Translate this page into:
Taifi rose extract improves the growth and physiology of cowpea seedling stage under drought stress
⁎Corresponding author at: Department of Physics, Faculty of Applied Science, Umm Al-Qura University, Makkah, Saudi Arabia. nmalabdallah@iau.edu.sa (Nadiyah M. Alabdallah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Drought stress is the most significant environmental stress factor affecting the growth of crops and farming sector in the present era. This study was carried out mainly in the rose water extracted from the essential oil of Taif rose (Rosa damascena “Trigintipetala”), which was explored for drought stress tolerance from industry. The aim of this study was to investigate how Taif rose water (RW) pretreatment affected cowpea (Vigna unguiculata var. California blackeye NO.46) seedlings under drought stress (65% and 35% of filed capacity). The cowpea seeds are immersed in rose water with 15%, 25% and 35% for a couple of hours before being implanted. Drought stress dramatically reduces morphological traits of root and shoot length, fresh and dry biomass and leaf area. However, pretreatment of RW seed dramatically improves cowpea growth conditions, relative water content, photosynthetic rate, and transpiration rate. However, under drought stress, RW pretreated seedlings showed a decreased levels in malondialdehyde (MDA), proline, total soluble proteins and carbohydrates. However, drought stress increases the quality of antioxidant enzyme activities like superoxide dismutase and catalase in pretreated RW cowpea seedlings. This study concludes that pretreating with RW could increase the drought tolerance in cowpea seedlings by stretching their antioxidant defense mechanisms. In addition, this study holds the potential to play a vital role in ensuring food security in the near future, as it provides valuable insights into improving crop resilience and productivity under drought conditions.
Keywords
Antioxidant
Malondialdehyde
Photosynthetic rate
Relative water content
Total soluble carbohydrate
1 Introduction
Cowpea (Vigna unguiculata L. Walp) is a very famous legume crop which is formed initially in Africa and furtherly extended to global world for using as one of the diets by humans. It takes up a limited area for cultivation and has a much lower yield than cereals. The prediction for cowpea production assumed a global coverage of around 14 million hectares by 2025. It belongs to Fabaceae family and it is well-known for warm season, as a vascular annual pulse crop (Carvoalho et al 2019; Alexandre et al 2016). Cowpeas are essential in the nourishment of the impoverished since they are a reasonably cheap source of substantial protein (Carvoalho et al 2019). Thus, it is a critical issue for research in Saudi Arabia due to its financial and dietary value.
Environmental variables known as abiotic stressors may have negative effects on agricultural yields (Jahan et al., 2021a). Agricultural plants alter their morphology, cellular structure, physiological function, biochemical and molecular level responses in response to abiotic stressors (Jahan et al., 2021b). Among abiotic stressors, drought is a significant constraint on vegetable crops, impairing its efficiency and threatening its yield. Oxidative stress, characterized by the excessive production of activated atmospheric oxygen (O2), also known as reactive oxygen species (ROS), can be brought on by both climate change and the resulting drought stress (Choudhury et al., 2017; Alabdallah et al., 2021; Hasan et al., 2021a; Hasan et al., 2021b). Possible side effects of ROS generation include oxidative stress and lipid peroxidation, both of which can cause cell death (Alabdallah and Hasan, 2021; Jumrani and Bhatia, 2019). Antioxidants play an important part in keeping ROS production and removal in cells at a steady equilibrium (Hasan et al., 2021c; Hasan et al., 2023). In plants, the removal of excess ROS via a variety of antioxidant defense after systems plays a crucial role in the evolution of drought tolerance, and it also aids in maintaining the cell's full metabolic function during drought (Rahman et al., 2022). Increase in proline and sugar levels during the adaptation process is possible because these compounds contribute significantly in the osmoregulation, the redox balance, and the scavenging of ROS under stressful conditions (Krasensky and Jonak, 2012). It has been demonstrated that drought stress causes changes in the morpho-physiological features of plants as well as the composition of their chlorophyll content (Jaleel et al., 2009). As a result, it appears that drought stress is implicated in the loss in photosynthetic efficiency in crops (Ashraf and Harris, 2013).
Several studies have demonstrated that extracts from leaves and flowers can help plants recover from stressful situations by resetting their physiological systems to normal (Basra et al., 2011; Khan et al., 2020). In light of rose's usefulness as a natural chemical and effective growth stimulant, it has been the subject of numerous studies recently (Nadeak et al., 2021; Tatke et al., 2015). The use of RW has the potential to improve plant tolerance to drought stress by lowering oxidative stress. The impacts of phytohormones and other plant extracts on plants have been extensively researched; However, pretreatment RW's impacts on plants under drought stress have not been adequately explored. The objective of this study was to examine the impact of RW on antioxidant enzymes and biochemical responses in cowpea plants exposed to various levels of drought stress. By understanding how RW affects antioxidant enzymes and biochemical responses in cowpea plants subjected to varying degrees of drought stress, we could develop an innovative agricultural practices and sustainable farming methods to boost cowpea yields and mitigate the impacts of drought stress on food production, thereby securing adequate food supplies for an ever-growing global population. Therefore, in our research, we investigated the following: cowpea's physiological reactions to drought, including its relative water content (RWC), proline content, total soluble carbohydrate, and antioxidant enzymes, and its morphological responses to RW treatment.
2 Materials and procedures
2.1 Extract by the traditional technology and phytochemical analysis of waste rose water
Roses from the Al Qurashi Rose Industry in Al Hada, Taif, Saudi Arabia (Rosa damascina var. trigintipetala) were gathered. The traditional technology as depicted in Fig. 1, includes a copper distillation system with a volume of 70–100 L. Cooling process is achieved by a single inclined pipe moving inside a separated water bath and then reaching outside connected with 20–25 L glass bottles. The copper system consumes 8–10 Kg of rose flowers (24000 – 30,000 flowers), which are first pressed by hands and then rose water added to them. The system is tightly closed by sticky tape. The mix is heated by a gas source (propane and butane) for 12–16 h at the boiling stage. The first period of heating is about 40 min for 100 °C after that the temperature gradually decreases to become 50–70 °C. Limited rose oil factories in the region utilize firewood as a source for heating the distillation system. The arising water vapor passes through the pipe and then condenses forming the distillate. Finally, the resultant products gather in the glass bottle, in which the oil floats at the top. Samples from rose water residue in the copper container have been collected and cooled in a refrigerator for the study. Phytochemical analysis of obtained samples of Taif rose water waste presented in Table 1.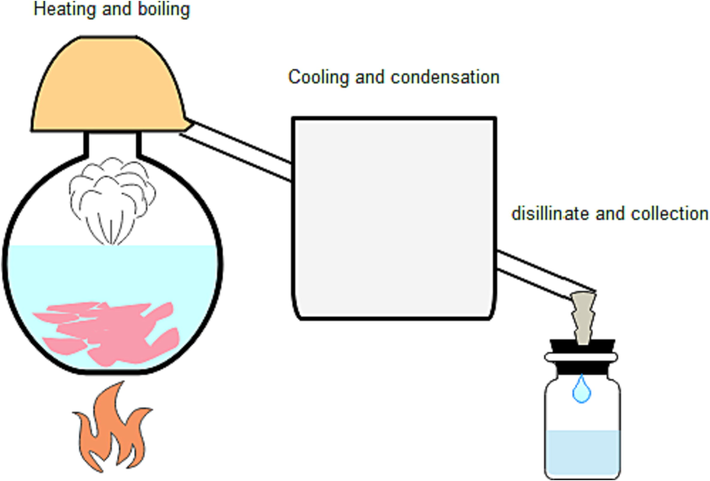
Schematic view of Taif rose traditional distillation technology.
Chemicals
Concentration
Phenol
0.58 ppm
Styrene
0.36 ppm
Toluene
0. 12 ppm
Mesitylene
0.15 ppm
Volatile Organic Compounds
0.23 ppm
2.2 Plants and experimental treatments
Research was conducted in a greenhouse on the campus of Imam Abdulrahman Bin Faisal University in Saudi Arabia. Seeds of cowpea (Vigna unguiculata var. California blackeye NO.46) were obtained in Altuajri, Saudi Arabia. Seeds were immersed in 0%, 15%, 25%, and 35% RW solutions for 2 h under optimum temperature (22 °C) conditions for the rose water (RW) treatments before planting. For the purpose of germination and subsequent growth, cowpea seeds were planted in equal amounts of mixture of soil in plastic containers (2 kg). Seedlings were watered twice weekly until saturation before receiving drought remedies. To simulate drought conditions, cowpea seedlings were exposed to several watering schedules (control at 85% FC, 65% FC, and 35% FC). Constant weighing of the pot ensured that the field capacity (FC) remained constant. RW was applied two times to both drought-stressed and control plants before conducting morphological and physiological measurements. The plants had been grown for a period of 35 days before they were exposed to drought conditions. Following 35 days, morphological and physiological measurements (such as photosynthetic and transpiration rate) were taken, and after harvesting period (45 days), biochemical parameters were analyzed. While the daytime and overnight temperatures were always around 22°/16° C, the relative humidity (RH) ranged from 60% to 70%. After being washed in double-distilled water to remove any remaining traces of sand, the cowpea seedlings' branches and roots were separated. The analytical balance (HR-200) was used to calculate the fresh and dry weights of the shoots and roots, and the results are shown in grams (gm). A portable leaf area meter was used to calculate the total leaf area of cowpea seedlings (LI-3000C).
2.3 Measurements of relative water content
Randomly chosen leaves were employed to obtain three measurements from each treatment. Fresh weight (FW) was determined by weighing leaf discs that were taken from cowpea seedlings. After 4 h, the turgid weight (TW) of the leaf discs was measured. Afterwards, the discs of leaves were dried in an oven at 75 °C (DW). The RWC % was determined using the following formula.
2.4 Determination of gas exchange parameters
Gas exchange measurements (photosynthesis (Pn) and transpiration (E) were taken solely from mature, healthy cowpea leaves. The leaf was measured under ambient conditions between 12:00 and 14:00. A CIRAS 3 portable photosynthesis system (PP Systems, Amesbury, Massachusetts, USA) was used to measure photosynthesis (Pn) and transpiration (E). The relative humidity in the leaf chamber was 62.8%, and the light intensity was 1200 mol m−2 s−1. 400 ppm CO2 was used as the condition in the CIRAS-3 leaf chamber.
2.5 Determination of proline
In this study Proline determination was studied completely using spectrophotometer as described by the documented study (Bates et al 1973).
2.6 Determination of total soluble proteins (TSP)
The concentration of TSP was completely followed by the old method of Bradford (1976).
2.7 Total soluble carbohydrates (TSC) determination
TSC concentration was determined by crushing 500 mg of leaf sample with 5 mL of 95% ethanol. Three milliliters of an anthrone mixture (72% sulphuric acid (100 ml) and 150 mg of anthrone) were added to 100 ml of the alcoholic extract. After that, the samples spent 15 min in a hot water bath. The TSC was determined by measuring the absorbance of the samples at 625 nm against a glucose standard curve (Wardlaw and Willenbrink,1994).
2.8 MDA concentration determination
Determination of MDA concentration was estimated in this study based on Health and Packers et al. (1968).
2.9 Estimation of antioxidant enzyme activity
In this study, this technique was completely following the previous published study (Mukherjee and Chaudhary et al., 1983).
2.9.1 Analysis of SOD activity
The complete protocol for SOD activity was followed from Hasanuzzaman et al. studies (2011) using a spectrophotometer.
2.9.2 Catalase (CAT) activity measurement
CAT activity was evaluated using the Aebi method (1984). Forty milliliters of the enzyme extract were mixed with three milliliters of hydrogen peroxide (30%) and ten milliliters of a phosphate buffer solution (10 mM) (pH 7). When all else failed, a spectrophotometer was used to measure the absorbance at 240 nm (LKB-Biochrom 4050).
2.10 Statistical analysis
One-way Anova analysis was studies using Minitab statistical software (Version 17.0). All the obtained results were presented in categorical variables. In this study, LSD test confirms bars with dissimilar letters, which differ significantly associated (p < 0.05).
3 Result
We investigated the favorable effects of pretreatment of RW on cowpea seedlings across a range of morphological and physiological parameters. According to the current study, increasing RW dose not only benefited plants subjected to drought stress, but also increased the growth and physiology of control plants. Drought stress significantly reduced shoot and root length, shoot fresh and dry weight, root fresh and dry weight, and leaf area in cowpea plants (Table 2). These growth metrics, however, were significantly enhanced in cowpea plants that had been pretreated with RW after experiencing drought stress (Table 2). Significant reductions in RWC of 4% and 4% were observed in cowpea seedlings subjected to drought treatments (65% FC and 35% FC, respectively) compared to control seedlings. However, relative to the RWC in the drought-stressed cowpea plants alone, RWC was raised by 4%, 8%, and 13% after being pretreated with RW (15%, 25%, and 35% RW), and by 4%, 12%, and 15% after being pretreated with RW (35% FC) (Fig. 2). Both the net photosynthetic rate (Pn) and transpiration rate (E) were significantly reduced by the drought treatments (65% and 35% FC), with Pn being reduced by 37% and E by 63%, respectively. Nevertheless, cowpea seedlings exposed to 35% FC after being pretreated with RW (15%, 25%, or 35% RW) saw increases in Pn of 7%, 41%, and 67% and E of 23%, 45%, and 69% (Fig. 2).
Growth parameters
Treatment
Rose water (%)
0% RW
15% RW
25% RW
35% RW
Shoot Length (cm)
Control
46.8 ± 3.3d
49.1 ± 3.2c
59.2 ± 4b
70.3 ± 4.6a
65% FC
30.2 ± 1.3d
38.5 ± 1c
36.8 ± 1.8b
47.1 ± 2.1a
35% FC
11.3 ± 1.9d
13.49 ± 1.4c
19.3 ± 1.2b
25.4 ± 1a
Root Length (cm)
Control
30.3 ± 1.2d
35.2 ± 1.1c
41.4 ± 1.1b
53.7 ± 0.92a
65% FC
25.1 ± 1.52d
27.6 ± 1.4c
32.3 ± 1.32b
39.4 ± 1.13a
35% FC
10.7 ± 0.6d
13.6 ± 0.72c
17.5 ± 0.81b
20.5 ± 0.9a
Shoot Fresh Weight, SFW (g plant−1)
Control
3.3 ± 0.23d
4.35 ± 0.19c
9.2 ± 0.18b
15.1 ± 0.09a
65% FC
2 ± 0.04d
2.51 ± 0.06c
7.3 ± 0.08b
10.6 ± 1.1a
35% FC
1.7 ± 0.05d
2.1 ± 0.06c
4.4 ± 0.07b
7.4 ± 0.77a
Shoot Dry Weight, SDW (g plant−1)
Control
1.7 ± 0.04d
2.2 ± 0.05c
4.8 ± 0.08b
7.5 ± 0.05a
65% FC
1.4 ± 0.02d
1.9 ± 0.03c
2.1 ± 0.06b
4.1 ± 0.08a
35% FC
1.1 ± 0.04d
1.2 ± 0.033c
1.9 ± 0.06b
2.4 ± 0.07a
Root fresh weight, RFW (g plant−1)
Control
2.4 ± 0.07d
2.6 ± 0.08c
3.2 ± 0.08b
4.5 ± 0.09a
65% FC
1.2 ± 0.02d
1.4 ± 0.02c
1.7 ± 0.03b
3.1 ± 0.07a
35% FC
0.8 ± 0.03d
1.04 ± 0.04c
1.47 ± 0.04b
2.1 ± 0.06a
Root dry weight, RDW (g plant−1)
Control
0.8 ± 0.07d
0.99 ± 0.06c
1.2 ± 0.05b
1.65 ± 0.05a
65% FC
0.52 ± 0.02d
0.66 ± 0.02c
0.84 ± 0.01b
1.06 ± 0.018a
35% FC
0.32 ± 0.04d
0.48 ± 0.04c
0.73 ± 0.038b
0.9 ± 0.03a
Leaf area (cm2)
Control
17.1 ± 0.8d
18.7 ± 0.9c
19.07 ± 0.95b
26.8 ± 1.1a
65% FC
10.8 ± 0.7d
12.6 ± 0.83c
13.1 ± 1.67b
19.5 ± 1.54a
35% FC
9.7 ± 2.6d
10.8 ± 2.4c
14.6 ± 2b
18.2 ± 1.8a
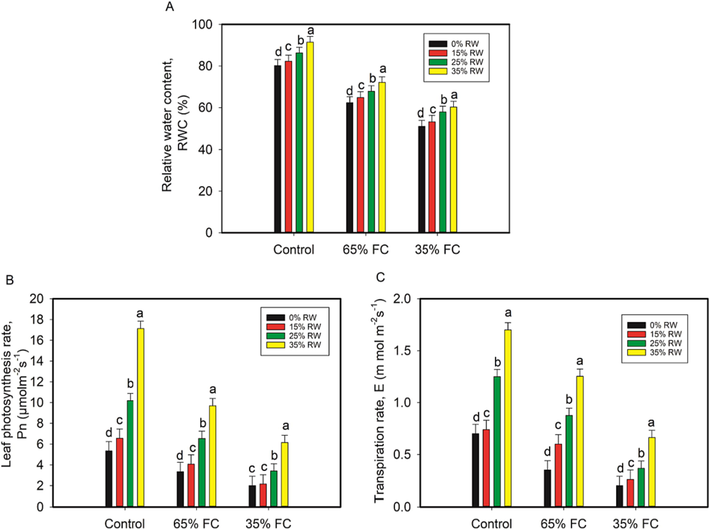
Pretreatment of cowpea seedling leaves with rose water (RW) affects relative water content (A), leaf photosynthetic rate (B), and transpiration rate (C) A p < 0.05 indicates a significant difference between RW with letters that aren't the same as each other in the data, which are the mean from three independent experiments.
The proline content was 24% and 46% higher under drought stress, respectively, compared to the controls. Total soluble protein (TSP) and total soluble carbohydrate (TSC) concentrations were also raised in drought-stressed (65% and 35% FC) cowpea plants by 25%, 45%, and 41%, 53%, respectively, compared to those in untreated plants. A reduction in proline, total soluble protein, and total soluble carbohydrate was seen in RW pretreated cowpea plants compared to drought-stressed (65% and 35% FC) seedlings (Fig. 3).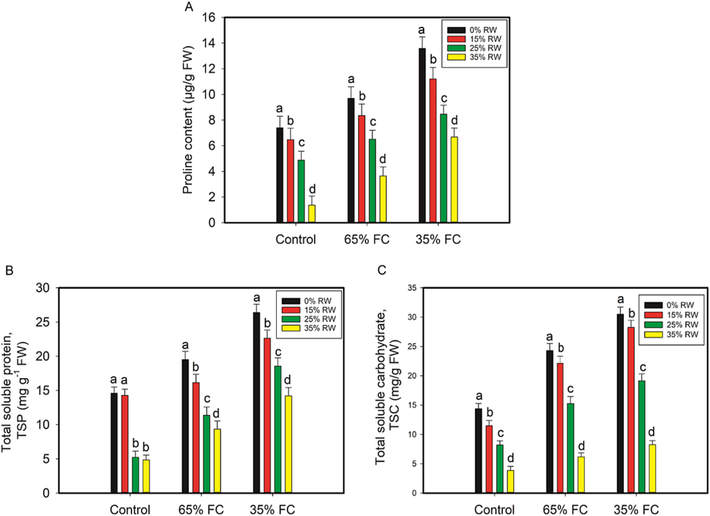
For drought stress, pretreatment with rose water (RW) had an effect on the levels of proline and total soluble protein in the leaves of cowpea seedlings, as well as the levels of total soluble carbohydrate. A p < 0.05 indicates a significant difference between RW with letters that aren't the same as each other in the data, which are the mean from three independent experiments.
Drought treatments of 65% FC and 35% FC significantly enhanced the malondialdehyde (MDA) concentration in cowpea seedlings that had been subjected to salt stress without first being pretreated with RW. Nonetheless, pretreatment of RW (15%, 25%, and 35% RW) decreased the MDA content by 14%,32% and 50% at 65% FC and 24%,35%,48%, respectively, at 35% FC. Cow pea plants subjected to drought conditions of 65% and 35% FC showed significant increases in antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT), as compared to control. Moreover, RW pretreated drought stressed seedlings showed increased SOD and CAT activity (Fig. 4) (See Fig. 5.)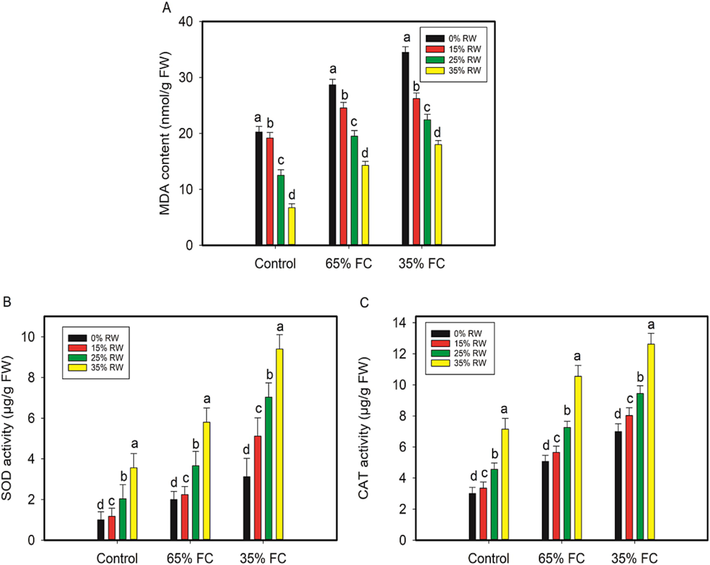
MDA concentration, SOD activity, and CAT activity in the leaves of drought-stressed cowpea seedlings were all affected by RW pretreatment (Fig. 3). P < 0.05 indicates a significant difference between RW with letters that aren't the same as each other in the data, which are the averages (±SE) from three independent experiments.
A schematic depicts how rose water improves the growth and physiology of cowpea seedlings under drought stress.
4 Discussion
We evaluated morphological and physiological characteristics of cowpea to see how drought stress affects growth. Cowpea growth was considerably reduced by drought stress (Table 1). Past studies reported that cowpea showed similar findings when subjected to abiotic stressors (Matsui and Singh, 2003).
Due to drought conditions, the relative water content of cowpea leaves reduced significantly. Earlier studies have also documented that drought leads to a reduction in relative water content (RWC) in cowpea plants (Zegaoui et al., 2017). Cowpea plants pretreated with RW had higher water content after being subjected to drought, suggesting that this may have boosted the plants' ability to absorb water. The rate of carbon dioxide assimilation is affected by drought stress and other environmental conditions, which has a negative effect on biomass accumulation (Hasan et al., 2020). Photosynthetic and transpiration rates of cowpea were shown to be decreased in our study as a result of drought stress. These decreases in gas exchange characteristics might be a result of a decreased rate of water and nutrient intake. Pretreatment with RW enhanced photosynthesis and transpiration rates, indicating that RW helped mitigate the adverse impacts of drought on gas exchange-related parameters, most likely by decreasing hydrogen peroxide formation (H2O2) (Kumari et al., 2018).
Proline is just one of several organic molecules that plants either create or store (Hasan et al., 2018; Hasan et al., 2020). Protecting plant cells from osmotic and other environmental stresses, proline is an osmoprotectant. Cowpea seedlings have a higher concentration of the amino acid proline after being subjected to drought stress, as discovered by our research. Under drought stress, RW pretreatment decreased proline content, most likely because of a decrease in osmotic stress. RW may be able to maintain the proline concentration that protects plant cells under drought. The impact of drought stress on plants' soluble proteins has been investigated in a number of different ways. Some research has shown that drought stress reduces the amount of soluble protein in plants (Zahoor et al., 2017), whereas other research has shown that a lack of water actually causes an increase in the amount of soluble protein in plants (Aziz et al., 2018; Zhang et al., 2019). Cowpea seedlings' soluble protein content increased during the drought, which is consistent with previous studies. Similar to other osomo-protectants like proline, RW lowers the amount of soluble protein in cowpea seedlings under drought stress, which may be due to a reduction in protein biosynthesis. Plants use carbohydrates as their main source of energy for their metabolic processes. During environmental stresses, carbohydrates accumulate in large amounts, and photosynthesis is inhibited, leading to a decrease in plant growth (Rook et al., 2010; Richter et al., 2015). Based on our findings, cowpea leaves accumulate more soluble carbohydrates when subjected to drought stress. Nevertheless, RW reduces the buildup of total soluble carbohydrates in cowpea leaves in response to drought stress, suggesting that it improves soluble sugar transport across cell membranes. Such carbohydrate contents may aid in the osmoregulation of plants and help in their water intake during times of stress (Farhangiabriz and Torabian, 2017; Abdellaoui et al., 2018).
Most research has focused on malondialdehyde (MDA), an aldehydic lipid peroxidation product. Similar to our findings, Hasan et al. (2018) reported that Moringa species experiencing drought stress had a higher total MDA content. The MDA concentration in cowpea was reduced, however, after being treated with RW, most likely because of the antioxidants' ability to scavenge free radicals (Hasan et al., 2018). Despite the fact that environmental influences have a major effect on the activity of antioxidant enzymes, these enzymes are crucial for maintaining health (Abdulmajeed et al., 2021, Alharbi et al., 2021). Our research showed that the effectiveness of RW varies with the amount administered. Antioxidant enzyme activity was boosted when RW-pretreated seed was used. Our results demonstrated that pretreatment of cowpea seed with RW at varying doses increased SOD and CAT activity when the seeds were subjected to drought stress.
5 Conclusion
Our findings offer novel insights into the physiological response to drought stress and the role of RW regulation in this process. These results lead us to propose that pretreating cowpea plants with RW is a highly effective approach for enhancing their drought tolerance. When cowpea plants were treated with different concentrations of RW (15%, 25%, 35%), their biomass, relative water content, gas exchange parameters, and antioxidant levels all increased. The highest RW concentration of 35% exhibited the most significant enhancement in cowpea plants' ability to withstand drought stress.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physiological, anatomical and antioxidant responses to salinity in the mediterranean pastoral grass plant stipa lagascae. Crop Pasture Sci.. 2018;68(9):872.
- [Google Scholar]
- Alleviation of copper phytotoxicity by acetylsalicylic acid and nitric oxide application in mung bean involves the up-regulation of antioxidants, osmolytes and glyoxalase system. J. Plant Interac.. 2021;16(1):201-212.
- [Google Scholar]
- Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021
- [CrossRef] [Google Scholar]
- Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants. 2021;10:1730.
- [Google Scholar]
- Cowpea (Vigna unguiculata L. Walp), a renewed multipurpose crop for a more sustainable agri-food system: Nutritional advantages and constraints. J. Sci. Food Agric.. 2016;96:2941-2951.
- [Google Scholar]
- Exogenous application of melatonin alleviates salt stress-induced decline in growth and photosynthesis in Glycine max (L.) seedlings by improving mineral uptake, antioxidant and glyoxalase system. Plant Soil Environ.. 2021;67
- [Google Scholar]
- Photosynthesis under stressful environments: an overview. Photosynthetica. 2013;51(2):163-190.
- [Google Scholar]
- Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem.. 2018;123:192-203.
- [Google Scholar]
- Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol.. 2011;13:1006-1010.
- [Google Scholar]
- Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205e207.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Reactive oxygen species, abiotic stress and stress combination. Plant J.. 2017;90(5):856-867.
- [Google Scholar]
- Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf.. 2017;137:64-70.
- [Google Scholar]
- Effects of magnetized water on phenolic compounds, lipid peroxidation and antioxidant activity of Moringa species under drought stress. J. Animal Plant Sci.. 2018;28:803-810.
- [Google Scholar]
- Insights into 28-homobrassinolide (HBR)- mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Inter.. 2020;15:371-385.
- [Google Scholar]
- Spermine: Its emerging role in regulating drought stress responses in plants. cells. 2021;261:1-15.
- [Google Scholar]
- GABA: a key player in drought stress resistance in plants. Int. J. Mol. Sci.. 2021;22:10136.
- [CrossRef] [Google Scholar]
- Spermine-mediated tolerance to selenium toxicity in wheat (Triticum aestivum L.) depends on endogenous nitric oxide synthesis. Antioxidants. 2021;10:1835.
- [Google Scholar]
- Modification of starch content and its management strategies in plants in response to drought and salinity: current status and future prospects. J. Soil Sci. Plant Nutr.. 2023;23:92-105.
- [Google Scholar]
- Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol Rep.. 2011;5:353-365.
- [Google Scholar]
- Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 1968;125:189-198.
- [CrossRef] [Google Scholar]
- Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA and GA-mediated pathways. Front. Plant Sci.. 2021;12:381.
- [Google Scholar]
- Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem.. 2021;167:309-320.
- [Google Scholar]
- Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol. Plantarum. 2009;31:427-436.
- [Google Scholar]
- Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants. 2019;25(3):667-681.
- [Google Scholar]
- Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.) South Afric. J. Bot.. 2020;129:74-81.
- [Google Scholar]
- Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot.. 2012;63(4):1593-1608.
- [Google Scholar]
- Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma. 2018;255:139-152.
- [Google Scholar]
- Root characteristics in cowpea related to drought tolerance at the seedling stage. Exp. Agric.. 2003;39:29-38.
- [Google Scholar]
- Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plantarum. 1983;58:166-170.
- [Google Scholar]
- Nadeak, L., Girsang, E., Ginting, C.N., Chiuman, L., 2021. Activity of rose flower extract and resepthakulum as antioxidant and anti-tyrosinase. In: Proceedings of the International Conference on Health Informatics, Medical, Biological Engineering, and Pharmaceutical - HIMBEP, 17-22, 2020, Medan, Indonesia.
- Heat shock proteins and antioxidant genes involved in heat combined with drought stress responses in perennial rye grass. Life. 2022;12:1426.
- [Google Scholar]
- Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci.. 2015;233:107-115.
- [Google Scholar]
- Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J.. 2010;26:421-433.
- [Google Scholar]
- Phytochemical analysis, in-vitro antioxidant and antimicrobial activities of flower petals of Rosa damascene. Int. J. Pharmacog. Phytochem. Res.. 2015;7:246-250.
- [Google Scholar]
- Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Funct. Plant Biol.. 1994;21:255-271.
- [Google Scholar]
- Potassium application regulates nitrogen metabolism and osmotic adjustment in cotton (Gossypium hirsutum L.) functional leaf under drought stress. J. Plant Physiol.. 2017;215:30-38.
- [Google Scholar]
- Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol.. 2017;218:26-34.
- [Google Scholar]
- Grafting improves tomato drought tolerance through enhancing photosynthetic capacity and reducing ROS accumulation. Protoplasma. 2019;256(4):1013-1024.
- [Google Scholar]







