Translate this page into:
BCG vaccine-induced mucosal humoral immunity in human nasal associated lymphoid tissue
⁎Corresponding author. wmahallawi@taibahu.edu.sa (Waleed H. Mahallawi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Mycobacterium tuberculosis (Mtb) is the causative pathogen of tuberculosis (TB). TB vaccine studies have long been conducted. Since the discovery of the successful intradermally administered Mycobacterium bovis Bacillus Calmette–Guérin (BCG) vaccine against TB, no licensed TB mucosal vaccine has yet been approved. Our research aims to investigate the suitability of human NALT-derived MNCs as an in vitro human cell culture model to explore the mucosal humoral immune responses to the BCG vaccine. Moreover, to assure that BCG vaccine able to induce the different antibody isotypes including IgG, IgM and IgA.
Methods
Tonsils from 20 patients who underwent elective tonsillectomies at Madinah Germany Hospital were recruited. Tonsillar mononuclear cells (MNCs) were separated and co-cultured in the presence and absence of the BCG vaccine. ELISA was used to measure BCG-induced IgG, IgM and IgA antibodies in the cell culture supernatants following stimulation and culture.
Results
Nasal-associated lymphoid tissue (NALT) cell culture as an in vitro model was successfully used to evaluate the BCG-induced humoral immune response. Significant (p = 0.001, n = 20) levels of specific, anti-TB IgG, IgM, and IgA antibody responses were detected in MNCs stimulated with the BCG vaccine compared with unstimulated MNCs. Significant differences were found between anti-TB antibody classes. Anti-TB IgG antibody was significantly higher (mean = 2.33) than anti-TB IgM (mean = 1.37) antibody (p = 0.0001, n = 20). Anti-TB IgM antibody was significantly higher than IgA (mean = 0.93) antibody (p = 0.0018, n = 20).
Conclusions
The current human model will be beneficial for the future comprehensive study of other immune components such as cellular immune responses to the BCG vaccine. The model would be ideal to design, improve and study the possible intranasal use of the BCG vaccine.
Keywords
Tonsillar MNC
NALT
Humoral immunity
BCG
TB
1 Introduction
TB is one of the top 10 causes of death globally. The BCG vaccine protects children against extrapulmonary TB, but it displays inconsistent protection against the disease in adults (Gopalaswamy & Subbian, 2022; Romano & Huygen, 2012).
An estimated of 25% of the worldwide population has been infected with Mtb and is at risk of developing TB.(Houben & Dodd, 2016) The TB prevalence in Saudi Arabia expected to reach about 14/100,000(Al Qurainees & Tufenkeji, 2016).
Mucosal immunity is a vital element of the immune system (Kiyono & Fukuyama, 2004). Of the numerous mucosal control sites, the nasal cavity is one of the most remarkable. It contains very large epithelial layers with a massive surface area that can be used for vaccine delivery (Kang et al., 2013).NALT embedded in the nasal submucosa, is believed to be the essential inductive site for immune responses to natural infections and nasal vaccinations. NALT is an essential immune component for mucosal and systemic immunity against upper respiratory tract pathogens (Beniova et al., 2014; Kiyono & Fukuyama, 2004). It plays a major role in immune protection against several respiratory infections (Tamura & Kurata, 2004). Studies have shown that tonsils, as part of the mucosal immune system, comprise predominantly B cells (nearly 65% of tonsillar tissue), in addition to approximately 30% CD3+ T cells and roughly 5% macrophages. The T cells are mainly CD4+ (Boyaka et al., 2000; Sada-Ovalle et al., 2012).
New studies have focused on developing mucosal vaccines, such as intranasal vaccines, targeting respiratory tract infections (Lycke, 2012). One study experimentally demonstrated in mice that airway vaccination triggered greater efficacy than routine parenteral administration of the BCG immunisation (Kaveh et al., 2020). A century since we began using BCG, it remains the only clinically permitted TB vaccine, but little is known about BCG-induced protective immunity mechanisms. Thus, investigating the immunological interactions of the BCG vaccine might reveal its link to immunity against TB. Therefore, much research remains to enable a workable design and develop new TB vaccines.
In the last several decades, extensive work has been performed towards developing novel TB vaccine candidates (Schrager et al., 2020). However, most of these vaccines were proposed to be administered parenterally, which provokes weak respiratory mucosal immunity (Jeyanathan et al., 2018). Therefore, designing a novel mucosally administered TB vaccine that mimics the natural route of TB infection is important.
Cell lines are frequently used to investigate the pathogenesis of respiratory viruses. They originate from animals (Kaye, 2006). However, no human mucosally derived cells have been used to investigate immunological interactions of the current authorised BCG vaccine. Thus, this study used a human NALT-derived cell culture model of tonsillar tissue as an alternative to the currently used cell lines to examine the humoral immune response to the BCG vaccine. To develop this cell culture model, we isolated a single-cell suspension from fresh NALT derived from tonsillar tissues and measured the tendency of NALT-derived lymphocytes to produce specific IgG, IgM, and IgA antibodies following BCG vaccine stimulation. We used enzyme-linked immunosorbent assay (ELISA) to detect the induced antibody in cell culture supernatants.
2 Materials and methods
2.1 Inclusion and exclusion chriteria
Patients who experienced snoring or obstructive sleep apnea were included in this study. Patients with recurrent tonsillitis were excluded. Patients with any history of immune suppression were also excluded.
2.2 Tonsillar samples and isolation of tonsillar mononuclear cells
Twenty patients who underwent an elective tonsillectomy at the ear, nose, and throat department at Madinah, Saudi Arabia, were recruited for this study. Signed informed consent was taken from all adult patients and the parents of child patients. Following the operation, the tonsils were immediately retained in HANKS transport medium (Sigma-Aldrich).
Cell suspensions were performed using a previous procedure (Mahallawi & Aljeraisi, 2021a). In brief, tonsils were processed within one hour of surgery. After adding 5 mL of RPMI complete medium (RPMI 1640 medium,Sigma-Aldrich), the tissues were minced and checked completely. The cell suspension was then passed through a 70-μm nylon mesh. MNCs were then isolated using Ficoll-Paque (Premium GE Healthcare, United Kingdom) gradient centrifugation (400 × g for 30 min). The MNCs then washed twice in PBS and resuspended in 5 mL of RPMI complete medium for culture with and without the BCG vaccine.
2.3 BCG vaccine
The BCG vaccine is a live freeze-dried vaccine containing an attenuated strain of Mtb. According to the manufacturer, each 0.1 mL contains between 2 × 105 and 8 × 105 cell-forming units (CFU; Serum Institute of India Pvt. Ltd). After reconstitution, we used serial dilutions to reach the optimal vaccine concentration for cell stimulation and ELISA.
2.4 Cell culture and stimulation for antibody production
Following the isolation of MNCs, we adjusted the cell concentration to 4 × 106 cells/mL. MNCs were then co-cultured in RPMI complete medium after adding the BCG vaccine, and unstimulated MNCs were used as a negative control. Different BCG vaccine concentrations were used to stimulate lymphocytes. We transferred 250 µL of each MNC suspension and cultured them in a sterile 96-well cell culture plate (Costar). We added 10 µL of BCG vaccine per well and the plate was then placed in the incubator in a 5% CO2 atmosphere at 37 °C. Cell culture supernatants were collected after 10 days and stored at − 70 °C until analysis.
2.5 Positive and negative controls
For specificity and sensitivity purposes, we used leftover serum samples from BCG-unvaccinated newborns (n = 7) as negative controls. We also used serum samples from adults (n = 10) who had received the BCG vaccine, taking the presence of scars as a confirmation of having received it.
2.6 Enzyme-linked immunosorbent assay
We used a previously described ELISA method with some modifications (Mahallawi, 2020). In brief, in a 96-well ELISA plate (Costar; Corning, NY, USA), we varied the BCG vaccine coating with different concentrations to reach the optimal concentration. We found that 10 µL/mL was the optimal concentration. Following that, 100 µL/well was added to the plate, which was then covered and kept overnight at 4 °C. The contents of that plate were discarded into a bucket containing 1% Virkon, and the plate was washed five times with washing buffer (PBS containing 0.05% Tween-20; Sigma-Aldrich, St. Louis, MO, USA). The plate was then blocked by adding 150 µL/well of blocking buffer (PBS containing 0.05% fetal bovine serum; Sigma-Aldrich) for 1 h at room temperature. Cell culture supernatant samples were diluted at 1:5, and serum samples were diluted at 1:100 with blocking buffer. Following sample dilutions, 100 µL was added to each well. One well was left without adding any samples and used as a blank. Alkaline phosphatase–conjugated goat anti-human IgG, IgM and IgA (secondary antibodies; Sigma-Aldrich) were prepared at 1:1,000, 1:2,000 and 1:1,000, respectively, and then 100 µL of each was added to all wells. The plate was incubated at room temperature for 30 min, then washed five times with washing buffer. Next, 100 µL/well of p-NPP, (Sigma-Aldrich) was added. The plate was kept in the dark until colour developed. After 30 min, 100 µL of stopping solution (1.2 N sodium hydroxide; Reagecon, UK) was added. The optical density at 450 nm (OD450) was measured. A blank reading was subtracted from all the readings to eliminate the plate background. The sample was defined as positive if the OD450 value was three standard deviations (SDs) above the mean value of the negative controls.
The study was approved by the Taibah University Ethical Committee (IRB No. MLT 2021080).
2.7 Statistical analysis
All calculations and statistical analyses were performed using GraphPad Prism statistical software (version 9, USA). Data are expressed as the mean ± SD. Comparisons between two groups were performed using Student’s t-test, and p < 0.05 was considered significant.
3 Results
3.1 Participant demography
A total of 20 participants were included in the study, of whom 47.6% (n = 12) were adults. The mean age for children was 8.59 ± 3.95 years, and the mean age for adults was 25.0 ± 6.20 years. Overall, 62% of the sample were male.
3.2 Measurment of BCG vaccine-induced mucosal humoral immunity of tonsillar MNCs
To evaluate the tendency of the BCG vaccine to induce a humoral immune response in NALT-derived MNCs, we stimulated the cells with the vaccine. Different vaccine concentrations were used to reach the optimal concentration, which was 10 µL. Additionally, cell culture supernatants were collected and tested using ELISA. As expected, the BCG vaccine was immunogenic to stimulate mucosal immunity in NALT MNCs. This is the first human in vitro cell culture model to be used successfully to evaluate the BCG-induced humoral immune response. Significant (p < 0.001, n = 20) levels of specific, anti-TB IgG, IgM and IgA antibody responses were detected in MNCs stimulated with the BCG vaccine compared with unstimulated MNCs (Fig. 1). Our result supports the future use of this model for further immunological studies using NALT MNCs.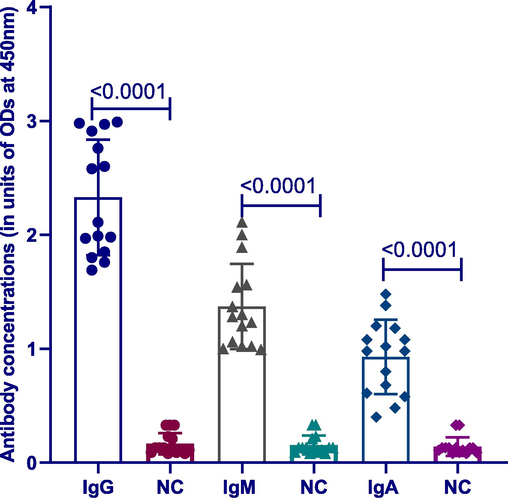
Differences between BCG-induced antibody classes with negative control. Significant differences existed between anti-TB IgG, IgM and IgA antibodies in MNCs stimulated induced by BCG versus unstimulated negative control (NC) (p < 0.0001, n = 20). Lines on the figure represent mean with SD.
3.3 Comparison between mucosally BCG-induced MNCs of different antibody classes
Following the stimulation of tonsillar MNCs, we measured BCG-induced anti-TB IgG, IgM and IgA antibodies using ELISA. BCG induced a strong humoral immune response in the NALT cell culture model. Significant differences were found between anti-TB antibody classes. Anti-TB IgG antibody was significantly higher (mean = 2.33)than anti-TB IgM (mean = 1.37) antibody (p < 0.0001, n = 20). Anti-TB IgM antibody was significantly higher than IgA (mean = 0.93) antibody (p = 0.0018, n = 20; Fig. 2).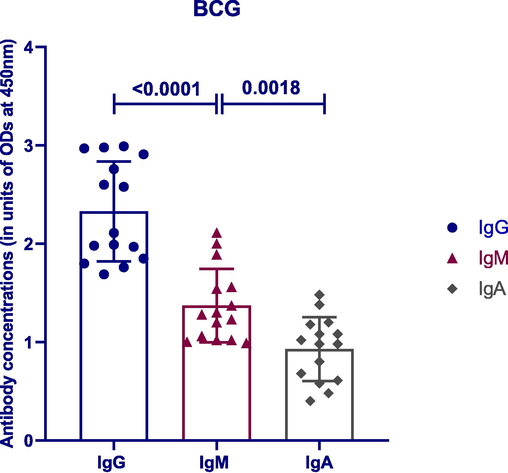
Comparison between BCG-induced antibody classes. Significant differences existed between anti-TB antibody classes. Lines on the figure represent mean with SD.
4 Discussion
Tuberculosis, caused by Mycobacterium tuberculosis, stands a lethal infectious diseas (Goud et al., 2020). The respiratory mucosal surfaces are the primary route of defence against many pathogens. Mucosal immunisation is a greatly favourable approach for vaccination against several pathogens, particularly those entering across the respiratory mucosa, such as Mtb (Troy et al., 2020). A recent study showed an encouraging TB immunisation method consisting of the current BCG parenteral priming followed by intrapulmonary mucosal vaccination with the TB subunit vaccine. It led to the stimulation of local pulmonary-localised, subunit-specific T cells in addition to systemic and lung mucosal IgA responses. This may have significant consequences for the design of mucosal vaccines proposed for safe airway administration (Thakur et al., 2020).
We investigated the suitability of the BCG vaccine to elicit a mucosal humoral immune response in tonsillar MNCs. The human upper respiratory tract comprises multiple lymphoid tissues, including draining lymph nodes and NALT (Pizzolla et al., 2017). Therefore, our investigation exploring the BCG vaccine’s immunogenicity at this mucosal immune compartment is of great interest. Intranasal vaccines primarily mimic the natural route of infection of respiratory pathogens. This is a much more appropriate route of vaccination against respiratory viruses, such as influenza and SARS-CoV-2 (Shim et al., 2020; Takaki et al., 2018).
Our study showed that the BCG vaccine induced a strong mucosal humoral immune response in the NALT model. We found significant differences between anti-TB antibody classes. The anti-TB IgG antibody level was significantly higher (mean = 2.33) than anti-TB IgM (mean = 1.37) antibody (p < 0.0001). Anti-TB IgM antibody was significantly higher than IgA (mean = 1.37 vs 0.93) antibody (p = 0.0018). Our findings emphasise that this human in vitro cell culture model was successfully established to investigate BCG immunogenicity to provoke different anti-TB antibody classes following stimulation.
The role of the humoral immune system in TB control is unclear. In humans, Mtb infection provokes Mtb-specific IgA and IgG antibodies in bronchoalveolar lavage fluid, although their exact role and degree of interaction with the infection remain undiscovered, especially at the level of mucosal immunity (Morrison & McShane, 2021). Therefore, vaccine delivery via respiratory paths has been revealed to provoke strong mucosal immune mechanisms that can be associated across species. It has shown increased antigen-specific cellular and humoral immune responses compared with peripheral blood delivery (Riste et al., 2021).
One study revealed that a B-cell deficit results in decreased recruitment of neutrophils, macrophages and CD8+ T cells to the lungs as a result of TB infection, proposing that B cells might impact the cellular structure here by controlling chemokines and adhesion molecules (Bosio et al., 2000; Troy et al., 2020). Additionally, a recent study verified that B-cell-deficient mice showed greater vulnerability to TB infection and worse disease. Early B-cell reduction in infected non-human primates modifies T-cell cytokine responses and results in increased bacterial loads in the lungs (Linge et al., 2023).
Intranasal vaccination is reportedly a successful way to stimulate the respiratory immune system (Chin-ichi Tamura 2004). Studies have revealed that many B cells exist in the tonsils (Passàli et al., 2003). In addition, CD4+ cells are a major constituent of tonsillar tissues, whereas smaller proportions of CD8+ cells are found here (Bernstein et al., 1999). Moreover, mucosal vaccination has been presented to induce humoral as well as cell-mediated antigen-specific immune responses. As an extra advantage, nasal vaccination needs a smaller antigen volume to stimulate antigen-specific mucosal and systemic immune responses compared to that used in parenterally administered vaccines (Gwinn et al., 2010).
Our finding of higher anti-TB IgG antibody levels compared with IgM and IgA isotypes is aligned with an earlier report that found B cells of the IgG isotype were predominant in tonsillar tissues, while B cells of the IgM and IgA isotypes were less common (Edwards et al., 1986). In our previous work, we showed that MNCs stimulated with SARS-CoV-2 spike protein primed a strong memory B cell–mediated immune response in NALT from individuals previously exposed to the virus (Mahallawi & Aljeraisi, 2021b). Thus, local mucosal sites such as tonsils retain a massive memory immune response that is triggered upon re-exposure via infection or vaccination. Therefore, relying on the promising mucosal vaccination for TB would be beneficial for ease of use and prolonged immunity.
To the best of our knowledge, this is the first study to investigate the suitability of human NALT-derived MNCs as an in vitro human cell culture model to explore the mucosal humoral immune response to the BCG vaccine. Therefore, the current study would contribute and add lots for researchers to deeply investigate other immune response such as cellular immunity to BCG using this model. Moreover, it could be useful to perform some functional antibody studies on the BCG-derived antibody.
Our study has several limitations. First, we did not examine the cellular immune response following BCG vaccine stimulation. Additionally, we did not test the BCG vaccine with added adjuvants, such as CpG, which have been shown in previous studies to induce a stronger and more prolonged immune response (Aichinger et al., 2011). Consequently, all our limitations could be future recommendations for further investigations.
5 Conclusions
This study highlights the significance of producing immune responses in the confined mucosal setting using the BCG vaccine. Moreover, it presents the immunological interaction in NALT-derived MNCs, which may have implications for future vaccine investigation.
Acknowledgment
Appreciation to the Deputyship for the Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number (IFKSURG-2-435).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adjuvating the adjuvant: facilitated delivery of an immunomodulatory oligonucleotide to TLR9 by a cationic antimicrobial peptide in dendritic cells. Vaccine. 2011;29(3):426-436.
- [CrossRef] [Google Scholar]
- A child with complicated Mycobacterium tuberculosis. Int. J. Pediatrics Adolescent Med.. 2016;3(1):28-33.
- [CrossRef] [Google Scholar]
- Specific clinical and immunological features of chronic diseases of the nasal-associated lymphoid tissue in the children. Vestn Otorinolaringol. 2014;4:36-38.
- [Google Scholar]
- Bernstein, J.M., Gorfien, J., Brandtzaeg, P., 1999. The Immunobiology of the Tonsils and Adenoids. In [Book].
- Infection of B cell-deficient mice with CDC 1551, a clinical isolate of Mycobacterium tuberculosis: delay in dissemination and development of lung pathology. J Immunol. 2000;164(12):6417-6425.
- [CrossRef] [Google Scholar]
- Human nasopharyngeal-associated lymphoreticular tissues. Functional analysis of subepithelial and intraepithelial B and T cells from adenoids and tonsils. Am. J. Pathol.. 2000;157(6):2023-2035.
- [CrossRef] [Google Scholar]
- Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Japanese J. Infectiouse Dis.. 2004;57
- [Google Scholar]
- Human adenoid organ culture: a model to study the interaction of influenza A with human nasopharyngeal mucosa [Research Support, Non-U.S. Gov't] J Infect Dis.. 1986;153(1):41-47.
- [Google Scholar]
- Molecular detection of Mycobacterium tuberculosis in pulmonary and extrapulmonary samples in a hospital-based study. Afr. Health Sci.. 2020;20(4):1617-1623.
- [CrossRef] [Google Scholar]
- Effective induction of protective systemic immunity with nasally administered vaccines adjuvanted with IL-1 [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] Vaccine. 2010;28(42):6901-6914.
- [CrossRef] [Google Scholar]
- The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152.
- [CrossRef] [Google Scholar]
- New tuberculosis vaccine strategies: taking aim at un-natural immunity. Trends Immunol.. 2018;39(5):419-433.
- [CrossRef] [Google Scholar]
- Characteristics of nasal-associated lymphoid tissue (NALT) and nasal absorption capacity in chicken. PLoS One. 2013;8(12):e84097.
- [CrossRef] [Google Scholar]
- Airway delivery of both a BCG prime and adenoviral boost drives CD4 and CD8 T cells into the lung tissue parenchyma. Sci. Rep.. 2020;10(1):18703.
- [CrossRef] [Google Scholar]
- SARS-associated coronavirus replication in cell lines. Emerg. Infect Dis.. 2006;12(1):128-133.
- [CrossRef] [Google Scholar]
- NALT- versus Peyer's-patch-mediated mucosal immunity. Nat. Rev. Immunol. 2004;4(9):699-710.
- [CrossRef] [Google Scholar]
- Prolonged B-lymphocyte-mediated immune and inflammatory responses to tuberculosis infection in the lungs of TB-resistant mice. Int. J. Mol. Sci.. 2023;24(2)
- [CrossRef] [Google Scholar]
- Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol.. 2012;12(8):592-605.
- [CrossRef] [Google Scholar]
- A serological assay to detect human SARS-CoV-2 antibodies. J. Taibah Univ. Med. Sci. 2020
- [CrossRef] [Google Scholar]
- In vitro cell culture model of human nasal-associated lymphoid tissue (NALT) to evaluate the humoral immune response to SARS-CoV-2 spike proteins. Saudi J. Biol. Sci.. 2021;28(8):4516-4521.
- [CrossRef] [Google Scholar]
- Infection with SARS-CoV-2 primes immunological memory in human nasal-associated lymphoid tissue. Clin. Immunol.. 2021;231:108850
- [CrossRef] [Google Scholar]
- Local pulmonary immunological biomarkers in tuberculosis. Front. Immunol.. 2021;12:640916
- [CrossRef] [Google Scholar]
- Recurrent and chronic inflammations of Waldeyer's ring in childhood: infectious, structural and immunological features. Int. Congr. Ser.. 2003;1257:239-252.
- [CrossRef] [Google Scholar]
- Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc. Natl. Acad. Sci. U.S.A.. 2017;114(20):5225-5230.
- [CrossRef] [Google Scholar]
- Phase I trial evaluating the safety and immunogenicity of candidate TB Vaccine MVA85A, delivered by aerosol to healthy M.tb-Infected Adults. Vaccines (Basel). 2021;9(4)
- [CrossRef] [Google Scholar]
- An update on vaccines for tuberculosis - there is more to it than just waning of BCG efficacy with time. Expert Opin. Biol. Ther.. 2012;12(12):1601-1610.
- [CrossRef] [Google Scholar]
- Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin. Exp. Immunol.. 2012;168(2):200-206.
- [CrossRef] [Google Scholar]
- The status of tuberculosis vaccine development. Lancet Infect. Dis.. 2020;20(3):e28-e37.
- [CrossRef] [Google Scholar]
- Induction of systemic immunity through nasal-associated lymphoid tissue (NALT) of mice intranasally immunized with Brucella abortus malate dehydrogenase-loaded chitosan nanoparticles. PLoS One. 2020;15(2):e0228463.
- [Google Scholar]
- Mucosal immune response in nasal-associated lymphoid tissue upon intranasal administration by adjuvants. J. Innate Immun.. 2018;10(5–6):515-521.
- [CrossRef] [Google Scholar]
- Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect Dis.. 2004;57(6):236-247.
- [Google Scholar]
- Intrapulmonary (i.pulmon.) Pull Immunization With the Tuberculosis Subunit Vaccine Candidate H56/CAF01 After Intramuscular (i.m.) Priming Elicits a Distinct Innate Myeloid Response and Activation of Antigen-Presenting Cells Than i.m. or i.pulmon. Prime Immunization Alone. Front. Immunol.. 2020;11:803.
- [CrossRef] [Google Scholar]
- Pulmonary mucosal immunity mediated through CpG provides adequate protection against pulmonary Mycobacterium tuberculosis infection in the mouse model. A role for type I interferon. Tuberculosis (Edinb). 2020;123:101949
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102773.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







