Translate this page into:
Synergistic antibacterial activity of biogenic AgNPs with antibiotics against multidrug resistant bacterial strains
⁎Corresponding authors at: Botany and Microbiology Department, Faculty of Science, Benha University, Benha 13511, Egypt. sara.m.elsayed@gmail.com (Sara Mohamed), myassin2.c@ksu.edu.sa (Mohamed Taha Yassin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The prevalence of hospital-acquired infections caused by drug resistant bacterial pathogens is a major health risk, leading to a considerable number of deaths and illnesses globally. Therefore, it is essential to develop new combinations of antimicrobial agents to effectively manage drug-resistant bacteria that are responsible for nosocomial infections. A current study aims to synthesize silver nanoparticles (AgNPs) from the seeds of Trigonella foenum-graecum (fenugreek). The AgNPs exhibited a spherical morphology and had an average particle size of 29.75 nm. In addition, XRD examination verified the presence of a face-centered cubic (fcc) lattice structure in the biosynthesized AgNPs, while FTIR study demonstrated the existence of several functional groups such as phenols, alkanes, and amines. The biogenic AgNPs exhibited the most potent antibacterial effect against E. coli strain, with relative inhibitory zone of 18.43 ± 0.35 mm and a minimum inhibitory concentration (MIC) of 50 µg/ml. In addition, the most potent antibacterial effect of AgNPs combined with colistin was observed against Acinetobacter baumannii strain, while the greatest combined efficacy of AgNPs with norfloxacin was found against Pseudomonas aeruginosa, resulting in a relative increase in the fold of inhibition area (IFA) of 0.53 and 0.35, respectively. In conclusion, the potent antibacterial and synergistic effect of AgNPs with antibiotics highlights their potential application of this combination in controlling nosocomial infections in health care settings.
Keywords
Fenugreek
Seed extract
Drug resistance
Antibacterial
Synergism
Colistin
Norfloxacin
1 Introduction
Nanotechnology involves the crafting, synthesis, and manipulation of materials on a nanoscale to attain distinct properties that can be effectively utilized for specific applications (Bora et al., 2024). Recently, a broad spectrum of applications and emerging disciplines across various fields, including optics, chemical industries, mechanics, electronics, space industries, single electron transistors, light emitters, energy science, nonlinear optical devices, photoelectrochemical catalysis, biomedical cosmetics, drug or gene delivery, and food and feed, has extensively embraced the capabilities of nanotechnology (Dhaka et al., 2023). Particles with dimensions ranging from 1 to 100 nm, possessing at least one dimension within this scale, are termed nanoparticles (NPs) (Calzolai et al., 2012; Calderón-Jiménez et al., 2017). In recent times, Metallic nanoparticles (MNPs) have garnered significant attention due to their distinctive physicochemical features, high monodispersity and small size (Shnoudeh et al., 2019; Nie et al., 2023). In this context, AgNPs are synthesized by various techniques including physical, chemical and biological. Limitations of physical and chemical synthesis methods for synthesizing AgNPs include costly procedures and hazardous substances that might lead to potential environmental and biological risks (Kakakhel et al., 2021). Hence, it was necessary to develop techniques to address these constraints and synthesize durable and pharmacologically effective AgNPs (Syafiuddin et al., 2017b). Nanoparticles are produced using various biological components such as plant extracts and microorganisms. The biological extract contains amino acids, proteins, vitamins, polysaccharides, enzymes, and phytoconstituents that function as reducing agents (Polash et al., 2021; Hamad et al., 2020). Trigonella foenum-graecum (L.), generally known as Fenugreek, is a fragrant leguminous plant cultivated in several countries. Fenugreek seeds have traditionally been used for their medicinal abilities to cure arthritis, diabetes, gastrointestinal issues, as well as for their anti-parasitic and anticancer characteristics (Awad et al., 2021). Previous studies reported the synergistic antibacterial activity of AgNPs with antibiotic as fosfomycin (Aljeldah et al., 2023), colistin (Yassin et al., 2022a), tigecycline, and linezolid (Al-Otibi et al., 2023). Prior research emphasized the eco-friendly production of biogenic silver nanoparticles using plant seed extracts, but few studies investigated the combined effect of these nanoparticles with antibiotics. The research aimed to assess the antimicrobial and combined effects of the eco-friendly synthesized AgNPs with norfloxacin and colistin antibiotics against multidrug-resistant bacterial strains. The fabricated AgNPs were synthesized using seed extract of Trigonella foenum-graecum and their combined activities with colistin and norfloxacin antibiotics were evaluated against multidrug–resistant gram negative bacterial strains namely, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and Escherichia coli strains.
2 Materials and methods
2.1 Preparation of Fenugreek seed extract
The seeds of Fenugreek were procured from a Riyadh, Saudi Arabian market, and the Herbarium of King Saud University's College of Science verified their authenticity. After being washed with tap water, the seeds were rinsed three times with distilled water for a complete cleaning procedure. They were then left to air dry. To make a fine powder out of the Fenugreek seeds, a mechanical mortar was used. After that, 50 g of powder were dissolved in 200 ml of distilled water, and the mixture was then heated in flasks at 50 °C for half an hour. The subsequent mixture was agitated with a magnetic stirrer for 24 h at 25 °C. To get a clear filtrate, the Fenugreek seeds extract was next filtered using Whatman filter paper (1). The extract was sterilized by passing it through a 0.45 µm Millipore membrane filter, and then it was stored in the fridge at 4 °C until later use.
2.2 Green synthesis of AgNPs
Initially, 10 mL of the water extract obtained from Fenugreek seeds was added to 90 mL of a 1 mM solution of colorless AgNO3 (Sigma-Aldrich, Missouri, USA). Subsequently, 90 mL of silver nitrate solution was mixed with 2, 4, 6, 8, and 10 mL of Fenugreek seed extract, each at a silver nitrate concentration of 1 mM. Additionally, different concentrations of AgNO3 (0.2, 0.4, 0.6, 0.8, and 1.0 mM) were used to synthesize silver nanoparticles while maintaining a constant extract concentration (2 mL).
Subsequently, the reaction mixture underwent incubation at room temperature in a shaking incubator under dark conditions. The biosynthesis process of AgNPs was visually monitored, observing a color shift from colorless to a light brown hue. AgNPs were separated from the reduced mixture through centrifugation at 10,000 rpm for 10 min. The collected AgNPs underwent a thorough washing process with deionized water, repeated three times to eliminate impurities. Subsequently, the extract underwent sterilization through filtration, employing a Millipore membrane filter with a pore size of 0.45 µm. Finally, the purified AgNPs were dried at 80℃ in an oven for subsequent physicochemical characterizations (Yassin et al., 2022b, 2022c).
2.3 Physicochemical properties of the biogenic AgNPs
The physicochemical features of the biogenically synthesized AgNPs were explored through diverse methodologies as UV–Vis, TEM, EDX, FTIR, XRD and zeta potential analysis of the biosynthesized AgNPs.
2.4 Screening of antimicrobial efficacy of AgNPs against bacterial pathogens
The American Type Culture Collection provided four bacterial strains: A. baumannii (ATCC 43498), K. pneumoniae (ATCC 700603), E. coli (ATCC 25922), and P. aeruginosa (ATCC 9027). The selection of bacterial pathogens for antimicrobial investigations was based on their substantial impact on worldwide mortality and morbidity, which is mostly caused by their extensive antibiotic resistance. The antibacterial efficacy of biogenic AgNPs was examined against the identified bacterial strains using the disc diffusion technique. The bacterial colonies were collected, mixed with a saline solution, and adjusted to a concentration of 108 cfu/mL using the 0.5 McFarland standard. After filling sterile Petri plates with sterile Mueller Hinton agar (MHA) media and inoculating them with the bacterial solution, 8 mm filter paper discs impregnated with 100 and 200 µg of AgNPs were added. Filter paper discs soaked with methanol solvent functioned as the negative controls, and Colistin sulfate as the positive control. Plates were incubated at 37 °C for 24 h after being refrigerated for 2 h to facilitate the diffusion of nanoparticles. A Vernier caliper was used to measure the inhibitory zone diameters. The broth microdilution test employing 96-well microtiter plates was used to evaluate the minimum inhibitory concentration (MIC) of AgNPs from Fenugreek seeds against the most sensitive strain of E. coli. In addition, the minimum bactericidal concentration (MBC) was determined by inoculating samples from MIC wells onto MHA plates. The MBC was defined as the lowest concentration at which no bacterial growth was observed.
2.5 Determination of synergistic potency of biogenic AgNPs with antibiotics
The combined efficacy of antibiotic-AgNPs against certain bacterial pathogens was evaluated via the use of disc diffusion assay. Fresh MHA plates were made and then inoculated with 0.5 mL of the bacterial solution that was prepared earlier. A separate set of discs was impregnated with both of colistin (10 μg) or norfloxacin (10 μg) and AgNPs (100 μg/disk) to examine their synergistic effect. The positive control discs were loaded with colistin (10 μg) and norfloxacin (10 μg) only, whereas the negative control discs were filled with filter paper discs soaked in methanol solvent. The moistened discs were allowed to dry before being positioned on the MHA plates that had been previously inoculated. Following that, the dishes were subjected to incubation at a temperature of 37 ℃ for a period of 24 h. The calculation of the increase in fold of inhibition area (IFA) was performed utilizing the subsequent equation:
(IFA) = (B2 − A2)/A2, where A was the diameter of the inhibition zone for colistin or norfloxacin antibiotics and B was the diameter of the inhibition zone for antibiotics and biogenic AgNPs combined together (Al-Otibi et al., 2023).
2.6 Determination of morphological cell deformations
Scanning electron microscopy (SEM) was utilized to assess the morphological changes in bacterial cells treated with biosynthesized AgNPs. The samples were processed for investigation as mentioned in a prior study (Almaary et al., 2023).
3 Results and discussion
3.1 Optimization of the reaction conditions
Various volumes of the plant extract and varying concentrations of AgNO3 were examined to determine the factors that contribute to achieving the highest yield of AgNPs. In this context, it was seen that the highest amount of AgNPs was produced when using a concentration of 1 mM of AgNO3 and 10 ml of the plant extract. This resulted in the development of AgNPs precipitates with concentrations of 0.57 ± 0.04 and 1.47 ± 0.09 mg/ml, respectively (Fig. 1).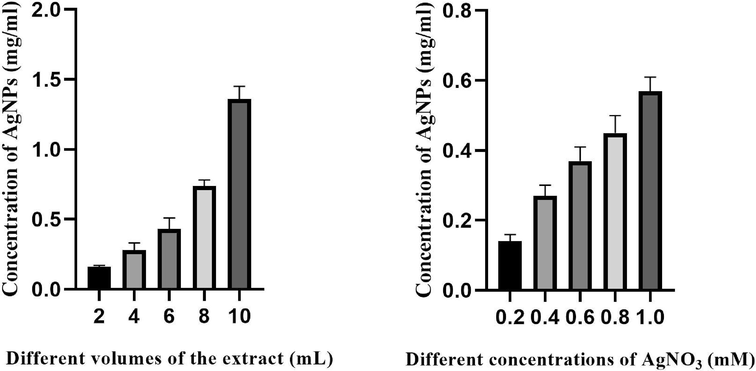
Effect of different concentrations of AgNO3 and plant extract on the yield of AgNPs.
3.2 Green synthesis of AgNPs
Fig. 2 showed the green fabrication of AgNPs using seed extract of Fenugreek. The colorless solution of silver nitrate (Fig. 2a) undergoes reduction when mixed with the liquid extract of Fenugreek seeds, leading to the creation of silver nanoparticles. The Fenugreek seeds include a variety of natural chemical components, including amino acids, flavonoids, saponins, alkaloids, and steroids. These components are responsible for the extensive usage of seed extracts in the environmentally friendly production of various metal and metal oxide nanoparticles (Jeevanandam et al., 2022). Furthermore, the extract phytochemicals function as capping and stabilizing agents, playing an important role in the production of AgNPs (Restrepo and Villa, 2021).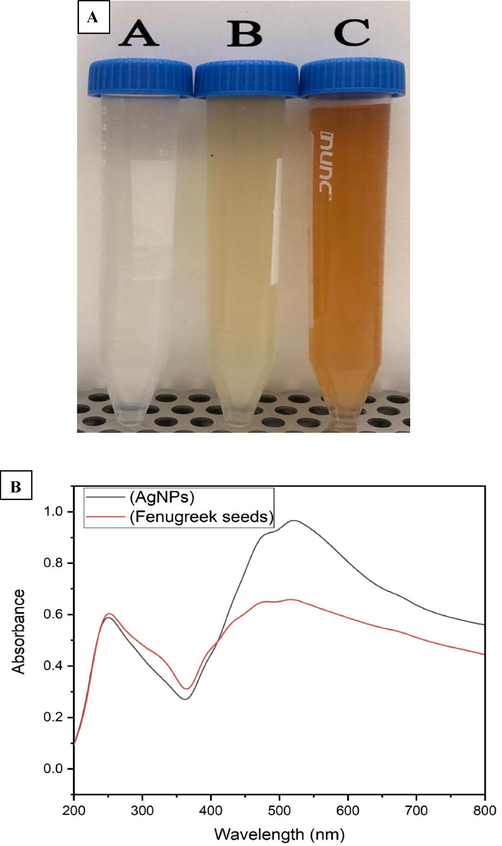
a) Green synthesis of AgNPs using T. foenum-graecum seeds extract, b) UV spectrum of Fenugreek seeds extract and their corresponding AgNPs.
3.3 UV analysis of biogenic AgNPs and Fenugreek seed extract
The surface plasmon resonance (SPR) of the biosynthesized AgNPs was determined by analysing the Fenugreek seed extract and AgNPs with UV analysis. The coordinated motion of electrons within the conduction band of nanoparticles upon absorption of an electromagnetic wave is referred to as surface plasmon resonance. In this setting, Fenugreek extract shown the existence of two absorption bands at 251 and 507 nm. Consistent with the outcomes of a prior study, our results confirmed the presence of a distinctive peak at 500 nm in the UV spectrum of the water extract of red currant (Rizwana et al., 2022). The absorption behavior seen in Fig. 2b is caused by SPR of AgNPs, which occurs when conduction band electrons of the AgNPs undergo coherent oscillation in response to the electromagnetic field. This explains the presence of a broad band with distinctive peaks at 476 and 509 nm in the UV spectrum of plant extract, which were shifted to a higher wavelength in the UV spectrum of AgNPs at 480 and 519 nm. The results of our study were consistent with those of a prior publication (Syafiuddin et al., 2017a).
3.4 TEM analysis of AgNPs
TEM micrographs of AgNPs showed that the nanoparticles were spherical in shape with average particle size of 29.75 nm (Fig. 3a). The histogram of the particle size distribution indicated that the size of the AgNPs varied between 10 and 70 nm (Fig. 3b). The findings of our study aligned with a previous publication that showed the successful production of silver nanoparticles (AgNPs) using olive leaf extract. The report reported that the average particle size of the AgNPs was around 30 nm (Khalil et al., 2014). Additionally, another research used transmission electron microscopy (TEM) to analyze AgNPs morphology made from an aqueous leaf extract of Azadirachta indica. According to the research, these AgNPs had an average size of 34 nm (Ahmed et al., 2016). The measured particle size was smaller than in earlier publications, showing the effectiveness of the present approach for synthesizing AgNPs using the seed extract of T. foenum-graecum.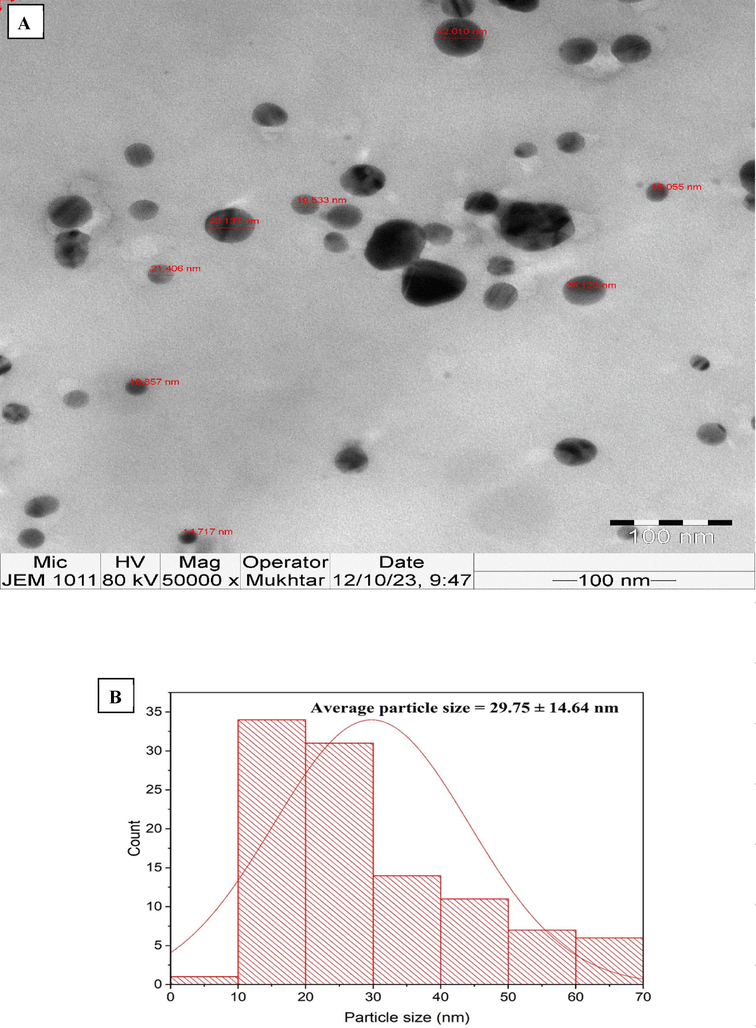
A) tem images of agnps, b). particle size distribution of agnps.
3.5 EDX analysis of the biogenic AgNPs
EDX analysis was used to conduct elemental mapping of the biogenic AgNPs (Fig. 4a). The study revealed that the proportion of elemental silver was 73.42 %, whereas the percentage of oxygen was 26.58 %. The strong signals at 2.983 keV were attributed to silver atoms, whereas the signal at 0.525 keV was allocated to oxygen atoms. The mass percentage of elemental Ag was more than that reported in a recent work on the green synthesis of AgNPs using the endophytic fungus Talaromyces purpureogenus, which was isolated from Taxus baccata Linn. The relative mass percentage was found to be 67.26 % (Sharma et al., 2022). Additionally, the EDX examination revealed that the AgNPs synthesized utilizing leaf extract of Holoptelea integrifolia had an elemental content of 71.32 % Ag (Kumar et al., 2019).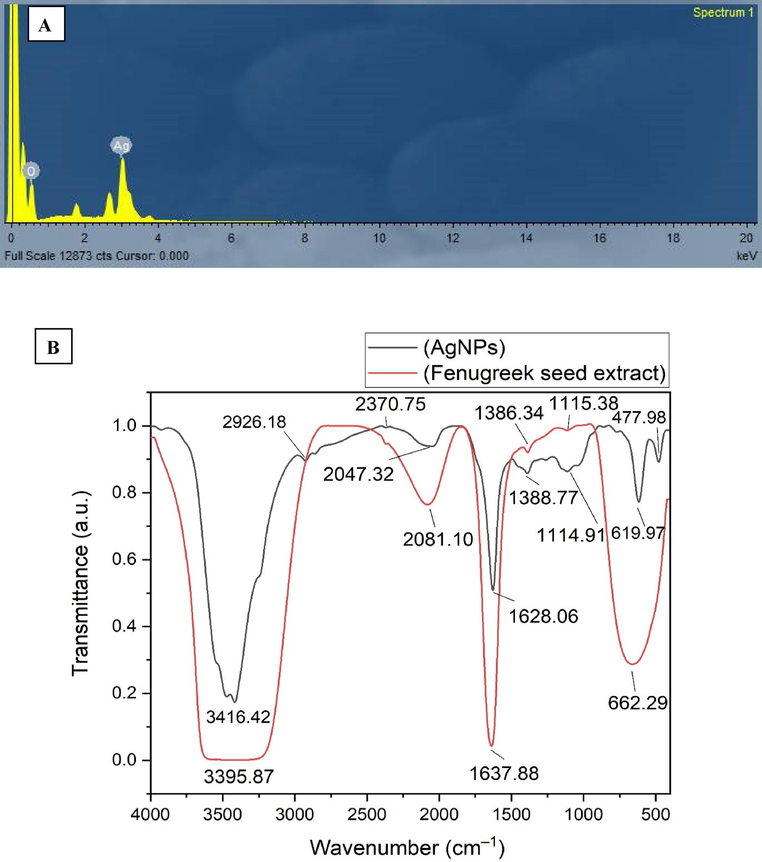
A) edx spectrum of agnps (x axis refereed to the energy level (kev) whereas y axis refer to the counts), b) FTIR spectrum of Fenugreek seeds extract and AgNPs.
3.6 FTIR analysis of the biogenic AgNPs
FTIR investigation of Fenugreek seed extract and AgNPs was done to detect the key functional groups contributing to the reduction, capping and stabilizing of AgNPs. In this setting, FTIR spectrum of the seed extract of Fenugreek displayed the existence of six peaks at 3395.87, 2081.10, 1637.88, 1386.34, 1115.38, and 662.29 cm−1 (Fig. 4b). On the other hand, the FTIR spectrum of AgNPs displayed the existence of nine absorption bands at wavenumbers of 3416.42, 2926.18, 2370.75, 2047.32, 1628.06, 1388.77, 1114.91, 619.97 and 477.98 cm−1. The wide spectral band observed at 3395.87 cm−1 in the Fenugreek spectrum was identified as the result of O–H stretching of phenolic biomolecules. In AgNPs spectrum, this peak was shifted to a higher wavenumber at 3416.42 cm−1, indicating that the extract phenols were adsorbed over nanoparticles surface (Hajebi et al., 2019). The spectra of AgNPs displayed two weak bands with wavenumbers of 2926.18 and 2370.75 cm−1. These peaks were identified as the result of stretching vibrations of C–H and N–H bonds in alkanes and amines, respectively (Oves et al., 2022). In contrast, the peak seen at 2081.10 cm−1 in the seed extract was shifted to a lower wavelength at 2047.32 cm−1, these bands were attributed to the C=S stretching of sulfur compounds (Mistry et al., 2021). Moreover, the absorption band seen at 1637.88 cm−1 in spectra of Fenugreek seed extract was shifted to a lower wavenumber at 1628.06 cm−1, corresponding to the amide I bands of proteins (Das et al., 2013). The peak seen at 1388.77 cm−1 in the spectra of AgNPs was displaced to a lower wavenumber of 1386.34 cm−1, indicating the C–H bending of alkanes. Nevertheless, the peak detected at 1114.91 cm−1 in the Fenugreek seed extract spectra was displaced to a higher frequency at 1115.38 cm−1 in the AgNPs spectra, indicating the C-N stretching of amines (Deivanathan and Prakash, 2022). The strong peak detected at 662.29 cm−1 was shifted to a lower wavenumber at 619.97 cm−1 corresponding to S-S stretching (Raghava et al., 2021).
3.7 XRD analysis of AgNPs
XRD investigation was performed to affirm the crystalline structure of the eco-friendly synthesized AgNPs. XRD analysis showcased the existence of six diffraction peaks at 2 theta angles of 21.19, 32.28, 37.31, 49.34, 57.48 and 77.55° corresponding to the orientation planes of (1 0 0), (1 1 0), (1 1 1), (2 0 0), (2 2 0) and (3 1 1), respectively (Fig. 5). The XRD outcomes were in line with that of previous reports (Varghese et al., 2019). The detected peaks validate the polycrystalline characteristic of the synthesized AgNPs and may be ascribed to the typical JCPDS data of crystalline silver with a face-centered cubic (fcc) lattice structure, identified by the code COD 2300113. The additional peaks seen in XRD pattern correspond to the organic stabilizers that inhibit the aggregation of AgNPs (Jalilian et al., 2020). The bioprepared AgNPs were found to have a crystalline size of 28.71 nm, which was determined using Scherrer's equation.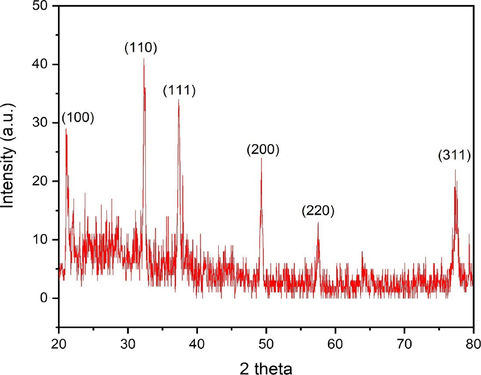
XRD spectrum of AgNPs.
The formula for calculating the crystalline size is given by D=0.9λ/ β cos θ, where λ represents the X-ray wavelength (1.54178 Å), k represents Scherrer's constant (k = 0.94), θ represents the diffraction angle (32.31°), and β represents the full width at half maximum (FWHM) of the most intense diffraction peak (0.301) (Yassin et al., 2023). The physicochemical features of the biogenic AgNPs were compared with that of previous literature as seen in Table 1.
Plants
Functional groups of AgNPs
Crystalline size
Antibacterial activity
References
Trigonella foenum-graecum seeds extract
Phenols, alkanes, amines, amide I bands of proteins, alkanes, and sulfur compounds
28 nm
Antibacterial activity against A. baumannii, E. coli, K. pneumoniae, and P. aeruginosa
This work
Datura stramonium leaf extract
Alcohols, phenols, carboxylic acids, proteins and aromatic compounds
18 nm
Antibacterial activity against E. coli and S. aureus
(Gomathi et al., 2017)
Croton sparsiflorus morong leaf extract
Amines, alcohols, ketones, aldehydes and carboxylic acids
16 nm
Antibacterial activity against S. aureus, E. coli, and B. substilis
(Kathiravan et al., 2015)
Pedalium murex leaf extract
Phenols, flavanones proteins, alkyl groups, aromatic and carboxylic compounds
14 nm
Antibacterial activity against E. coli, K. pneumoniae, B. subtilis and S. aureus
(Anandalakshmi et al., 2016)
Sesbania grandiflora leaf extract
Amines, alcohols, ketones and carboxylic acid.
ND
Antibacterial activity against S. enterica and S. aureus
(Das et al., 2013)
Fumaria officinalis aerial plant extract
Alcohols, phenols, amines, amides, peptides and proteins
20 nm
Antibacterial activity against gram positive bacteria as S. aureus, B. cereus, B. luteus, and L. monocytogenes; also against gram negative bacteria as E. coli, P. aeruginosa, K. pneumoniae and P. vulgaris
(Milorad Cakić et al., 2018)
P. nigrum leaf and stem extract
For leaf derived AgNPs: Ketones, secondary amines, nitro groups, esters, alkynes
For stem derived AgNPs: primary alcohols, primary and secondary amines, nitrile groups, and amides27 nm and 13.5 nm, respectively
Antibacterial activity against Citrobacter freundiiand Erwinia cacticida
(Paulkumar et al., 2014)
Syngonium podophyllum leaf extract
Amide II, protein, secondary amine, and aldehydes
ND
Antibacterial activity against S. aureus, B. subtilis, E. coli and P. aeruginosa
(Naaz et al., 2021)
3.8 Zeta potential analysis
Zeta potential analysis was done to estimate the zeta potential value of the eco-friendly synthesized AgNPs. The presence of high negative values suggests that anionic stabilizing agents are effectively coordinating with the AgNPs, resulting in a stable colloidal solution. This stability is achieved by the electrostatic repulsion between the negatively charged particles. The presence of negatively charged surfaces on AgNPs serves to inhibit the aggregation of nanoparticles and exert control over their shapes and sizes (Paosen et al., 2017). In this context, zeta potential value of AgNPs was detected to be –22.4 mV (Fig. 6a). However, the results from DLS displayed that the mean hydrodynamic nanosize of AgNPs was 166.9 nm (Fig. 6b). This size is larger than what was observed using TEM and XRD techniques. The difference might be accredited to the existence of capping biomolecules and the fact that DLS analysis measures both the nanoparticles diameter and the surrounding hydrate layers. The polydispersity index (PDI) of the biogenic AgNPs was estimated to be 0.364, which in agreement with the findings of a prior investigation that reported a PDI value of 0.365 for AgNPs synthesized using Ginkgo biloba leaf extract (Wang et al., 2019). This indicates that the synthesized AgNPs were effectively dispersed in water.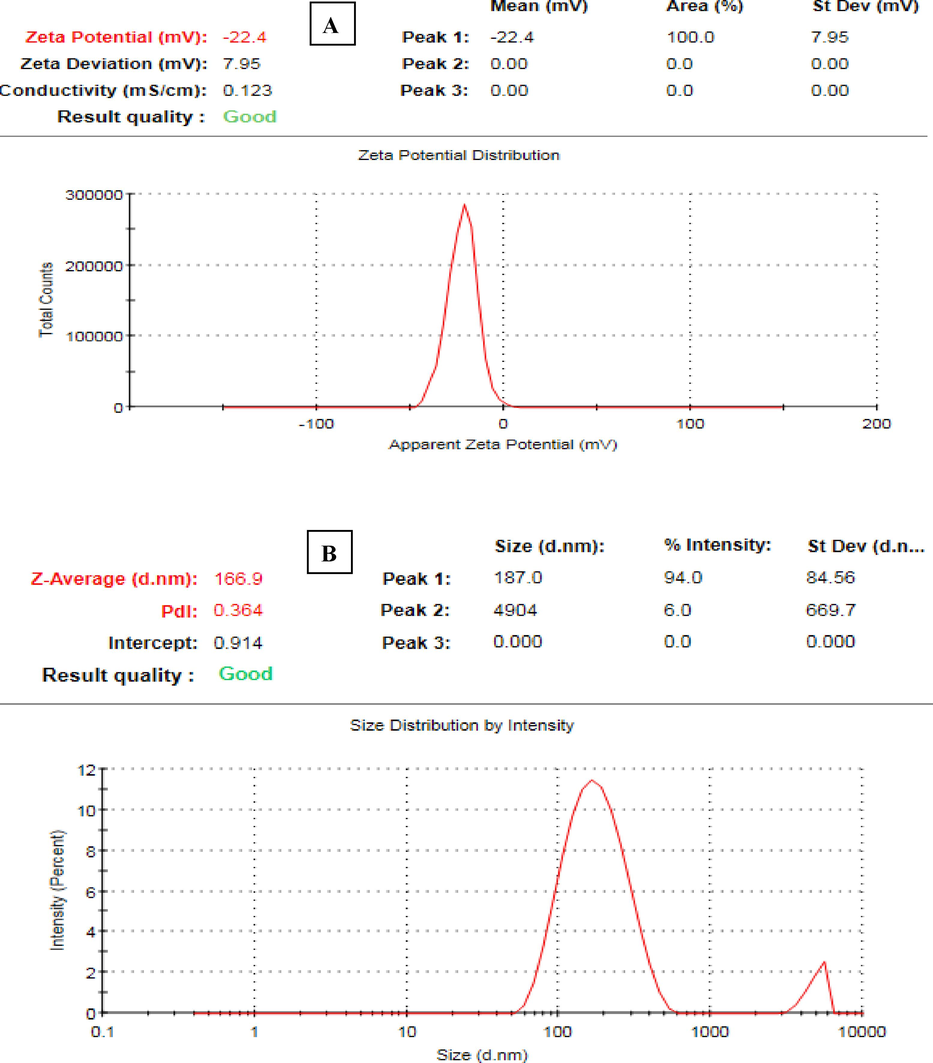
A) zeta potential value of agnps, b) DLS spectrum of AgNPs.
3.9 Antibacterial efficacy of AgNPs against the concerned microbial strains
The antibacterial efficacy of AgNPs was detected against A. baumannii, E. coli, K. pneumoniae, and MRSA pathogens using a disc diffusion assay. E. coli strain showed the highest antibacterial susceptibility to the biogenic AgNPs at the two tested concentrations of AgNPs (50 and 100 µg/disc), demonstrating relative suppressive zones of 18.43 ± 0.35 and 21.32 ± 0.19 mm, respectively (Table 2). Nevertheless, the MRSA strain exhibited the least sensitivity to the biogenic AgNPs at the tested doses, resulting in inhibitory zones of 10.24 ± 0.21 and 11.26 ± 0.51 mm, respectively. The study findings demonstrated that AgNPs exhibit greater antibacterial efficacy against Gram-negative bacteria compared to Gram-positive bacteria due to the presence of a peptidoglycan layer in the cell wall of Gram-positive bacteria (Ramalingam et al., 2016). This layer, consisting of glycan strands cross-linked by short peptides and anionic glycopolymers known as teichoic acid, acts as a natural barrier that hinders the penetration of nanoparticles. In contrast, Gram-negative bacteria possess a cell wall that is thinner and has a lower amount of peptidoglycan. Although lipopolysaccharides (LPS), a crucial element of the Gram-negative bacterial cell wall, greatly enhances the strength and integrity of the membrane, its negative charge promotes the attachment of nanoparticles (Tavares et al., 2020). Previous research has shown that reactive oxygen species (ROS) play a significant role in the antibacterial action of biosynthesized AgNPs, as shown in an evaluation of AgNPs' ROS-mediated antibacterial activity against multidrug-resistant E. coli and S. aureus. Consequently, the elevated amount of ROS caused the bacterial membranes to be disrupted by increasing their permeability. This ultimately led to the disruption of the electron transport chain and the leaking of cellular content, leading to the bacterial cell death (Das et al., 2017). The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were detected for E. coli strain which showed the highest sensitivity to AgNPs. The detected MIC and MBC values were found to be 50 and 100 µg/ml, respectively. The antimicrobial activity of AgNPs could be assigned to the capping agents adsorbed over the biogenic AgNPs as phenols, alkanes, amines, amide I bands of proteins, and alkanes as detected by FTIR analysis and this was in accordance with previous report (Roy et al., 2019). Different superscript letters indicated that values were significantly different at p ≤ 0.05.
Bacterial strains
AgNPs
(50 μg/disk)AgNPs
(100 μg/disk)Colistin
(10 µg/disk)Negative control
A. baumannii
12.19 ± 0.21 a
13.83 ± 0.14 a
14.02 ± 0.32 a
0.00 ± 0.00
E. coli
18.43 ± 0.35b
21.32 ± 0.19b
27.35 ± 0.29b
0.00 ± 0.00
K. pneumoniae
14.52 ± 0.43c
20.76 ± 0.31b
21.05 ± 0.41c
0.00 ± 0.00
P. aeruginosa
10.24 ± 0.21c
11.26 ± 0.51c
16.12 ± 0.24 a
0.00 ± 0.00
3.10 Synergistic antibacterial efficiency of AgNPs with antibiotics
The AgNPs exposed the uppermost synergistic efficacy with colistin against A. baumannii strain with relative IFA of 0.53 whereas AgNPs displayed the highest synergistic proficiency with norfloxacin against P. aeruginosa with relative IFA value of 0.35 (Table 3). Our outcomes were in line with that of a prior investigation which reported the antimicrobial action of silver nanoparticles conjugated with colistin against E. coli, K. pneumoniae, and P. aeruginosa (Muenraya et al., 2022). The combined action of AgNPs and colistin antibiotic against the gram negative bacterial strains is due to the impact of colistin antibiotic on the lipopolysaccharide, a key component of the outer membrane. This leads to the disruption of the outer membrane, allowing AgNPs to enter the bacterial cells. AgNPs then exert their killing effect by producing reactive oxygen species, that oxidize the bacterial DNA, proteins and lipids, ultimately resulting in the demise of the bacterial cells (Tripathi and Goshisht, 2022). An earlier work documented the synergistic effect of norfloxacin and gallotannin-capped iron oxide nanoparticles, showing an increase in inhibitory zones from 13 mm to 17 mm and 19 mm against the two strains, respectively (Ahmed et al., 2021). The combined effect of AgNPs and norfloxacin might be accredited to AgNPs accumulation on the outer membrane of bacterial cells, triggering their disruption. On the other hand, norfloxacin antibiotic inhibits bacterial growth by interacting with bacterial topoisomerase IV and DNA gyrase, thereby preventing DNA replication (Huang et al., 2024). IFA refers to increase in fold of inhibition area. Different superscript letters indicated that values were significantly different at p ≤ 0.05.
Bacterial strains
AgNPs (100 μg/disk) + Colistin (10 µg/disk)
Colistin (10 µg/disk)
Negative control
IFA
A. baumannii
20.27 ± 0.58 a
13.89 ± 0.52 a
0.00 ± 0.00
0.53
E. coli
34.94 ± 0.39b
25.18 ± 0.26b
0.00 ± 0.00
0.48
K. pneumoniae
27.18 ± 0.42c
20.52 ± 0.48c
0.00 ± 0.00
0.43
P. aeruginosa
19.16 ± 0.53 d
16.43 ± 0.18 d
0.00 ± 0.00
0.29
AgNPs (100 μg/disk) + norfloxacin (10 µg/disk)
norfloxacin (10 µg/disk)
Negative control
IFA
A. baumannii
11.83 ± 0.18 a
9.74 ± 0.26 a
0.00 ± 0.00
0.32
E. coli
32.65 ± 0.18b
28.79 ± 0.58b
0.00 ± 0.00
0.22
K. pneumoniae
30.47 ± 0.29c
24.96 ± 0.47c
0.00 ± 0.00
0.33
P. aeruginosa
27.13 ± 0.41 d
21.85 ± 0.34 d
0.00 ± 0.00
0.35
3.11 Detection of bacterial cell deformations using SEM analysis
The morphological deformations of E. coli cells were investigated using SEM analysis. The control cells of E. coli were found to be normal, rod-shaped, and with structural integrity without any signs of deformation. Moreover, it was observed that E. coli cells subjected to AgNPs treatment exhibited aberrant morphological characteristics, including cellular damage, wrinkling, malformation, as well as a cracked outer surface and distorted cell membranes (Fig. 7).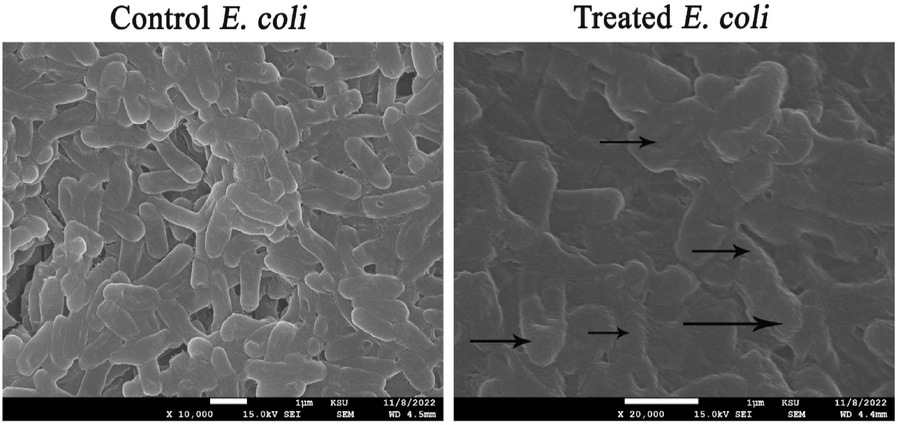
Morphological deformations of E. coli cells exposed to biogenic AgNPs (the morphological deformations were pointed out by arrows).
4 Conclusions
Trigonella foenum-graecum facilitated green biosynthesis of AgNPs with potential physicochemical techniques. The eco-friendly synthesized AgNPs were spherical in shape with mean nanosize of 29.75 nm and average zeta potential of –22.7 mV. Hence, the prepared AgNPs showed effective antimicrobial action against the concerned bacterial strains due to the potential physicochemical features of AgNPs such as the small particles size and the capping of biomolecules as phenols, alkanes and amines. The biogenic AgNPs showed a higher synergistic activity with colistin compared to norfloxacin antibiotic. The recorded synergistic activity of AgNPs with antibiotics highlights the potential use of these combinations to control nosocomial infections in health care settings. Moreover, the effective combination of AgNPs with colistin and norfloxacin antibiotics could offer potential therapeutic options against the drug resistant bacterial pathogens.
Funding
This research project was supported by a grant from the Researchers Supporting Project number (RSP2024R114), King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Khalid Maniah: Software, Resources, Investigation, Formal analysis, Data curation. Fatimah Olyan Al-Otibi: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources. Sara Mohamed: Visualization, Software, Investigation, Formal analysis, Data curation. Basant A. Said: Software, Methodology. Mohamed Ragab AbdelGawwad: Writing – review & editing. Mohamed Taha Yassin: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Conceptualization.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R114), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci.. 2016;9:1-7.
- [CrossRef] [Google Scholar]
- Synthesis of gallotannin capped iron oxide nanoparticles and their broad spectrum biological applications. RSC Adv.. 2021;11:9880-9893.
- [CrossRef] [Google Scholar]
- Synergistic Antibacterial Potential of Greenly Synthesized Silver Nanoparticles with Fosfomycin Against Some Nosocomial Bacterial Pathogens. Infect. Drug Resist.. 2023;16:125-142.
- [CrossRef] [Google Scholar]
- Synergistic antibacterial proficiency of green bioformulated zinc oxide nanoparticles with potential fosfomycin synergism against nosocomial bacterial pathogens. Microorganisms. 2023;11(3):645.
- [Google Scholar]
- Green Biofabrication of Silver Nanoparticles of Potential Synergistic Activity with Antibacterial and Antifungal Agents against Some Nosocomial Pathogens. Microorganisms. 2023;11:945.
- [CrossRef] [Google Scholar]
- Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci.. 2016;6:399-408.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles using Trigonella foenum-graecum seed extract: Characterization, photocatalytic and antibacterial activities. Sens. Actuators Phys.. 2021;323:112670
- [CrossRef] [Google Scholar]
- Bora, S., Pooja, D., Kulhari, H., 2024. Introduction of Nanoscience and Nanotechnology, in: Pooja, D., Kulhari, H. (Eds.), Nanotechnology Based Delivery of Phytoconstituents and Cosmeceuticals. Springer Nature, Singapore, pp. 1–38. https://doi.org/10.1007/978-981-99-5314-1_1.
- Green Synthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles Produced fromFumaria officinalis L. Plant Extract. Colloid J.. 2018;80:803-813.
- [CrossRef] [Google Scholar]
- Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Chem: Front; 2017. p. :5.
- Measuring nanoparticles size distribution in food and consumer products: a review. Food Addit. Contam. Part A. 2012;29:1183-1193.
- [CrossRef] [Google Scholar]
- Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem.. 2017;10:862-876.
- [CrossRef] [Google Scholar]
- Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta. a. Mol. Biomol. Spectrosc.. 2013;104:265-270.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using aqueous leaf extract of Guettarda Speciosa and its antimicrobial and anti-oxidative properties. Chem. Data Collect.. 2022;38:100831
- [CrossRef] [Google Scholar]
- A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem.. 2023;6:101108
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resour.-Effic. Technol.. 2017;3:280-284.
- [CrossRef] [Google Scholar]
- Rapeseed flower pollen bio-green synthesized silver nanoparticles: a promising antioxidant, anticancer and antiangiogenic compound. JBIC J. Biol. Inorg. Chem.. 2019;24:395-404.
- [CrossRef] [Google Scholar]
- Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater.. 2020;30:4811-4828.
- [CrossRef] [Google Scholar]
- Combined effects of norfloxacin and polystyrene nanoparticles on the oxidative stress and gut health of the juvenile horseshoe crab Tachypleus tridentatus. J. Hazard. Mater.. 2024;468:133801
- [CrossRef] [Google Scholar]
- Green synthesized silver nanoparticle from Allium ampeloprasum aqueous extract: Characterization, antioxidant activities, antibacterial and cytotoxicity effects. Adv. Powder Technol.. 2020;31:1323-1332.
- [CrossRef] [Google Scholar]
- Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale. 2022;14:2534-2571.
- [Google Scholar]
- Green synthesis of silver nanoparticles and their shortcomings, animal blood a potential source for silver nanoparticles: A review. J. Hazard. Mater. Adv.. 2021;1:100005
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using Croton sparsiflorus morong leaf extract and their antibacterial and antifungal activities. Spectrochim. Acta. a. Mol. Biomol. Spectrosc.. 2015;139:200-205.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem.. 2014;7:1131-1139.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using leaf extract of Holoptelea integrifolia and preliminary investigation of its antioxidant, anti-inflammatory, antidiabetic and antibacterial activities. J. Environ. Chem. Eng.. 2019;7:103094
- [CrossRef] [Google Scholar]
- Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnol. Lett.. 2021;43:307-316.
- [CrossRef] [Google Scholar]
- Silver Nanoparticles Conjugated with Colistin Enhanced the Antimicrobial Activity against Gram-Negative Bacteria. Molecules. 2022;27:5780.
- [CrossRef] [Google Scholar]
- Naaz, R., Siddiqui, V.U., Qadir, S.U., Siddiqi, W.A., 2021. Green synthesis of silver nanoparticles using Syngonium podophyllum leaf extract and its antibacterial activity. Mater. Today Proc., ICMN-2020 46, 2352–2358. https://doi.org/10.1016/j.matpr.2021.05.062.
- Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf.. 2023;253:114636
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci.. 2022;29:460-471.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater. Lett.. 2017;209:201-206.
- [CrossRef] [Google Scholar]
- Piper nigrum Leaf and Stem Assisted Green Synthesis of Silver Nanoparticles and Evaluation of Its Antibacterial Activity Against Agricultural Plant Pathogens. Sci. World J.. 2014;2014:829894
- [CrossRef] [Google Scholar]
- Biogenic Silver Nanoparticles: A Potent Therapeutic Agent. In: Singh S., ed. Emerging Trends in Nanomedicine. Singapore: Springer; 2021. p. :81-127.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles by Rivina humilis leaf extract to tackle growth of Brucella species and other perilous pathogens. Saudi J. Biol. Sci.. 2021;28:495-503.
- [CrossRef] [Google Scholar]
- Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces. 2016;8:4963-4976.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag.. 2021;15:100428
- [Google Scholar]
- Sunlight-mediated green synthesis of silver nanoparticles using the berries of Ribes rubrum (red currants): Characterisation and evaluation of their antifungal and antibacterial activities. Molecules. 2022;27:2186.
- [Google Scholar]
- Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv.. 2019;9:2673-2702.
- [Google Scholar]
- Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Lett.. 2022;10:2.
- [CrossRef] [Google Scholar]
- Chapter 15 - Synthesis, Characterization, and Applications of Metal Nanoparticles. In: Tekade R.K., ed. Biomaterials and Bionanotechnology, Advances in Pharmaceutical Product Development and Research. Academic Press; 2019. p. :527-612.
- [CrossRef] [Google Scholar]
- A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc.. 2017;64:732-756.
- [CrossRef] [Google Scholar]
- A purely green synthesis of silver nanoparticles using Carica papaya, Manihot esculenta, and Morinda citrifolia: synthesis and antibacterial evaluations. Bioprocess Biosyst. Eng.. 2017;40:1349-1361.
- [CrossRef] [Google Scholar]
- Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics. 2020;9:314.
- [Google Scholar]
- Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater.. 2022;5:1391-1463.
- [CrossRef] [Google Scholar]
- Silver nanopaticles synthesized using the seed extract of Trigonella foenum-graecum L. and their antimicrobial mechanism and anticancer properties. Saudi J. Biol. Sci.. 2019;26:148-154.
- [CrossRef] [Google Scholar]
- Extract of Ginkgo biloba leaves mediated biosynthesis of catalytically active and recyclable silver nanoparticles. Colloids Surf. Physicochem. Eng. Asp.. 2019;563:31-36.
- [CrossRef] [Google Scholar]
- Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic against Multidrug-Resistant Bacterial Pathogens. Crystals. 2022;12:1057.
- [CrossRef] [Google Scholar]
- Facile Green Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Origanum majorana with Potential Bioactivity against Multidrug Resistant Bacterial Strains. Crystals. 2022;12:603.
- [CrossRef] [Google Scholar]
- Synergistic Antifungal Efficiency of Biogenic Silver Nanoparticles with Itraconazole against Multidrug-Resistant Candidal Strains. Crystals. 2022;12:816.
- [CrossRef] [Google Scholar]
- Green Synthesis, Characterization, and Antifungal Efficiency of Biogenic Iron Oxide Nanoparticles. Appl. Sci.. 2023;13:9942.
- [CrossRef] [Google Scholar]







