Translate this page into:
Evaluation of possible palliative role of tamarixetin against cisplatin-induced renal toxicity by modulation of oxidative stress, inflammation and apoptosis in rats
⁎Corresponding author. drhaseebanwar@gcuf.edu.pk (Haseeb Anwar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cisplatin (CP) is a top-notch anti-cancerous agent that is used during the treatments of various types of tumors. Tamarixetin (TM) is a naturally occurring polyphenolic compound with versatile therapeutic and pharmacological abilities. The current investigation was purposed to elucidate the antagonistic effects of TM against CP-prompted renal intoxication. Sprague Dawley rats (n = 48) were separated into 4 equal groups i.e., Group 1st was designated as control group while the 2nd group was treated with CP (10 mg/kg) only, group 3rd received CP (10 mg/kg) + TM (50 mg/kg) and designated as a co-treated group while group 4th was administered with TM (50 mg/kg) only. Our results revealed that treatment of CP reduced the activity of catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione-disulfide reductase (GSR), glutathione S-transferase (GST) as well as glutathione (GSH) while elevate ROS and MDA levels. CP administration raised the level of urea, creatinine, KIM1 along with NGAL while significant reduction in creatinine clearance. Whereas, CP treatment substantially elevated the level of caspase-3, caspase- 9 and Bcl-2 associated X protein (Bax) while reducing the level of B cell lymphoma protein 2 (Bcl-2). CP administration significantly elevated the concentration of nuclear factor kappa-B (NF-kB), interleukin 6 (IL-6), interleukin 1 beta (IL-1β) as well as tumor necrosis factor α (TNF-α), and instigated histopathological damages in renal tissues. However, Co-treatment of CP + TM showed palliative effects against CP-induced impairments. The current study manifested that TM is a potential flavonoid that could be used as a therapeutic drug to ameliorate renal damages instigated by CP.
Keywords
Cisplatin
Tarmarixetin
Renal damages
Inflammation
Oxidative stress
1 Introduction
Cancer is one of the most prevalent cause of deaths across the globe while cisplatin (CP) is a potent chemotherapeutic agent used to treat various kinds of malignancies (Ijaz et al., 2020). Its therapeutic and pharmaceutical applications are restricted, due to the significant adverse effects during treatment of cancer, that include hair fall, diarrhea, anxiety, inflammatory disorders, neurodegeneration, ototoxicity, gastric toxicity, liver toxicity, and renal toxicity (Li et al., 2018). Cisplatin is reported to cause mitochondrial dysfunction in male Sprague Dawley rats (Ijaz et al., 2021). Oxidative stress induced lipid peroxidation which disrupts the integrity of intracellular as well as cellular membranes (Talas et al., 2009).
Among aforementioned toxicities, renal toxicity is the most prevalent form of toxicity following the treatment with cisplatin (Marcato et al., 2014). Triggering of vascular renal constriction, reduction in glomerular filtration rate (GFR), decline in the blood flow towards renal tissues, elevation in creatinine level, and drop in blood magnesium and potassium concentrations are all pathophysiological manifestations of cisplatin-induced renal damage (Pabla and Dong, 2008). Occurrence of renal toxicity is still significant despite the fact that diuretics and sufficient hydration of individuals can prevent (Oh et al., 2014).
Natural products are reported to decrease oxidative stress as well as hypertensive effects (Selamoğlu et al., 2015). Plants are considered as a prime source of medicines (Regginato et al., 2020), however flavonoids (plants derivatives) are the potential therapeutic compounds that exerts significant curative role against several xenobiotics (Omar et al., 2020). Tamarixetin is a flavonoid, known as methylated quercetin with tetra-hydroxy structural configuration (Parajuli et al., 2018). It has been documented that tamarixetin showed anti-tumorous (Nicolini et al., 2014), anti-inflammatory (Park et al., 2018) as well as cardioprotective activities (Hayamizu et al., 2018). However, evidences regarding tamarixetin’s curative effects against xenobiotics instigated renal damages are not much reported. Therefore, this study was proposed to investigate the mitigative action of tamarixetin to antagonize CP prompted kidney damages. 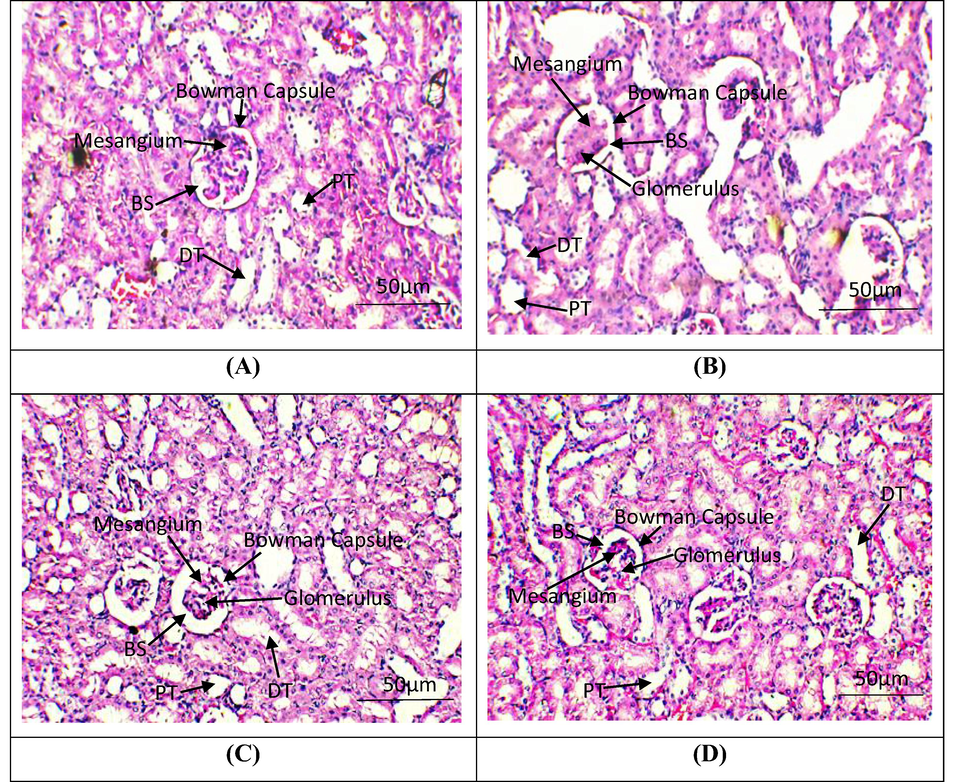
Histopathological examination of renal tissues. H&E stain; 40X (A) Group I; Normal glomeruli and tubules (B) Cisplatin intoxicated group; necrosis in glomeruli, tubular dilation and vacuolization of tubular epithelial cells (C) Cisplatin + Tamarixetin Co-administrated group; normal glomeruli, rare atrophic tubules in cortex (D) Tamarixetin supplemented group; Glomeruli and tubules seem normal. BS, Bowman Space; PT, Proximal convoluted tubules; DT, Distil convoluted tubules.
2 Materials and methods
2.1 Chemicals
Cisplatin & Tamarixetin were bought from Sigma-Aldrich (Germany).
2.2 Animals
Albino rats with 200 ± 20 g body weight were accommodated in animal research station at University of Agriculture Faisalabad. Both the temperatures as well as moisture were kept at 22 ± 1 °C and 40–60% respectively as well as 12 h dark/light period were sustained. Rats were also facilitated with standard food and tap water and study was approved by institutional ethical committee.
2.3 Experimental design
A dose of CP (10 mg/kg) was administrated being mixed in normal saline solution. Tamarixetin was administered orally to the rats for thirty days. Rats (n = 48) were apportioned into 4 groups of 12 each by following the sequence as 1st group (untreated) was nominated as control group. Group 2nd was intoxicated with only CP (10 mg/kg) on 1st day of the experiment (Bishr et al., 2019). Group 3rd was given the combine dose of CP (10 mg/kg) and tamarixetin (50 mg/kg) during the experiment. Group 4th was given only tamarixetin (50 mg/kg bw) by mixing in normal saline. The whole dose regimen was followed for seven days and on the eighth day rats were decapitated to collect blood sample by utilizing (retro-orbital venous plexus) after completion of experiment. One of the kidneys was preserved in solution of 10% formalin and other was collected in plastic bag for biochemical assays while stored at −80 °C.
2.4 Biochemical analysis of antioxidant enzymes
The method demonstrated by Aebi (1984) was employed to evaluate the activity of CAT. SOD activity was evaluated by the protocol illustrated by Kakkar et al. (1984). Lawrence & Burk method was used to determine the activity of GPx (Lawrence and Burk, 1976). Moron et al. (1979) methodology was used to assess the level of GSH. The procedure outlined by Younis et al. (2016) was compiled in order to determine activity of GST. The protocol explained by Carlberg and Mannervik (1975) employed to evaluate GSR activity.
2.5 Analysis of ROS and MDA
Level of MDA was assessed via the protocols outlined by Ohkawa et al., 1979. The approach proposed by Hayashi et al. 2007 was used to determine the level of ROS.
2.6 Evaluation of kidney markers
Levels of urea, creatinine as well as creatinine clearance were evaluated by employing Standard Randox laboratory kits (County Antrim, UK).
2.7 Determination of KIM1 and NGAL
KIM1 and NGAL levels were evaluated by employing Quantikine ELISA kits manufactured by R & D system, Co-Ltd. Changning, China.
2.8 Analysis of apoptotic markers
Concentrations Apoptotic markers (Caspase-3, Caspase-9, Bax as well as Bcl-2) were analysed by standard ELISA lab kits from Cusabio technology LLC in Houston, TX, in compliance to the instructions of manufacturers.
2.9 Evaluation of inflammatory markers
Inflammatory markers (TNF-α, IL-6, NF-kB, IL-1β levels and COX-2) were assessed by standard ELISA lab kits from Shanghai-YL-Biotech Co. Ltd. as per the instructions of manufacturer.
2.10 Histopathological assessment
A solution of 20% formaldehyde was used to fix the samples. Following the fixation, samples were fixed in blocks after being paraffin-embedded. Slides were prepared by fixing thin pieces (3–4 mm) of sample, Haemotoxylin was used to stained the sample and analyse at 40x under the light microscope.
2.11 Statistical analysis
Values (results) were displayed as Mean + SEM. ANOVA (one way), to compare the groups Tukey’s test were employed by Minitab software while P < 0.05 was set as the level of significance.
3 Results
3.1 Protective effects of tamarixetin on the activity of antioxidant enzymes
Results depicted (Table 1) that administration of only CP remarkably (P < 0.05) lowered the activity of anti-oxidant enzymes (CAT, GSR, SOD, GPx, GST) as well as level of GSH, in contrary to the untreated group. Combined administration of CP + TM substantially increased the level of antioxidants as compare to only CP administrated group. Administration of TM only demonstrated antioxidant enzymes activity as in the untreated group. Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
CAT (U/mg protein)
GPx (U/mg protein)
SOD (U/mg protein)
GSR (U/mg protein)
GST (U/mg protein)
GSH (mg/dl)
Control
8.86 ± 0.64a
19.70 ± 0.59a
7.22 ± 0.22a
5.39 ± 0.32a
25.92 ± 1.23a
16.25 ± 1.55a
CP
4.72 ± 0.33b
7.70 ± 0.82c
3.43 ± 0.22c
1.93 ± 0.18c
9.64 ± 0.85c
6.06 ± 0.54b
CP + TM
7.87 ± 0.29a
14.37 ± 1.07b
5.17 ± 0.32b
3.61 ± 0.27b
21.61 ± 0.87b
13.36 ± 0.88a
TM
8.89 ± 0.69a
19.76 ± 0.94a
7.24 ± 0.24a
5.43 ± 0.33a
26.51 ± 1.90a
16.49 ± 2.11a
3.2 Protective effects of tamarixetin on ROS and MDA levels
Kidneys of CP intoxicated group exhibited significant upsurge (P < 0.05) in the levels of ROS and MDA in contrary to untreated group. Administration of CP + TM lowered the level of ROS and MDA. However, treatment of only tamarixetin, maintained the ROS as well as MDA levels close to the values of control group (Table 2). Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
ROS (μmol/g tissue)
MDA (nmol/g tissue)
Control
1.37 ± 0.16c
0.78 ± 0.10c
CP
7.98 ± 0.61a
2.99 ± 0.12a
CP + TM
2.47 ± 0.42b
1.14 ± 0.11b
TM
1.35 ± 0.16c
0.73 ± 0.11c
3.3 Palliative effects of tamarixetin on kidney function markers
CP administrated group showed noticeable (P < 0.05) increased in the levels of urea, creatinine, on the other hand the level of creatinine clearance was decreased. Co-treatment of CP + TM lowered the levels of urea and creatinine while raised the serum creatinine clearance as compare to CP treated rats. Administration of TM only showed serum values remained similar as in untreated group (Table 3). Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
Urea (mg/dl)
Creatinine (mg/dl)
Creatinine Clearance (ml/min)
Control
14.27 ± 1.49c
1.29 ± 0.06c
1.75 ± 0.12a
CP
39.96 ± 3.90a
3.93 ± 0.21a
0.37 ± 0.08c
CP + TM
22.78 ± 1.33b
2.19 ± 0.170b
1.02 ± 0.11b
TM
14.11 ± 1.11c
1.28 ± 0.05c
1.77 ± 0.14a
3.4 Palliative effects of tamarixetin on renal function markers
Only CP administrated group showed considerable (P < 0.05) increase in the levels of KIM1 as well as NGAL in contrast with control group. The group administrated with CP + TM showed reduction in aforementioned markers. However, administration of TM only maintained the levels of both above mentioned cytokines as compare to untreated group (Table 4). Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
KIM-1 (mg/ml)
NGAL (ng/day)
Control
0.26 ± 0.05c
0.55 ± 0.07c
CP
1.81 ± 0.10a
2.40 ± 0.16a
CP + TM
1.10 ± 0.07b
1.01 ± 0.12b
TM
0.24 ± 0.06c
0.53 ± 0.07c
3.5 Palliative effects of tamarixetin on Caspase-3, Caspase-9, Bax and Bcl-2
Results presented (Table 5) elaborates that only CP treated group manifested remarkable (P < 0.05) elevation in caspase-3, Bax as well as caspase-9, whereas reduced Bcl-2 level in contrary to control group. Combined administration of CP + TM decreased the concentration of aforementioned pro-apoptotic markers while enhanced level of Bcl-2. Treatment of TM only kept the same levels of all these markers as in the untreated group (Table 5). Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
Caspase-3 (pg/g tissue)
Bax (pg/g tissue)
Bcl-2 (ng/g tissue)
Caspase-9 (pg/g tissue)
Control
1.33 ± 0.09c
2.47 ± 0.11c
18.05 ± 1.32a
3.34 ± 0.20c
CP
13.94 ± 0.82a
7.85 ± 0.73a
4.50 ± 0.50c
19.91 ± 1.71a
CP + TM
3.29 ± 0.23b
4.06 ± 0.09b
11.69 ± 1.45b
9.84 ± 1.25b
TM
1.28 ± 0.13c
2.43 ± 0.10c
17.36 ± 1.60a
3.30 ± 0.20c
3.6 Palliative effects of tamarixetin on inflammatory markers
Administration of CP notably (p < 0.05) increased the activity of inflammatory cytokines (TNF-α, IL-6, NF-kB, IL-1β levels and COX-2). Combined treatment of CP + TM noticeably (P < 0.05) lowered the levels of above-mentioned inflammatory cytokines. TM administrated group showed no significant difference from the values of untreated group (Table 6). Values exhibiting dissimilar superscripts vary substantially from other groups.
Groups
NFkB (ng/g tissue)
IL-1β (ng/g tissue)
TNF-α (ng/g tissue)
COX-2 (ng/g tissue)
IL-16 (ng/g tissue)
Control
15.83 ± 1.95c
29.68 ± 2.54c
6.59 ± 0.38c
23.91 ± 2.67c
7.76 ± 0.77c
CP
73.41 ± 2.15a
88.80 ± 3.51a
23.83 ± 2.82a
78.04 ± 4.18a
25.31 ± 2.19a
CP + TM
29.74 ± 3.18b
41.20 ± 4.00b
13.44 ± 2.03b
33.84 ± 2.71b
11.48 ± 0.84b
TM
15.63 ± 1.75c
29.41 ± 2.33c
6.53 ± 0.36c
23.51 ± 3.10c
7.71 ± 0.75c
3.7 Palliative effect of tamarixetin on renal tissues histology
Histopathological evaluations revealed that control group and TM treated group exhibited normal histology of renal tissues. Structure of glomeruli, distal as well as convoluted tubules and Bowman’s capsule was normal in these groups. However, CP treated group exhibited histopathological irregularities in parenchyma cells, reduction in glomerular filtration rate, appearance of cortical segments on Malpighian tubules, reduction in reabsorption from proximal/distal convoluted tubules and collecting duct and accumulation of necrotic cells. Co-treated group showed significant protection of renal tissues against CP induced renal damages (Fig. 1).
4 Discussion
In animals, kidneys are vital organs for the removal of metabolic waste from the body (Jensen-Jarolim et al., 2013). Reactive oxygen species (ROS) and oxidative stress (OS) are regarded to be the main causes of several lethal disease in living system including acute kidney injury (Dennis and Witting, 2017). The antioxidant enzymes (CAT, GPx, SOD, GSR, & GST) activity and level of GSH was decreased while level of ROS and MDA increased after CP treatment. It is revealed that generation of ROS induces oxidative stress which ultimately elevates the level of MDA (Talas et al., 2014). An investigation designed by Widowati et al. (2022) elaborated that oxidative stress is the core factor behind nephrotoxicity. In our study, Co-treatment of CP + TM enhanced the antioxidant enzymes activity as well as diminished the ROS & MDA concentrations in kidney due to its polyphenolic structural configuration. Our results are in accordance with the investigation executed by Fan et al. 2019, who documented that administration of TM showed palliative action against cardiac hypertrophy by decreasing ROS production, thus showing its anti-oxidative ability.
Urea, creatine as well as creatinine clearance are designated kidney function markers which are by-product of metabolism. Levels of Urea and creatinine were noticeably elevated as a result of intoxication caused by CP treatment, while creatinine clearance was significantly decreased. Renal intoxication caused by CP is characterized by considerably impaired renal activity, shown by raised serum creatinine & concentration of urea in the blood (Farooqui et al., 2017). In addition, elevated concentration of blood urea & serum creatinine following decreased in the level of creatinine clearance are indicators of severe oxidative renal damages (Khan et al., 2010). Our investigation is in compliance with the experimented intended by Ijaz et al. (2021) who demonstrated that CP intoxication disrupts the urine & serum profile. However, our examination revealed the reno-protective role of tamarixetin by regulating urine and serum profile.
NGAL & KIM1 concentration in (CP treated rats) were increased substantially. These are most prominent indicators behind acute kidney injury (Lei et al., 2018). After damage to kidney, NGAL is frequently released into the blood stream and causes damages to other parts of body and eliminates via urine (Yim, 2015). Levels KIM1 and NGAL in kidney tissues were noticeably elevated when exposed to cisplatin (platinum-based medicines) instigating sever renal damages (Abdelsalam et al., 2018). Our investigations revealed that co-treated group (CP + Tamarixetin) maintained the concentration of KIM1 & NGAL in the renal tissues which previously endorsed by the study of shin et al. (2015) while the group which was treated with only tamarixetin showed marvellous curative properties by diminishing the levels aforesaid markers.
Current study revealed that CP administration escalated pro-apoptotic (Bax, caspase-9 & caspase-3) marker’s concentration while lowered the concentration of Bcl-2 (anti-apoptotic protein). Moreover, cytochrome C transfer from the mitochondrial membrane to the cytoplasmic matrix is prompted by decreased level of Bcl-2 & higher concentration of Bax which in turn stimulate the activation of caspase-3 and caspase-9 which led towards acute apoptotic response (Katiyar et al., 2005). Therefore, TM administration attenuated damages due to considerable regulations in aforesaid irregularities which is near to the findings of Lei et al. (2015).
In the existing investigation, administration of CP showed increased concentration of the inflammatory cytokines activity in renal tissues. CP administration significantly increased levels of TNF-α, IL-6, NF-kB, IL-1β, and COX-2 which ultimately showed acute inflammatory responses (Rehman et al., 2014). It is documented that natural compounds have ability to degrade I‐κB which is the inhibitor of NF‐κB ultimately reduce oxidative stress owing to its antioxidative activity (Salmas et al., 2017). Treatment of CP + tamarixetin remarkably decrease the concentration of above-mentioned markers. These results validated the anti-inflammatory role of TM as previously investigated during in-vitro and in-vivo analysis by Kaleemann et al. (2011).
According to histopathological evaluations, the control and TM (only) treated group showed normal histology of renal tissues while CP treated group showed, tubular dilatation, inflammation of epithelial cells, peritubular infiltration, and tubular necrosis. Acute kidney injury prompted by CP has remarkable inflammatory influence on renal tissues, proximal tubular damages, oxidative stress, and vascular injuries (Ozkok and Edelstein, 2014). However, in the co-treated group, tamarixetin prevented all the aforementioned renal damages.
5 Conclusion
The current investigation demonstrated that tamarixetin have ability to protect the kidneys against CP-induced damages. These findings demonstrated that tamarixetin showed protective effect against oxidative stress and apoptosis, which are the two pivotal contributors in CP-induced kidney injury. The disruption in the degree of antioxidants, renal damage biomarkers, apoptotic markers and inflammatory cytokines as well as histopathological impairments was successfully prevented by tamarixetin administration. Therefore, reno-protective capability of tamarixetin could be attributed to its anti-oxidant as well as anti-apoptotic potential.
Acknowledgement
This work was funded by Researchers Supporting Project number (RSP2023R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Urinary biomarkers for early detection of platinum-based drugs induced nephrotoxicity. BMC Nephrol.. 2018;19:1-8.
- [Google Scholar]
- Ambroxol attenuates cisplatin-induced hepatotoxicity and nephrotoxicity via inhibition of p-JNK/p-ERK. Can. J. Physiol. Pharmacol.. 2019;97:55-64.
- [Google Scholar]
- Tamarixetin protects against cardiac hypertrophy via inhibiting NFAT and AKT pathway. J. Mol. Histol.. 2019;50:343-354.
- [Google Scholar]
- Protective effect of Nigella sativa oil on cisplatin induced nephrotoxicity and oxidative damage in rat kidney. Biomed. Pharmacother.. 2017;85:7-15.
- [Google Scholar]
- Cardiotonic actions of quercetin and its metabolite tamarixetin through a digitalis-like enhancement of Ca2+ transients. Arch. Biochem. Biophys.. 2018;637:40-47.
- [Google Scholar]
- Cardiotonic actions of quercetin and its metabolite tamarixetin through a digitalis-like enhancement of Ca2+ transients. Arch. Biochem. Biophys.. 2018;637:40-47.
- [Google Scholar]
- Casticin Alleviates Testicular and Spermatological Damage Induced by Cisplatin in Rats. Pak. Vet. J.. 2020;40:234-238.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res.. 2007;631:55-61.
- [Google Scholar]
- Orientin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. Pak. Vet. J. 2021;41:574-578.
- [Google Scholar]
- Ameliorative effects of morin on cisplatin-induced toxicity in renal mitochondria isolated from rats. J. King Saud Univ. Sci.. 2021;33
- [CrossRef] [Google Scholar]
- Volume and clearance: kidneys and excretory systems. In: Comparative Medicine: Anatomy and Physiology Vienna. Vienna: Springer; 2013. p. :171-177.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Silymarin induces apoptosis primarily through a p53-dependent pathway involving Bcl-2/Bax, cytochrome c release, and caspase activation. Mol. Cancer Ther.. 2005;4:207-216.
- [Google Scholar]
- Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol.. 2010;48:2469-2476.
- [Google Scholar]
- Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis. 2011;218:44-52.
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [Google Scholar]
- Neuroprotective effects of quercetin in a mouse model of brain ischemic/reperfusion injury via anti–apoptotic mechanisms based on the Akt pathway. Mol. Med. Rep.. 2015;12:3688-3696.
- [Google Scholar]
- Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep.. 2018;8:1-9.
- [Google Scholar]
- Xanthohumol attenuates cisplatin-induced nephrotoxicity through inhibiting NF-κB and activating Nrf2 signaling pathways. Int. Immunopharmacol.. 2018;61:277-282.
- [Google Scholar]
- Cisplatin properties in a nanobiotechnological approach to cancer: A mini-review. Curr. Cancer Drug Targets.. 2014;14:458-476.
- [Google Scholar]
- Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lungs and liver. Biochim. Biophys. Acta.. 1979;582:67-71.
- [Google Scholar]
- Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells. Mol. Carcinog.. 2014;53:939-950.
- [Google Scholar]
- Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolyte Blood Press.. 2014;12:55-65.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- In vitro haemostatic efficacy of aqueous, methanol and ethanol plant extracts of three medicinal plant species in Palestine. Braz. J. Biol.. 2020;80:763-768.
- [Google Scholar]
- Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int.. 2014;2014
- [CrossRef] [Google Scholar]
- Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int.. 2008;73:994-1007.
- [Google Scholar]
- Regiospecific biosynthesis of tamarixetin derivatives in Escherichia coli. Biochem. Eng. J.. 2018;133:113-121.
- [Google Scholar]
- Tamarixetin exhibits anti-inflammatory activity and prevents bacterial sepsis by increasing IL-10 production. J. Nat. Prod.. 2018;81:1435-1443.
- [Google Scholar]
- Antidiabetic and hypolipidemic potential of Campomanesia xanthocarpa seed extract obtained by supercritical CO2. Braz. J. Biol.. 2020;81:621-631.
- [Google Scholar]
- Alleviation of hepatic injury by chrysin in cisplatin administered rats: probable role of oxidative and inflammatory markers. Pharmacol. Rep.. 2014;66:1050-1059.
- [Google Scholar]
- Effects of propolis, caffeic acid phenethyl ester, and pollen on renal injury in hypertensive rat: An experimental and theoretical approach. CellBiochem.. 2017;35:304-314.
- [Google Scholar]
- Protective effects of quercetin against HgCl2-induced nephrotoxicity in Sprague-Dawley rats. J. Med. Food.. 2015;18:524-534.
- [Google Scholar]
- Antioxidative effects of novel synthetic organoselenium compound in rat lung and kidney. Ecotoxicol. Environ. Saf.. 2009;72:916-921.
- [Google Scholar]
- Role of propolis on biochemical parameters in kidney and heart tissues against L-NAME induced oxidative injury in rats. Clin. Exp. Hypertens. 2014;36 492-496.salmas
- [Google Scholar]
- Protective Effect of Ethanolic Extract of Jati Belanda (Guazuma ulmifolia L.) by Inhibiting Oxidative Stress and Inflammatory Processes in Cisplatin-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2022;42:376-382.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin and kidney diseases. Child. Kidney Dis.. 2015;19:79-88.
- [Google Scholar]
- Protective effects of Fraxinus xanthoxyloides (wall.) leaves against CCl4 induced hepatic toxicity in rat. BMC Comp. Alter. Med.. 2016;16:407-420.
- [Google Scholar]







