Translate this page into:
Chorein-dependent microfilament organization in tumor cells
⁎Corresponding author. salkahtani@ksu.edu.sa (Saad Alkahtani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Chorein is variably expressed in different cancer cells. In various non-malignant cell types, this protein regulates cytoskeleton microstructure and signaling. However, the role of chorein in regulating the actin cytoskeleton in tumor cells remains elusive. Here, we investigated chorein expression in various breast tumor cells and the involvement of this protein in microfilament organization. We used laser scanning microscopy to analyze microfilaments architecture, Triton X-100 fractionation to quantify G and Total actin levels, and quantitative RT-PCR to assess chorein gene transcription. We show that in line with previous observations, the less differentiated MCF7 breast cancer cells exhibited the highest relative expression of chorein compared to MDA-MB231 and T47D cell lines. Contrastingly, in less differentiated ZF rhabdomyosarcoma cells expressing high chorein levels, silencing of this protein was followed by clear depolymerization of actin microfilaments as apparent from IF morphological analysis. Quantification of G- and F-actin levels by Triton X-100 fractionation that revealed a significant increase in this ratio fully supported this finding. These results disclose that chorein is highly expressed in less differentiated tumor cells. In addition, silencing of this protein induces significant structural disorganization of the actin network, providing clear evidence that chorein regulates microfilament cytoskeleton architecture in tumor cells of higher malignant potential.
Keywords
Chorein
Tumor
Actin
Microfilament
Gene Silencing
Polymerization
1 Introduction
Chorein protein is encoded by Vacuolar Protein Sorting 13A (VPS13A) gene (Velayos-Baeza et al., 2004), and is present in several tissues (Kurano et al., 2007; Alesutan et al., 2013; Schmidt et al., 2013). Lack of chorein causes the neurodegenerative disease chorea-acanthocytosis (ChAc) (Burgunder, 2014; Dobson-Stone et al., 2004), manifested by severe movement disorder (Lang et al., 2017). Functional analysis revealed that chorein regulates various cellular functions (Saiki et al., 2007), including exocytosis (Pelzl et al., 2017a), cytoskeletal organization (Honisch et al., 2015a,b), and cell survival (Pelzl et al., 2017b). Recent studies addressed in detail the molecular basis of chorein-governed actin cytoskeletal alterations. According to these reports, chorein is partially effective by binding to phosphatidylinositol lipids (Foller et al., 2012), followed by the activation of phosphoinositide-3-kinase (PI3K)-a p85-subunit subsequent increase of Ras-related C3 botulinum toxin substrate (Rac1) activity and phosphorylation of p21 protein-activated kinase 1 (PAK1) (Foller et al., 2012). These studies cemented the fundamental function of chorein as a regulator of cytoskeletal microstructure.
Earlier studies indicated that alteration in actin cytoskeletal microstructure and cellular signaling pathways in tumor cells (Katsantonis et al., 1994; Stournaras et al., 1996) is a decisive dedifferentiation step (Papakonstanti and Stournaras, 2008; Stournaras et al., 2014; Kotula, 2012; Araki et al., 2015) governing key cellular functions. Previous studies disclosed a correlation between actin polymerization dynamics and tumorigenic behavior (Stournaras et al., 2014). Chorein is interacting with cytoskeletal proteins (Shiokawa et al., 2013) regulating cytoskeletal organization in various non-malignant cells (Schmidt et al., 2013; Honisch et al., 2015a,b) and modifies key signaling pathways (Yamazaki et al., 2005; Yilmaz and Christofori, 2009; Alesutan et al., 2013).
Previous studies revealed that chorein transcription varies in rhabdomyosarcoma cell lines with diverse differentiation stages (Yu et al., 2016). Indeed, chorein transcription levels were higher in drug-resistant ZF rhabdomyosarcoma cells with poor differentiation, compared to RH, RD, and A204 rhabdomyosarcoma cells (Yu et al., 2016). Additionally, it was witnessed that silencing of chorein induced a solid apoptotic response in ZF cells, implying that chorein may regulate cell survival (Honisch et al., 2015a,b). However, it remains elusive, whether chorein expression varies in other tumor cells and may correlate to tumor differentiation grade. In addition, although previous studies addressed the chorein-dependent regulation of actin cytoskeleton in non-malignant cells, it is still unclear whether chorein differential expression in various tumor cells affects microfilament organization.
In this research, we addressed the chorein expression in various breast cancer cell lines and investigated the function of chorein in regulating actin architecture in tumor cells expressing high chorein levels. We report here that less differentiated breast cancer cells express relatively high chorein levels, while silencing of this protein induced large deregulation of actin in diverse human rhabdomyosarcoma cancer cells, establishing a substantial role that chorein protein might play in tumors.
2 Materials and methods
2.1 Cells
The cell line of ZF multifocal alveolar rhabdomyosarcoma (established by Dr. Sabine Schleicher at the Children’s Hospital Tubingen), the cell line of embryonal rhabdomyosarcoma RD (DSMZ, Braunschweig, Germany) and the alveolar rhabdomyosarcoma cell line RH30 (DSMZ, Braunschweig, Germany) were used and nurtured in Dulbecco's Modified Eagle Medium (DMEM), comprising 10% of fetal bovine serum (FBS), 1% L-glutamine and complemented with 1% penicillin–streptomycin at 37 °C temperature and 5% CO2. MCF7, MDA-MB231, and T47D cells were from ATCC. Likewise, they were cultured in 1:1 DMEM/Ham’s F12 medium accompanied with 10% of FBS, 2 mM L-glutamine, 30 mM NaHCO3, 16 ng/ml insulin, and 50 mg/ml penicillin–streptomycin.
2.2 Chorein silencing
Before transfection, cells (1 × 105) were harvested in 6-well culture plates for a whole day. Afterward, transfection was done with siRNA for VPS13A (chorein) (ID# s23342, Ambion, Darmstadt, Germany) or with negative (control) siRNA (ID#4390843, Ambion) through siPORT amine transfection agent (Ambion™) as per manufacturer’s guidelines.
2.3 RNA isolation, cDNA synthesis and quantitative real time PCR
Cells were raised at 3 × 105 cells/ml and grown for 48 h before isolation of RNA under DMEM comprising 10% FBS and 1% penicillin–streptomycin. For determination of tubulin transcription levels, entire RNA was extracted at 24 h, 48 h, and 72 h post-transfection through TriFast™ (Peqlab, Erlangen, Germany). Total RNA was isolated from the breast cancer cell lines MDA-MB231, T47D and MCF7, as well as from HEK (human embryonic kidney cell line). RNA was extracted using RNAiso plus (Takara, Japan). According to the manufacturer's instructions. cDNA was synthesized from 2 μg of total RNA, using random hexamer priming and the SuperScript II, Reverse Transcriptase kit (Invitrogen, Life Technologies, USA).
Real-time PCR was performed using KAPA SYBR Green/ROX qPCR Master Mix (Fermentas, USA) and a 7500 Real-Time System (Applied Biosystems). Relative quantification of gene expression was calculated using the relative standard curve method. HEK cell line was chosen for standard curve generation due to its high expression of Vps13A (chorein). Thus, serial dilutions of HEK cDNA were used in order to obtain standard curves, for both target gene (Vps13A (chorein)) and endogenous reference gene (β-actin). For each individual sample, input amounts (relative copy number) of the target and reference genes were calculated using the equations of the standard curves. To normalize target gene expression to the endogenous reference gene, the value of target gene input amount was divided by the value of the reference gene input amount.
For the amplification the following primers were used (5′-3′ orientation):
Actin, forward CGGCATCGTCACCAACTG.
Actin, reverse GGCACACGCAGCTCATTG.
Vps13A (chorein), forward TGGAGAGAAGCACGAAAACTC.
Vps13A (chorein), reverse TGGGCATCCTTACATCCATGA.
2.4 Confocal laser scanning microscopy
All the cells were diluted in phosphate-buffered saline (PBS) keeping cell density at 5 × 107 cells/ml. Next, 10 μl of the suspended cells were then smeared that were air-dried for half an hour, and then methanol fixation was performed for two minutes. After rinsing with PBS, the sample was processed for actin staining (Stournaras et al., 2014). For analysis of microfilament staining, cells underwent fixation with 4% paraformaldehyde (PFA) for 15 min. After another round of dual PBS washing, the specimen was suspended with blocking buffer (1xPBS/5% normal goat serum/0.3% Triton™ X-100) for another 60 min. After washing thrice, using DRAQ5 dye (BioStatus), the nuclei were dyed for 10 min. Then, ProLong™ Gold Antifade Mountant (Invitrogen) was employed to mount slides. Confocal microscopy was conducted using a LSM 5 EXCITER and image analysis was done.
2.5 Measurement of G/total actin ratio
Triton™ X-100 G-actin comprising and total-actin comprising fractions of rhabdomyosarcoma cells were primed as explained earlier (Papakonstanti and Stournaras, 2007). Specimens were nurtured in 50 ml of Triton extraction buffer (0.3% Triton X-100, 300 mM sucrose, 5 mM Tris, 2 mM EGTA, 1 mM PMSF, 2 μM phalloidin, 10 μg/ml leupeptin, 1 mM sodium orthovanadate, 50 mM NaF, 20 μg/ml aprotinin) at pH 7.4 on ice. The soluble proteins in the supernatant were isolated by aspiration. From the plate, the triton-insoluble pellet was scratched into 50 ml radio-Immunoprecipitation (RIPA) buffer (1% Triton X-100, 0.1% SDS, 50 mM Tris/HCl, 1% sodium deoxycholate, 1 mM DTT, 0.15 M NaCl, 1 mM EDTA, and 1 mM sodium orthovanadate) at pH 7.4. Insoluble materials were eradicated via centrifugation. Identical volumes of each were then exposed to SDS-PAGE and western blot through monoclonal anti-beta-actin antibodies. A decrease of the triton-soluble (G−) over the total (T−) actin ratio is indicative of actin polymerization.
3 Results
Previous studies discovered that the transcription levels of chorein were higher in drug-resistant, poorly differentiated ZF rhabdomyosarcoma cells (Yu et al., 2016; Honisch et al., 2015a,b). Interestingly, this report revealed that the 3 families of rhabdomyosarcoma cells express different amounts of chorein, while the highest expression was determined in the less differentiated ZF rhabdomyosarcoma cell line (Yu et al., 2016). We further analyzed whether chorein expression correlates to tumor differentiation grade. For this, we performed similar analyses in various breast tumor cell lines. We report here that following previous observations, gained in rhabdomyosarcoma cells, among all tested breast cancer cell lines, the less differentiated MCF7 expresses the relatively highest chorein level, compared to MDA-MB231 and T47D cells (Fig. 1). This finding supports the hypothesis that chorein expression correlates to the grade of differentiation of various tumors. Indeed, in the second part of this project, we assessed the spectrum of actin polymerization in ZF rhabdomyosarcoma tumor cells that demonstrate the highest expression of chorein. For this we have used both, confocal analysis of actin microfilaments, along with measurements of G- and F-actin levels by using Triton X-100 fractionation analysis.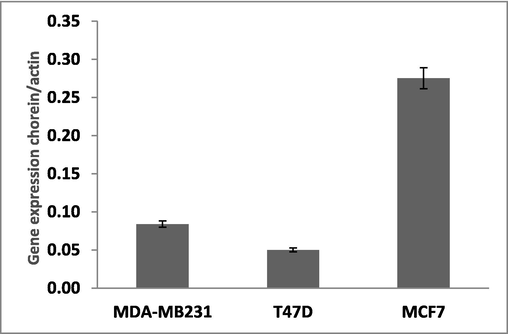
Vsp13A (chorein) gene expression in different breast cancer cell lines: Vsp13A (chorein) gene expression in three different breast cancer cells lines, MDA-MB231, T47D and MCF7 was determined by quantitative real time RT-PCR, with β-actin as the reference gene, using the relative standard curve method.
Confocal laser scanning microscopy showed diminished filamentous structures in chorein-silenced ZF cells (Fig. 2A lower panels) in disagreement with corresponding wild-type cells (Fig. 2A upper panels). This finding indicates that chorein silencing in ZF cells provokes the reorganization of actin microfilaments.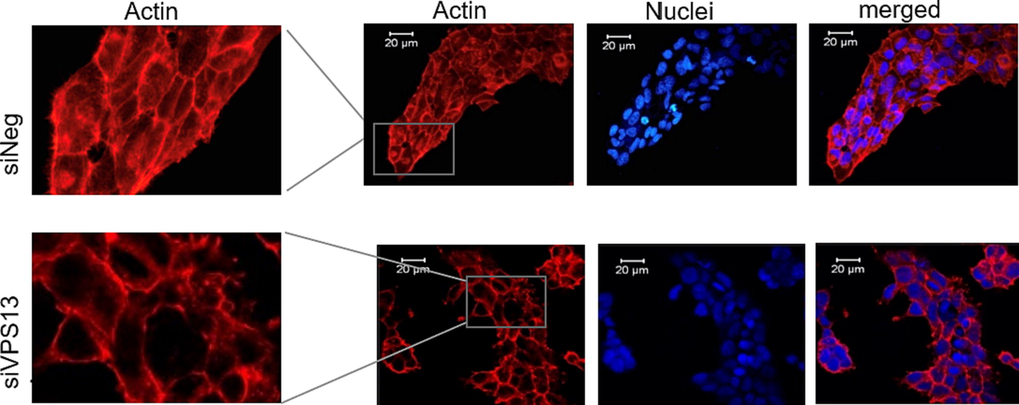
Chorein silencing influences microfilament organization in ZF cells. Confocal laser scanning microscopy of actin and nuclei in chorein-silenced (siVPS13A, upper panels) and control (siNeg, lower panels) ZF rhabdomyosarcoma tumor cells. Scale bars represent 20 μm.
We further specified this finding through detailed quantification of G- and F-actin levels. For this, we have used the G/F actin ratio analysis based on Triton X-100 fractionation. This previously established method (Papakonstanti and Stournaras 2007) was largely used to determine the actin polymerization dynamics in various cell types of malignant or non-malignant origin in the past. By applying this technology, we revealed a substantial increase in the G/F actin ratio (Fig. 3). This finding implies important actin depolymerization and thus it is supporting the morphological changes presented in Fig. 2.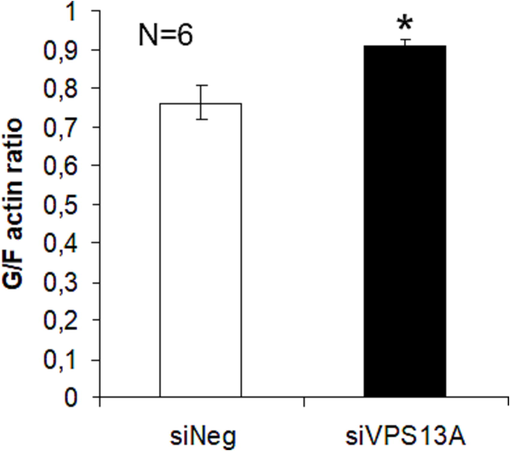
Chorein silencing correlates with microfilament depolymerization in ZF cells. Quantification of G- and F-actin levels using G/F actin ration analysis by Triton X-100 fractionation from chorein-silenced (siVPS13A) and control (siNeg) ZF rhabdomyosarcoma tumor cells, from n = 6 independent experiments. *(p < 0.05) indicates statistical significance.
4 Discussion
In the present study, we report differential expression of chorein in various breast tumor cells. Interestingly, a correlation between expression levels and cellular tumorigenic potential became evident. Indeed, mRNA transcripts of chorein were highly expressed in poorly differentiated MCF7 breast tumor cells, in comparison with the chorein transcription levels measured in T47D and MDA-MD231 cells. These findings indicate a potential correlation of chorein expression with the differentiation grade of breast tumor cells. In addition, they fully support previously published experimental observations in rhabdomyosarcoma cells (Honisch et al., 2015a,b). Indeed, the chorein gene was reported to be principally transcribed in drug-resistant ZF rhabdomyosarcoma cells with poor differentiation compared to well-differentiated RD and A204 rhabdomyosarcoma cells (Honisch et al., 2015a,b). Altogether, these results support the idea that chorein is expressed highly in tumor cells with low differentiation grade, implying that chorein might play a substantial part in the tumorigenic promise of poorly differentiated tumor cells.
Furthermore, we are the first to report herein that chorein deficiency, by silencing this protein, disrupts the cytoskeletal protein network of microfilaments in tumor cells. These observations became evident by applying both qualitative morphological analysis by confocal laser scanning microscopy, along with quantitative identification of the monomeric (G−) and filamentous (F−) actin content after Triton X-100 fractionation. These results emphasize a significant activity of chorein protein in regulating cytoskeletal organization and function, supporting previous findings. Indeed, it was earlier cited that actin microfilaments are depolymerized in erythrocytes, fibroblasts, and blood platelets isolated from chorea-acanthocytosis (ChAc) patients (Pelzl et al., 2017b, Foller et al., 2012). From these findings, it was concluded that lack of functional chorein in platelets, fibroblasts, or erythrocytes of ChAc patients leads to substantial structural disorganization of cytoskeletal components contributing to the pathogenomic erythrocyte shape changes in ChAc (Lang et al., 2017, Pelzl et al., 2017b). Our present findings provide novel insights, highlighting the regulatory involvement of chorein in actin organization in tumor cells as well. Since actin reorganization is a crucial regulatory step in governing apoptotic responses in tumor cells (Papakonstanti and Stournaras, 2008, Grzanka et al., 2003) including their migratory potential (Kallergi et al., 2007, Yilmaz and Christofori, 2009), our present findings intimate an imperative regulatory function of chorein in poorly differentiated malignant cells with pharmacological or clinical potential. Additional studies are now needed to address in detail the possible role of chorein in the tumorigenic activity of less differentiated tumor cells with high metastatic potential.
5 Conclusions
Our findings support previous observations, denoting that the expression of chorein protein may vary in correlation to the differentiation grade of diverse tumor cells, together with breast cancer cells. In addition, we report that this protein regulates the restructuring of the actin cytoskeleton in rhabdomyosarcoma cells, indicating a potential implication of chorein protein in well-established actin regulating apoptotic responses in tumor cells.
Availability of data
The data generated or analyzed in this article are online and publicly available without request.
Authors’ contributions
Saad Alkahtani and Saud Alarifi cultured cell lines, performed treatments, and evaluated gene expression. Abdullah Alkahtani performed silencing of chorein and data analysis. Abdullah Alkahtani and Christos Stournaras tested chorein by confocal laser scanning microscopy and measured the G/total actin ratio through Triton™ X-100.
Acknowledgements
This Work was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdul-Aziz City for Science and Technology, Kingdom of Saudi Arabia, grant number 14-MED-1893-02. Also authors would like to thank Dr, Anna Tsapara and Dr. Nefeli Zacharopoulou (University of Crete Medical School) for important technical support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chorein sensitivity of actin polymerization, cell shape and mechanical stiffness of vascular endothelial cells. Cell. Physiol. Biochem.. 2013;32:728-742.
- [Google Scholar]
- p53 regulates cytoskeleton remodeling to suppress tumor progression. Cell. Mol. Life Sci.. 2015;72(21):4077-4094.
- [CrossRef] [Google Scholar]
- Genetics of Huntington's disease and related disorders. Drug Discov. Today. 2014;19:985-989.
- [Google Scholar]
- Chorein detection for the diagnosis of chorea-acanthocytosis. Ann. Neurol.. 2004;56:299-302.
- [Google Scholar]
- Chorein-sensitive polymerization of cortical actin and suicidal cell death in chorea-acanthocytosis. FASEB J.. 2012;26:1526-1534.
- [Google Scholar]
- Cytoskeletal reorganization during process of apoptosis induced by cytostatic drugs in K-562 and HL-60 leukemia cell lines. Biochem. Pharmacol.. 2003;66(8):1611-1617.
- [Google Scholar]
- Chorein sensitive arrangement of cytoskeletal architecture. Cell. Physiol. Biochem.. 2015;37:399-408.
- [Google Scholar]
- Chorein addiction in VPS13A overexpressing rhabdomyosarcoma cells. Oncotarget. 2015;6:10309-10319.
- [Google Scholar]
- Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell. Physiol. Biochem.. 2007;20(6):977-986.
- [Google Scholar]
- Differences in the G/total actin ratio and microfilament stability between normal and malignant human keratinocytes. Cell. Biochem. Funct.. 1994;12(4):267-274.
- [Google Scholar]
- A critical molecule coordinating actin cytoskeleton reorganization with PI-3 kinase and growth signaling. FEBS Lett.. 2012;586(17):2790-2794.
- [CrossRef] [Google Scholar]
- In vivo distribution and localization of chorein. Biochem. Biophys. Res. Commun.. 2007;353:431-435.
- [Google Scholar]
- Neurons, erythrocytes and beyond – the diverse functions of chorein. Neurosignals. 2017;25(1):117-126.
- [Google Scholar]
- Actin cytoskeleton architecture and signaling in osmosensing. Methods Enzymol.. 2007;428:227-240.
- [Google Scholar]
- Cell responses regulated by early reorganization of actin cytoskeleton. FEBS Lett.. 2008;582(14):2120-2127.
- [CrossRef] [Google Scholar]
- Lithium sensitivity of store operated Ca2+ entry and survival of fibroblasts isolated from chorea-acanthocytosis patients. Cell. Physiol. Biochem.. 2017;42:2066-2077.
- [Google Scholar]
- Lithium sensitive ORAI1 expression, store operated Ca2+ entry and suicidal death of neurons in chorea-acanthocytosis. Sci. Rep.. 2017;7:6457.
- [Google Scholar]
- Primary skeletal muscle involvement in chorea-acanthocytosis. Mov. Disord.. 2007;22:848-852.
- [Google Scholar]
- Chorein sensitivity of cytoskeletal organization and degranulation of platelets. FASEB J.. 2013;27:2799-2806.
- [Google Scholar]
- Chorein, the protein responsible for chorea-acanthocytosis, interacts with beta-adducin and beta-actin. Biochem. Biophys. Res. Commun.. 2013;441:96-101.
- [Google Scholar]
- Altered actin polymerization dynamics in various malignant cell types: evidence for differential sensitivity to cytochalasin B. Biochem. Pharmacol.. 1996;52(9):1339-1344.
- [Google Scholar]
- The actin cytoskeleton in rapid steroid hormone actions. Cytoskeleton (Hoboken). 2014;71(5):285-293.
- [CrossRef] [Google Scholar]
- Regulation of cancer cell motility through actin reorganization. Cancer Sci.. 2005;96(7):379-386.
- [Google Scholar]
- The cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.. 2009;28(1–2):15-33.
- [CrossRef] [Google Scholar]
- Chorein sensitive orai1 expression and store operated Ca2+ entry in rhabdomyosarcoma cells. Cell. Physiol. Biochem.. 2016;40(5):1141-1152.
- [Google Scholar]







