ± 1.21
672.40 ± 1.49
627.30 ± 1.21
133.79 ± 1.12*
–
885.63 ± 1.19
458.79 ± 1.21
MetOH
205.60 ± 1.37
141.68 ± 1.21**
236.10 ± 2.16
–
149.91 ± 1.79**
138.92 ± 1.15*
157.22 ± 1.18***
n-But
141.50 ± 1.19*
152.38 ± 1.12**
211.16 ± 2.66
264.56 ± 0.66
–
–
–
EtAc
162.90 ± 1.61*
151.52 ± 1.44**
172.80 ± 1.21*
702.43 ± 0.92
–
128.51 ± 1.24***
143.14 ± 1.43**
n-Hex
–
148.20 ± 1.62
468.25 ± 1.91
–
284.27 ± 1.27
–
141.68 ± 1.21**
DCM
157.50 ± 1.36**
461.76 ± 1.21
–
–
687.30 ± 0.33
367.56 ± 1.12
138.33 ± 1.14***
Floral
Aqu
421.18 ± 1.89
240.14 ± 0.96
678.23 ± 1.49
611.32 ± 0.14
235.80 ± 2.12
476.50 ± 1.32
138.79 ± 1.09**
MetOH
138.97 ± 1.79**
139.51 ± 1.14
128.2 ± 1.14***
137.60 ± 1.42**
574.31 ± 1.93
136.83 ± 1.16**
127.32 ± 0.18***
n-But
355.09 ± 1.45
140.26 ± 1.21
362.23 ± 1.71
133.09 ± 1.48*
268.77 ± 0.89
239.88 ± 1.18
198.34 ± 0.082**
EtAc
–
463.11 ± 1.54
239.24 ± 1.98
832.53 ± 0.69
796.84 ± 0.41
195.39 ± 0.92**
–
n-Hex
404.90 ± 1.71
147.26 ± 1.18
474.20 ± 0.97
623.92 ± 0.51
491.91 ± 0.61
432.60 ± 0.87
941.68 ± 1.51
DCM
138.10 ± 2.21**
458.50 ± 1.16
127.20 ± 2.24**
129.78 ± 1.13**
137.15 ± 1.81*
128.01 ± 1.83**
238.33 ± 1.14
Control
93.34 ± 1.87
31.70 ± 0.98
117.6 ± 1.65
34.2 ± 1.26
58.7 ± 2.05
131.40 ± 1.79
130.71 ± 1.29
3.5 Light and scanning electron microscopy of bacterial biofilm
The untreated control biofilm of both S. aureus and S. aureus MDR displayed a dense and well-developed network of bacterial cells within the biofilm under light microscope (40X magnification). However, on treatment with standard drugs or MetOH floral extract, the cells appeared scattered and disrupted (Fig. 3). Particularly, on treatment with MetOH extract, S. aureus MDR exhibited more significant disruption of biofilm compared to standard drug, moxifloxacin. These results indicate that the extracts not only reduced the overall mass of biofilm, as assessed by 96-well crystal violet assay but also induced the changes in cell density. Furthermore, the bioactive extracts from Carum copticum demonstrated the ability to reduce the biofilm development in MDR β-lactamase producing enteric bacteria (Maheshwari et al., 2019).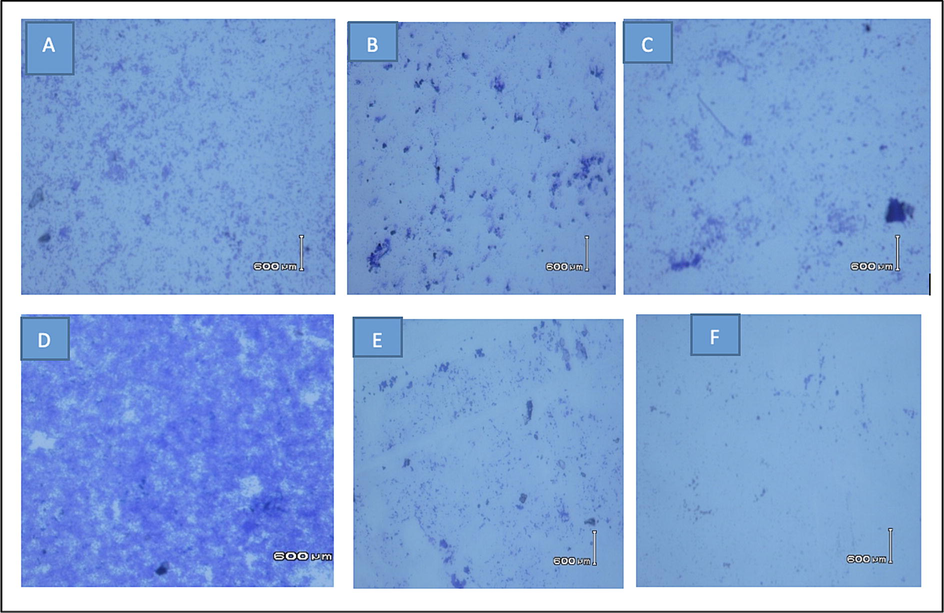
The Light microscopy images (40X) of S. aureus biofilm stained with crystal violet dye. (A) untreated biofilm, (B) S. aureus treated biofilm with ampicillin, (C) S. aureus treated biofilm with MetOH floral extract, (D) S. aureus MDR untreated biofilm, (E) S. aureus MDR treated biofilm with moxifloxacin and (F) S. aureus MDR treated biofilm with MetOH floral extract. Untreated control biofilms of both S. aureus and S. aureus MDR strains have a well-developed dense network of bacterial cells compared to the cells treated with standard drugs or MetOH floral extract seems to be scattered and disrupted.
These findings were also confirmed through scanning electron microscopy (SEM). Untreated control bio-film was appeared as amorphous matrix with well-developed shape while the treated cells appeared as non-amorphous extra-cellular polymeric substance (EPS) matrix and showed crystalline structural changes (Fig. 4). Previous studies also demonstrated structural morphological alterations in the biofilms of both Gram-negative and Gram-positive bacteria following treatment with phenolic acids derived from plant extracts (Campos et al. 2009). Additionally, the SEM analysis confirmed the bactericidal and anti-biofilm efficacy of medicinal plant extracts against Streptococcus pyogenes biofilms (Wijesundara and Rupasinghe 2019).
Scanning electron micrographs of S. aureus: (A) untreated biofilm, (B) S. aureus treated biofilm with ampicillin, (C) S. aureus treated biofilm with MetOH extract, (D) S. aureus MDR untreated biofilm, (E) S. aureus MDR treated biofilm with moxifloxacin and (F) S. aureus MDR treated biofilm with MetOH extract. Untreated control biofilm showed well-developed amorphous matrix while the treated cells appeared as non-amorphous polymeric substance with crystalline structural changes.
3.6 In vivo toxicity assay
The toxicity effects of floral-MetOH extract were examined in rats for determination of extract safety at different selected doses (Table 6). No toxicity symptoms were noted in rats after oral administration of plant at the dose of 0.5–2 g/kg BW. However, rats taking the dose of 3 g/kg BW showed the toxicity signs like salivation, jerks and writhes with 25 % mortality. Furthermore, at the dose of 4 g/kg and 5 g/kg BW, increased toxicity was found with 75 % and 100 % mortality within few hours. Thus, the lethal dose (LD50) was calculated to be 3872.98 mg/kg BW in rats and hence, the safe dose of extract was found up-to 2 g/kg BW (Table 6). Similarly, Tridax procumbens also showed LD50 > 2000 mg/kg BW in rats (Abubakar et al., 2012). According to OECD guidelines, MetOH floral extract of N. procumbens belongs to class 5 (LD50 > 2000–5000 mg/kg BW) that is designated as low toxicity class. In another study, similar LD50 of 3942 mg/kg BW was observed by MetOH leaf extract of Abrus precatorius and LD50 > 2000–5000 mg/kg BW was reported by plant extracts of Ageratum conyzoides (Agaie et al., 2000). Hence, it might be the reason that only the floral part of N. procumbens is typically used by Cholistani nomads to treat fever and diabetes, as they might well aware of safe dose and toxicity effects of this plant due to their personal life long experience. Median lethal dose (LD50) was found to be 3872.98 mg/kg BW in rats. The MetOH floral extract of the plant was found to be safe up to 2 g/kg body weight (BW) dose. LD50 = Lethal dose that cause 50% mortality in animal trial.
Extract dose
(mg/kg BW)Toxicity signs
Mortality
(%)LD50
(mg/kg BW)
500
None
0
3872.98
1000
None
0
2000
None
0
3000
Jerks, fits, writhing
25
4000
Coma, convulsion, salivation
75
5000
Convulsion and expired
100
4 Conclusions
The current study provides a scientific rationale regarding medicinal importance of N. procumbens through its in-depth phytochemical, anti-oxidant, anti-bacterial, anti-biofilm activities and in-vivo toxicity assay of its aerial and floral extracts. MetOH and DCM extracts of floral part contain high concentrations of 2,3-di methoxy benzoic acid (38.21 µg/mg), chlorogenic acid (26.59 µg/mg) and catechin (14.24 µg/mg) exhibit comparatively high anti-oxidant (IC50 < 100 μg/ml), anti-bacterial (MIC 62.5–125 µg/ml) and anti-biofilm (IC50 < 150 μg/ml) activities against P. aeruginosa and S. aureus MDR and better anti-biofilm potential than Moxifloxacin especially against MDR strains (confirmed through light and scanning electron microscopes). The floral-MetOH extract dose was found safe up-to 2 g/kg BW in toxicity studies in rats. Hence, it has been concluded that the floral part of this medicinal plant could be safely employed in various pharmaceutical preparations to get the benefits of its important medicinal compounds with least toxicity.
Funding
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R677), King Saud University, Riyadh, Saudi Arabia, for financial support.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R677), King Saud University, Riyadh, Saudi Arabia, for financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Acute and sub chronic toxicity of Tridax procumbens in experimental animals. J. Environ. Sci. Toxicol. Food Technol.. 2012;1:19-27.
- [Google Scholar]
- Functional and Phytochemical potential of Berberis. Pak-Euro J. Medical. Life Sciences. 2021;4(Special Issue):S25-S34.
- [Google Scholar]
- Toxicological effects of the water extract of Ageratum conyzoides in rats. Sokoto J. Vet. Sci.. 2000;2:27-31.
- [Google Scholar]
- Free radical-scavenging capacity and HPLC-DAD screening of phenolic compounds from pulp and seed of Syzygium claviflorum fruit. J. Agri. Food Res.. 2021;6:100203
- [Google Scholar]
- Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. Journal of Infection and Public Health. 2020;13(11):1734-1741.
- [Google Scholar]
- Phytochemicals: Extraction, isolation and identification of bioactive compounds from plant extracts. Plants. 2017;6(4):42.
- [Google Scholar]
- In vitro antioxidant, antimicrobial and antiproliferative studies of four different extracts of Orthosiphon stamineus, Gynura procumbens and Ficus deltoidea. Saudi J. Biol. Sci.. 2020;27(1):417-432.
- [Google Scholar]
- In vitro and in vivo anti-inflammatory potential of Octhochloa compressa extracts in carrageenan induced rats. Pak-Euro J. Med. Life Sci.. 2021;4(4):206-214.
- [Google Scholar]
- Assessing the in vitro and in vivo antioxidant and anti-inflammatory effects of aerial and floral extracts of Neurada procumbens. J. King Saud Univ. Sci 2023:102822.
- [Google Scholar]
- Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156-164.
- [Google Scholar]
- Antioxidant and antimicrobial activity of methanol, dichloromethane and ethyl acetate extracts of Scutellaria litwinowii. Science Asia. 2011;37:327-334.
- [Google Scholar]
- Study of phytochemical, anti-microbial, anti-oxidant and anti-cancer properties of Allium wallichii. BMC Complementary and Alternative Medicine. 2017;17(1):102.
- [Google Scholar]
- Anti-biofilm activity against Staphylococcus aureus MRSA and MSSA of neolignans and extract of Piper regnellii. Revista Brasileira de Farmacog.. 2017;27(1):112-117.
- [Google Scholar]
- Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Inter. J. Food Microbiol.. 2009;135(2):144-151.
- [Google Scholar]
- A review of the traditional use of southern African medicinal plants for the treatment of selected parasite infections affecting humans. Journal of Ethnopharmacology. 2018;220:250-264.
- [Google Scholar]
- Solubility of gallic acid in methanol, ethanol, water and ethyl acetate. Journal of Chemical & Engineering Data. 2008;53(3):776-778.
- [Google Scholar]
- Effect of in vitro digestion on the bioaccessibility and bioactivity of phenolic compounds in fractions of Eugenia pyriformis fruit. Food Res. Internat.. 2021;150A:110767
- [Google Scholar]
- Anti-biofilm activity of hydromethanolic plant extracts against Staphylococcus aureus isolates from bovine mastitis. Heliyon. 2019;5(5):e01728.
- [Google Scholar]
- SEM Analysis of Surface Impact on Biofilm Antibiotic Treatment. Scanning. 2017;11:2960194.
- [Google Scholar]
- In vitro antibacterial and antibiofilm activity of selected medicinal plants and spices extracts against multidrug resistant Pseudomonas aeruginosa. J. Pharmacognosy Phytochem.. 2018;7(3):2114-2121.
- [Google Scholar]
- Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Central J.. 2012;6(1):43.
- [Google Scholar]
- Staphylococcus aureus: A common threat to fish and its products. Pak-Euro J. Med. Life Sciences. 2021;4(Special Issue):S35-S40.
- [Google Scholar]
- Phytochemical composition and in vitro pharmacological investigations of Neurada procumbens L. (Neuradaceae): A multidirectional approach for industrial products. Industrial Crops Products. 2019;142:111861
- [Google Scholar]
- Orostachys japonicus ethyl acetate fraction suppresses MRSA biofilm formation. Asian Pacific J. Trop. Med.. 2020;13(1):38-45.
- [Google Scholar]
- Optimization of Aqueous Extraction and Biological Activity of Harpagophytum procumbens Root on Ex Vivo Rat Colon Inflammatory Model. Phytotherapy Research. 2017;31(6):937-944.
- [Google Scholar]
- Bioactive extracts of Carum copticum and thymol inhibit biofilm development by multidrug-resistant extended spectrum β-lactamase producing enteric bacteria. Biofouling. 2019;35(9):1026-1039.
- [Google Scholar]
- Inhibition of biofilm formation, quorum sensing and other virulence factors in Pseudomonas aeruginosa by polyphenols of Gynura procumbens leaves. Journal of Biomolecular Structure & Dynamics. 2022;40(12):5357-5371.
- [Google Scholar]
- Ethnomedicinal uses of herbs from northern part of Nara desert. Pakistan. Pak. J. Bot.. 2010;42(2):839-851.
- [Google Scholar]
- Confocal microscopy imaging of the biofilm matrix. Journal of Microbiological Methods. 2017;138:50-59.
- [Google Scholar]
- Study of Storage Conditions on the Nutraceutical Products And its Implementation at Retail and Whole Sale Stores of Quetta. Pak-Euro J. Med. Life Sci.. 2021;4(3):94-98.
- [Google Scholar]
- Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS-Wageningen J. Life Sci.. 2016;78:21-28.
- [Google Scholar]
- Medicinal plants used in traditional medicine by ethiopians: A review article. J. Respir. Med. Lung Dis.. 2019;4(1):1-3.
- [Google Scholar]
- Identification of catechin as one of the flavonoids from Combretum albiflorum bark extract that reduces the production of quorum-sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Applied and Environmental Microbiology. 2010;76(1):243-253.
- [Google Scholar]
- Bactericidal and anti-biofilm activity of ethanol extracts derived from selected medicinal plants against Streptococcus pyogenes. Molecules. 2019;24(6):1165.
- [Google Scholar]
- Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine. 2017;37:27-48.
- [Google Scholar]
- In-Vitro Propagation of Neurada procumbensl L (Chipri Booti): An Endangered Medicinal Plant from Cholistan Desert. Pak. J. Agri. Res.. 2018;31(1):1-6.
- [Google Scholar]
- Synergism of prenylflavonoids from Morus alba root bark against clinical MRSA isolates. Phytomedicine. 2018;39:93-99.
- [Google Scholar]







