Translate this page into:
Valorisation of Citrus limetta peel for Aspergillus terreus FP6 mediated pectinase fermentation and application in grape juice clarification
⁎Corresponding author at: Department of Microbiology and Botany, School of Sciences, JAIN (Deemed-to-be University), 34, 1st Cross, J.C. Road, Bangalore 60027, Karnataka, India. bhattsourav3011@gmail.com (Sourav Bhattacharya)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Objectives
The present investigation highlights the process parameters influencing the pectinase fermentation by a newly isolated Aspergillus terreus using valorised agro-wastes and determination of the efficacy of the pectinase in grape juice clarification.
Methods
The fungal pectinase was produced under submerged fermentation with the optimisation of carbon and nitrogen sources, initial pH of the medium, inoculum load, incubation time and effect of agitation. The pectinase was later used for the clarification of grape juice and improvement of its total phenolic content.
Results and Conclusions
Medium supplemented with sweet lime peel and yeast extract (1 % w/v), at initial pH 6.0, when incubated for 72 h under agitated conditions favoured the highest pectinase production by the fungal culture. Following the pectinase action for 1 and 3 h, the grape juice clarification percentages and total phenolic content were 34.21 %, 69.54 %, 3004, and 3484 mg GAE/L, respectively. The results highlight the valorisation of sweet lime peel for the industrial production of fungal pectinase. The study pronounces the cost-effective pectinase fermentation due to the fungi’s non-fastidious nutritional requirement and ubiquitous nature. The increase in the grape juice clarification and total phenolic content following the A. terreus FP6 pectinase treatment was significantly superior to similar commercial enzyme formulations. The result advocates the candidature of the enzyme in the beverage industry, where it can help mediate a greener approach for fruit juice clarification.
Keywords
Agro-wastes
A. terreus FP6
Juice clarification
Pectinase
Total phenolic content
Valorisation
Data availability
Data will be made available on request.
1 Introduction
The increasing population has been a major reason behind the constant surge in food production, leading to abundant agricultural leftovers (bagasse, husks, hulls, peels, pulps, shells, stem, and straw). These leftovers mostly decompose in the environment or the biomass is reduced by incineration process. Both these activities can be potentially hazardous from the environmental view point as they mediate the greenhouse gas and particulate matter emission in significant excess (Varghese et al., 2023). The growing interest in sustainable circular bioeconomy ushered the valorisation of agro-wastes to generate high-value products (such as carotenoids, enzymes, essential oils, polyphenols, single-cell proteins) and biofuels, thereby minimising the overall carbon footprint (Blasi et al., 2023).
Pectinases constitute high molecular weight pectin hydrolysing enzymes produced from diverse microbial cultures. Fungal pectinases gain industrial relevance due to the extracellular nature, increased activity, and tolerance. Constant efforts are being made to explore different pectin-rich agro-wastes to improve process productivity and practicability to satisfy industrial demands (Kaur et al., 2023). Exploring a reliable pectinase-producing fungal starter culture may involve members belonging to the Aspergillus genera since many species prominently degrade natural polysaccharides. Moreover, Aspergillus species get utilised in industrial fermentation owing to the capacity to secrete exoenzymes in large quantities and the feasibility of being cultivated in industrial bioreactors. Additionally, they have been tagged ‘generally regarded as safe / GRAS’ cultures by the United States Food and Drug Administration. Furthermore, based on the optimisation flexibility (by numerous bioprocessing strategies), scalability, and downstream processes, submerged cultivation is the most favourable fermentation system to up-scale pectinase production (El-Gendi et al., 2022).
Pectinases find a significant application in fruit juice clarification and hence pectinases from various sources have been extensively explored to evaluate the enzyme’s catalytic efficiency. Unprocessed fruit juices appear cloudy as the inherent polysaccharides interact with the natural proteins leading to the colloid (haze) formation. This hampers juice clarification during processing. Pectinases hydrolyse the pectin polysaccharides present in the fruit cell wall. Further, this releases the phenolic entities trapped within the cell vacuoles, thereby increasing the juices’ total phenolic content (TPC) and aiding in the clarification without using harmful chemical solvents (acetone, diethyl ether, ethanol or toluene) (Chen et al, 2023).
The present work was conceptualised towards achieving maximum pectinase production through the valorised agro-wastes by a newly isolated thermophilic Aspergillus terreus FP6 under submerged fermentation conditions. The produced pectinase from this novel fungal isolate exhibited high efficiency as established by the enhanced TPC content and colour developed in the clarified grape juice. Based on the existing literature, the A. terreus FP6 pectinase proved to be ‘fast acting’ than the commercial pectinases employed for the clarification of fruit juices.
2 Materials and methods
2.1 Chemicals and reagents
Citrus pectin (with ≥85 % esterification) was purchased from Merck, Germany. Chemicals (analytical grade) and dehydrated media were purchased from HiMedia, Mumbai, India.
2.2 Isolation of fungi
Compost soils were subjected to serial dilution (10-1 to 10-6) and spread plating (100 µL of serially diluted aliquots) on pectin agar (g/L: pectin, 5.0; yeast extract, 1.0; KH2PO4, 4.0; NaCl, 2.0; MgSO4, 1.0; MnSO4, 0.05; FeSO4, 0.05; CaCl2, 2.0; NH4Cl, 2.0; pH 6.0 ± 0.2). All plates were incubated (28 ± 2 °C, 96 h) and following incubation, the plates were checked for the presence of fungal colonies. The fungal isolates with discrete morphology were maintained on pectin agar slants (as pure culture) for further use (Mat Jalil et al., 2023).
2.3 Screening of pectinolytic fungi
Pectinolytic fungi were screened by inoculating individual isolates (as point inoculation) on pectin agar followed by incubation (28 ± 2 °C, 72 h). Immediately after incubation, the cultures were flooded with an aqueous iodine solution (1 % w/v) and rested (28 ± 2 °C, 15 min). A clear zone of pectin hydrolysis (surrounding the fungal growth) against the dark media background was considered ‘positive’ for pectinase production. The diameters of the colony and hydrolytic zone diameters were measured (to the nearest millimeter), and the enzyme index was calculated. The isolates exhibiting the enzyme index > 2 were considered as potent pectinase producer. The isolate demonstrating the maximum zone index was selected for optimization studies (Davanso et al., 2019).
2.4 Molecular identification and bioinformatics analysis of selected fungal isolate
The genomic DNA of the selected isolate was extracted using the Fungal Genomic DNA Spin-50 isolation kit (Chromous Biotech Pvt. Ltd., India). PCR amplification of the 18S rRNA gene sequence was performed with universal forward and reverse 18S rRNA primers (5′-GTAGTCATATGCTTGTCTC-3′ and 5′-GAAACCTTGTTACGACTT-3′, respectively). Genetic Analyser (ABI 3500 XL, Applied Biosystems, USA) and the SeqScape software (version 5.2) were used for sequencing the PCR amplicon (Bhattacharya et al., 2020). The nucleotide sequence was aligned using BLASTn with other lines available in the NCBI database (Altschul et al., 1990). Finally, the nucleotide sequence was submitted to GenBank (NCBI, USA), providing an accession number.
The gene sequence of the selected isolate and the sequences obtained from GenBank were aligned using the Molecular Evolutionary Genetic Analysis software (MEGA, version 10.1.7). Gap introduction and manual adjustment of the alignment allowed the maximum sequence similarity. The Neighbor-joining method (by Maximum Likelihood statistical method) was used to determine the best-fit substitution model for the evolutionary analysis. Partial deletion (site coverage cut-off of 95 %) was used to treat the gaps/missing data. The maximum likelihood statistical method was applied using the bootstrap method (replications = 500) to reconstruct the phylogeny. Further validation of the phylogenetic relationship between the sequences was assessed by maximum parsimony. Nearest-Neighbor- Interchange method was used to determine the tree inference (Chakraborty and Shivakumar, 2021).
2.5 Optimisation of pectinase production
One factor at a time approach (OFAT) was adopted to study the influence of process parameters on pectinase production. The effect of agro-wastes (1 % w/v: apple, grapefruit, orange, sweet lime or tomato peels) and nitrogen sources (1 % w/v: ammonium chloride, ammonium sulphate, beef extract, casein, peptone or yeast extract) were studied. Physical parameters: initial pH of the medium (4, 5, 6, 7, 8 or 9), inoculum load (0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 or 5.0 % v/v), incubation time (24, 48, 72, 96, 120 or 144 h), and effect of agitation (0, 50, 100, 150 or 200 rpm) were optimised.
2.6 Pectinase assay
Pectinase assay involves detecting D-galacturonic acid (liberated due to enzymatic pectin hydrolysis) by the dinitrosalicyclic acid method. The standard curve of D-galacturonic acid (10–100 µg/mL) was prepared. The absorbance at 540 nm was measured using a spectrophotometer (UV‐1800, Shimadzu, Japan). A unit of pectinase activity is the amount of enzyme that liberated 1 μmol of D-galacturonic acid per minute under standard experimental conditions and expressed as units per milliliter (U/mL) (Queirós et al., 2023).
2.7 Application of pectinase
The pectinase produced under submerged fermentation was subjected to the four-step purification namely ammonium sulphate precipitation (70 % saturation), dialysis, ion exchange chromatography (using DEAE‐cellulose), and gel filtration chromatography (using Sephadex G-100). With this purification strategy, the enzyme was purified 20.85-fold (to homogeneity). The partially purified pectinase exhibited 13.50 U/mL and 450 U/mg of enzyme activity and specific activity, respectively (Bhattacharyya et al., 2021).
2.7.1 Grape juice clarification
The undamaged berries of Vitis labrusca (Bangalore blue grapes) procured from the Horticultural Producers' Cooperative Marketing and Processing Society, Bangalore, India, were surface sterilised by potassium permanganate solution, rinsed multiple times in sterile deionised water and macerated to obtain the juice. The experimental conditions were: control (500 μL of physiological saline +10 mL of grape juice); test (500 μL of partially purified pectinase +10 mL of pre-heated grape juice). Pre-heating of the grape juice (85 °C, 3 min) was carried out to inactivate the natural plant enzymes that might interfere with the result. All the screw cap tubes (15 mL) were incubated (28 ± 2 °C, 1 and 3 h) and centrifuged (3864 × g, 28 ± 2 °C, 5 min). The fluids from individual tubes were aspirated, the solid residue dried, and the tubes were re-weighed. The clarification percentage was calculated as per the formula given by Sudeep et al. (2020).
2.7.2 Total phenolic content (TPC) estimation
For the TPC estimation, the 'test,' i.e., pectinase treated clarified grape juice samples (500 μL) were mixed with distilled water (4 mL), Folin-Ciocalteau reagent (400 μL) and incubated (28 °C, 5 min). After incubation, sodium carbonate solution (7 % w/v, 4 mL) was added, and the mixture volume was 10 mL with distilled water. The mixture was incubated in the dark (40 °C, 20 min). The fruit juice sample was replaced with an equal quantity of distilled water for the blank'. The standard curve of gallic acid (10–100 µg/mL) was prepared. The 'blank' and 'test' absorbance were measured at 750 nm using a UV‐1800 spectrophotometer (Shimadzu, Japan). The TPC was expressed as gallic acid equivalent or GAE (mg/L) (Molina-Cortés et al., 2020).
2.8 Statistical analysis
Statistical validation of data is the process of examining the accuracy of the data collected and determines the degree of acceptance of the results of the conducted experiments. In the present study, experiments were performed in triplicate (n = 3) and data presented graphically (mean ± standard deviation). GraphPad Prism software (version 9.4.0) was used for one-way ANOVA. One-way ANOVA was used to investigate if variations in factors have a measurable effect on the pectinase production. Post hoc analysis was run using Tukey’s multiple comparison test to compare means of all possible pairs using a studentised range distribution. Bonferroni p-value adjustment was used to determine the significant variable within a particular parameter. p-values < 0.05 were considered statistically ‘significant’.
3 Results
3.1 Isolation and screening of potent pectin utilising fungi
Pectin agar supported the growth of eleven pectin utilising fungi (designated FP1 to FP11). After calculating the zone index, isolate FP6 demonstrated a maximum value (2.57) and was chosen for the study.
3.2 Molecular identification and phylogenetic analysis of potential pectinase producer
The obtained PCR amplicon was ∼1.5 kbp. By BLASTn analysis, the resulting nucleotide sequence (1094 bp), compared with 18S rRNA gene sequences in the NCBI database, and matched with an Aspergillus sp cluster. A 96 % sequence similarity with Aspergillus terreus strain Naj522019 was recorded. Hence isolate FP6 was designated as Aspergillus terreus FP6. The A. terreus FP6 ribosomal sequence was provided an accession number (MZ068227) by GenBank (NCBI, USA).
By screening similar sequences (100 numbers) from the NCBI database, four sequences with high (100) bootstrap support values formed well-supported clades of Aspergillus sp. Jukes-Cantor (as indicated for the best-fit model for evolutionary analysis) was applied to generate the phylogenetic tree of the nucleotide dataset screened. Within individual clades, A. terreus FP6 formed a well-supported clade with the A. terreus JC-1-2-1 gene sequence (Fig. 1).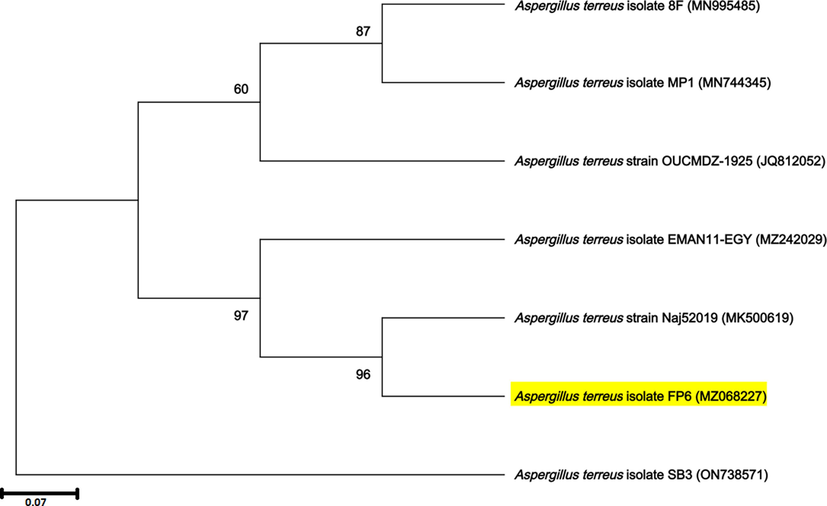
Phylogenetic tree derived from maximum likelihood and maximum parsimony analysis of gene sequences of A. terreus FP6 (analysed in the study) and its relatives from GenBank.
3.3 Effect of agro-wastes
A. terreus FP6 showed higher pectinase synthesis (24–57.3 U/mL) in medium fortified with fruit peels as compared to ‘control’ (p < 0.0001). Citrus fruit peels (orange and sweet lime) supported higher enzyme production than apple, grapefruit, and tomato peels. The highest activity (57.3 U/mL) was documented for the medium supplemented with sweet lime (Citrus limetta) peel (p < 0.0001). The succession of the pectinase production by A. terreus FP6 was as follows: sweet lime peel > orange peel > apple peel > grapefruit peel > tomato peel. The production of pectinase using tomato peel was least significant, with p value 0.0343 (Fig. 2a).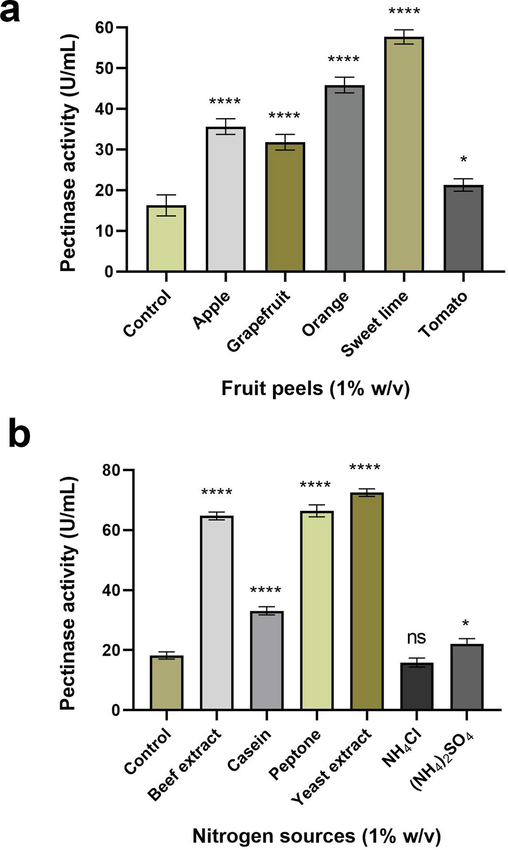
(a) Effect of agro-wastes on pectinase production by A. terreus FP6 (b) Effect of nitrogen supplements on pectinase production by A. terreus FP6. Data represent mean ± S.D. (n = 3). ns = not significant, * = p ≤ 0.05, **** = p < 0.0001.
3.4 Effect of nitrogen sources
While studying the effect of different nitrogen sources on pectinase fermentation, A. terreus FP6 could result in significant enzyme production when supplemented with organic nitrogen as compared to ‘control’ (p < 0.0001). Better enzyme production was recorded when supplemented with organic nitrogen than inorganic ones. The culture yielded the highest enzyme activity (74.6 U/mL) with yeast extract (p < 0.0001). Beef extract (63.9 U/mL; p < 0.0001) and peptone (69.3 U/mL, p < 0.0001) favoured high enzyme production. Casein moderately favoured the enzyme synthesis (35.9 U/mL; p < 0.0001). Ammonium sulphate had negligible influence on enzyme production (23.9 U/mL; p value 0.0316), while the influence of ammonium chloride (18.6 U/mL, p value 0.2580) was found to be non significant (Fig. 2b).
3.5 Effect of initial pH of the medium
The enzyme was better produced (58.7–92 U/mL) at acidic pH, and a decline in the production (73.3–41.3 U/mL) resulted as the pH values approached the alkaline range. A. terreus FP6 exhibited maximum pectinase activity (92 U/mL) at pH 6.0 (p < 0.0001). As compared to pH 6, highly significant decrease in pectinase production (p < 0.0001) was noted across extremely acidic and alkaline pH. pH 6.0 favoured the least (41.3 U/mL) enzyme production (Fig. 3a).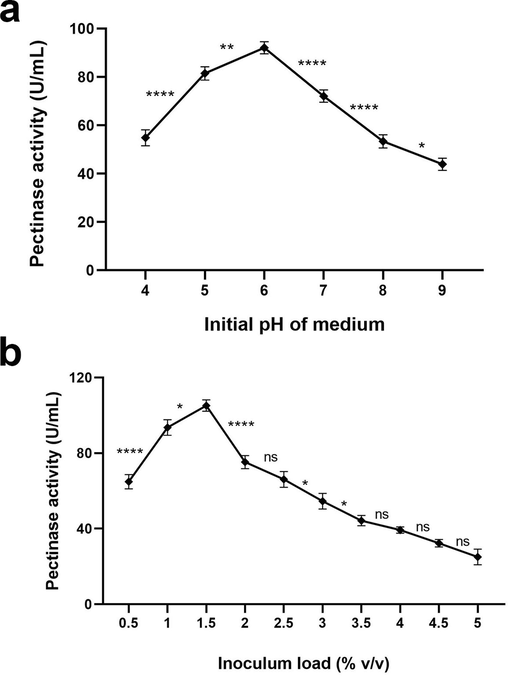
(a) Effect of initial pH of media on pectinase production by A. terreus FP6 (b) Effect of inoculum load on pectinase production by A. terreus FP6. Data represent mean ± S.D. (n = 3). ns = not significant, * = p ≤ 0.05, ** = p ≤ 0.01, **** = p < 0.0001.
3.6 Effect of inoculum load
The inoculum load consisting of different volumes of A. terreus FP6 showed variation in terms of enzyme production (69.3–20 U/mL), among which 1.5 % v/v inoculum favoured maximum pectinase activity (108 U/mL, p < 0.0001). As the inoculum load was increased, a gradual dip in enzyme production was noted with 5 % v/v inoculum resulting in least pectinase activity (20 U/mL) (Fig. 3b).
3.7 Effect of incubation time
Results demonstrated that A. terreus FP6 pectinase synthesis increased significantly and steadily with an increase in incubation time to reach a maximum (113.3 U/mL; p < 0.0001) at 72 h. Enzyme production was observed to be 28 and 84 U/mL for 24 and 48 h of incubation, respectively (p < 0.0001). Likewise, upon prolonged incubation, pectinase activity significantly decreased with the least (45.3 U/mL; p value 0.0046) recorded at 144 h (Fig. 4a).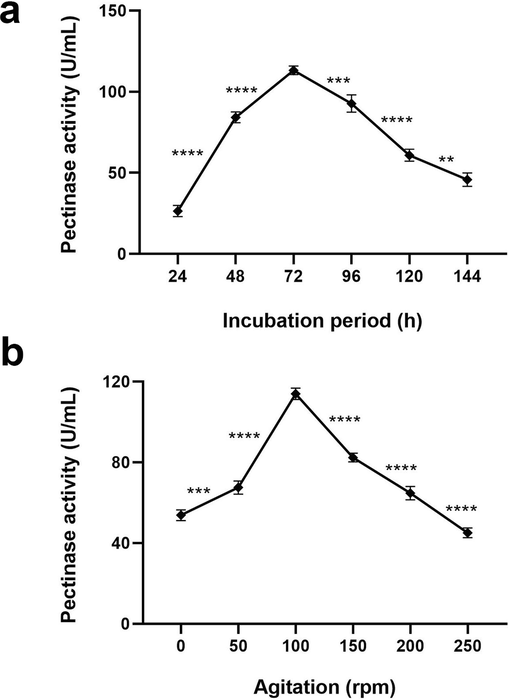
(a) Effect of incubation time on pectinase production by A. terreus FP6 (b) Effect of agitation conditions on pectinase production by A. terreus FP6. Data represent mean ± S.D. (n = 3). ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p < 0.0001.
3.8 Effect of agitation
As fermentation of pectinase by fungi is an oxygen-requiring process, optimisation of agitation speed was essential in correlating the role of aeration in pectinase synthesis. A. terreus FP6 when incubated under moderate shaking conditions, 100 rpm supported the maximum enzyme production (116 U/mL) as compared to static incubation condition (56 U/mL) (p < 0.0001). However, under the higher agitations (150 to 250 rpm), pectinase production decreased significantly (p < 0.0001), possibly due to the less contact time between the cells and pectin (Fig. 4b).
3.9 Clarification of grape juice
Following the enzymatic action, reduction in turbidity was seen as the decreasing amount of solid precipitated in the grape juice, thus making it appear clearer. With 1 and 3 h of treatment, the percentage of clarification was 34.21 % and 69.54 %, respectively. No reduction in turbidity was observed in the ‘control’. With the increase in time for pectinase treatment, the reduction in the weight of the residual plant fibres (seen as precipitate) supported the increase in clarification percentage (Fig. 5a).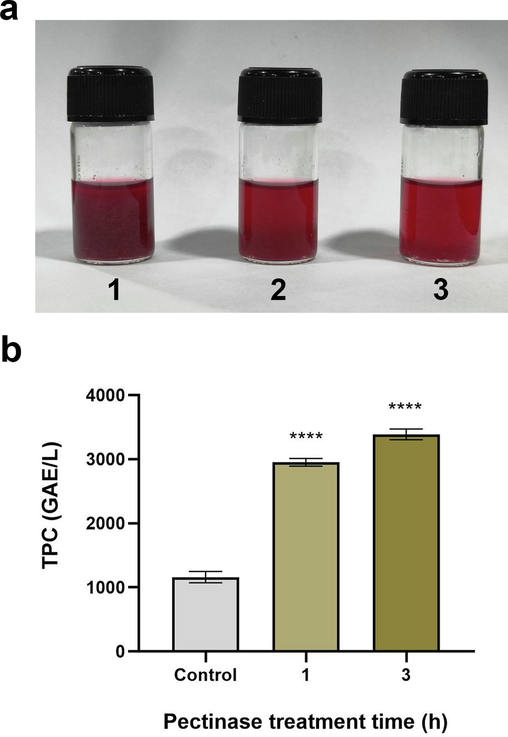
Treatment of grape juice by partially purified A. terreus FP6 pectinase (a) fruit juice clarification study (1, control; 2, treatment for 1 h; 3, treatment for 3 h) (b) TPC of grape juice pre- and post-pectinase treatment. Data represent mean ± S.D. (n = 3). **** = p < 0.0001.
3.10 Determination of TPC of treated grape juice
The TPC of the grape juice significantly increased after the treatment with A. terreus FP6 pectinase (p < 0.0001). 3004, 3484, and 1259 mg GAE/L was estimated to be the TPC in the sample treated for 1 h and 3 h and in the 'control,' respectively (Fig. 5b). Compared to the 'control,' a 2.38- and 2.76-fold increase in the TPC was recorded following 1 and 3 h of A. terreus FP6 pectinase treatment.
4 Discussion
Microbial production of primary metabolites is highly influenced by their growth, which is determined by the availability of specific nutrients. Agro-wastes are generally considered economical substrates for industrial bioprocessing. Hence, pectinase fermentation involving fruit wastes (like apple pomace, banana waste, lemon peel, and orange peel) is a lucrative option due to the easy availability of such substrates (Haile and Ayele, 2022). Fruit peels are abundant in methyl-esterified galacturonic acid (negatively charged), where the esterification level changes depending on the source. Thus, the ability of some pectinolytic fungi to produce a plethora of pectinolytic enzymes (differing in their substrate specificity) depends on the amount of available pectin in the fruit peels (Patidar et al., 2018).
The highest pectinase production by A. terreus FP6 in the medium containing C. limetta peels was documented since the substrate served as a source of heterogeneous nutrients and contains (on a dry weight basis) ash, 3.5 %; calcium pectate, 30.28 %; crude fiber, 17 %; fat, 1.7 %; lignin, 5.4 %; moisture, 12.5 %; total sugar, 14 % (Younis et al., 2015). Simultaneously, it is interesting to note that the natural oils in the C. limetta peels did not inhibit the growth of the fungal culture, which is a challenge when such agronomic wastes are considered. An earlier study showed that under favourable fermentation conditions, A. niger was a highly potent strain in endo polygalacturonase production using citrus peels (Dhillon et al., 2004). Furthermore, during the bioethanol production using sweet lime peel, cellulase and pectinase enzymes were produced by A. niger under solid-state fermentation (John et al., 2019).
Most industrially relevant microbes involved in enzyme production utilise nitrogen sources in organic forms/ inorganic forms/ both. Since nitrogen supplements profoundly influence microbial metabolism, they have been preferred for enhancing the production of extracellular pectinase (Mat Jalil et al., 2023).
Organic nitrogen sources have been documented to facilitate better fungal growth and metabolite production than their inorganic counterparts. Yeast extract being yeast cell hydrolysate, the natural attributes and chemical qualities of amino acids (alanine, arginine, aspartic acid, cystine, glutamic acid, glycine, histidine, isoleucine, phenylalanine); vitamins (biotin, cobalamin, niacin, pantothenic acid, pyridoxal, riboflavin, thiamin, tocopherol); macrominerals (calcium, magnesium, phosphorus, potassium, sodium, sulphur) and microminerals (copper, chloride, iron, magnesium, zinc) is maintained. During pectinase fermentation, A. terreus FP6 probably hydrolysed yeast extract efficiently, utilising the stored mineral component and other growth factors that can be easily incorporated into the cellular metabolism. Hence, yeast extract enhanced the growth-promoting conditions and facilitated increased pectinase production by A. terreus FP6 (Riesutea et al., 2021).
An earlier study discussing the effect of nutritional parameters on pectinase production by A. niger IBT-7 reported yeast extract (1 % w/v) to be the best-supplemented nitrogen source yielding the highest pectinase production (39.1 U/mL/min) (Abdullah et al., 2018). While studying pectinase and polygalacturonase production by a thermophilic A. fumigatus isolated from decomposing orange peels, the media supplemented with yeast extract and ammonium sulphate supported the highest enzyme production (415 U/g) (Phutela et al., 2005). During the submerged fermentation using passion fruit peel as a substrate for pectin hydrolysis, A. aculeatus URM4953 produced the highest pectinase in a medium containing yeast extract (0.2 % w/v) (Silva et al., 2019).
Microbial cells are sensitive to the concentration of hydrogen ions in the medium, as these cells lack any mechanism for adjusting their internal pH. As individual cultures vary in their pH optima, any alteration in pH on either side of the optimum value during the fermentative process results in altered microbial growth and metabolite instability. The pH of the fermentation medium plays a crucial role in enhancing pectinase production, transporting various components across the cell membrane, and managing vital cellular metabolism (Lund et al., 2020).
In the present study, the highly significant decrease in pectinase production above and below the optimum pH might have resulted from the altered cell membrane polarity resulting in the inconsistent transport of ions across the cell that affected the growth and pectinolytic enzyme synthesis by the culture. The present findings corroborate with a previous study investigating the effect of pH (5.0 to 9.0) on exo-polygalacturonase production by A. tamarii, where the results showed that the exo-polygalacturonase production reached a maximum (101.05 U/mL) at pH 6.0. On the other hand, suboptimal enzyme production was recorded at alkaline conditions (Shanmugavel et al., 2018). A. flavus-mediated pectinase production and biomass development were influenced differently by the initial pH of the medium. Results indicated that pH 5.5 acted as the best pH for pectinase production. Either an elevation or decrease in pH value beyond the optimum showed a gradual decline in enzyme production (Nsude et al., 2019). While investigating the effect of the initial pH of the medium on A. niger-mediated pectinase production, the maximum pectinase production and concentration of reducing sugar released were recorded (117.1 ± 3.4 μM/mL/min and 3.54 ± 0.9 mg/mL, respectively) at pH 5.0 (Ahmed et al., 2019).
Besides the physiological condition, the fermentation profile of any starter culture is governed by the proportion of the inoculum involved. Being transferred at its logarithmic growth stage, a higher percentage of inoculum is reported to usher microbial overcrowding and rapid exhaustion of supplements during fermentation. Alternatively, a smaller inoculum size facilitates a longer time for the cells to multiply to a significant number for better substrate utilisation through enzyme production. Thus, balancing the increasing biomass and accessible nutrient would yield optimal enzyme production (Diaz-Tang et al., 2022).
In the present study, with a particular increase in the inoculum percentage, pectinase production steadily escalated probably due to effective utilisation of the substrate by the actively growing microbial cells. On the other hand, a decline in enzyme production was noticed at higher inoculum volumes due to the development of oxygen stress and competition for nutrients among the fungal cells. The present findings are in accordance with a previous report where an increase in spore suspension of A. fumigatus (from 0.5 to 4.0 mL) improved the pectinase production. 1.5 mL of inoculum volume resulted in the highest enzyme production (3.52 U/mL) (Okonji et al., 2019). The analysis of different inoculum sizes of A. niger indicated a maximal pectinase yield (1.64 U/mL) using the inoculum size of 1 × 106 spores/mL (Darah et al., 2013).
The growth rate of microbial culture governs incubation time and primarily enables the steady state of growth followed by metabolite synthesis. Therefore, altering the incubation time can result in varying concentrations of the metabolite yield (Gonzalez and Aranda, 2023).
In the present study, upon prolonged incubation, pectinase activity substantially decreased, possibly due to an impaired fungal metabolism resulting from the depletion of indispensable nutrients and agglomeration of toxic auxiliary metabolites in the medium. Thus, it resulted in the inactivation of the secreting machinery of the pectinase. An earlier study studied different incubation times (0 to 120 h) for A. niger IBT-7 to determine the maximum pectinase productivity. As incubation proceeded, the enzyme production gradually increased and reached its maximal point after 72 h (Abdullah et al., 2018). While optimising the fermentation conditions for A. flavus pectinase production using African star cherry pectin, the incubation period varied for five periods (till five days) at 35 °C. The highest specific activity of 2.13 U/mg was obtained at 96 h (Nsude et al., 2019).
During submerged fermentation, agitation under the controlled condition not only improves biomass (cells/hyphae) and oxygen exchange between the various phases but also maintains homogeneous chemical and physical conditions in the medium by continuous mixing and thereby promoting the optimal cellular growth and improved production of enzymes (Lizardi-Jiménez and Hernández-Martínez, 2023).
In the present study, the moderate agitation of the medium not only helped inadequate mixing of the fungal mass but also played a role in better nutrient availability, oxygen, and heat transfer to the starter culture. With the higher agitations (150 to 250 rpm), pectinase production decreased, possibly due to the less contact time between the cells and pectin. Under static conditions, inadequate/ no mixing of the broth affected the enzyme synthesis due to the developed oxygen tension, as oxygen acts as a terminal electron acceptor for oxidative reactions to provide energy for cellular activities. In addition, changes in the morphology of microorganisms caused by agitation might have also influenced microbial growth and enzyme production (Umar et al., 2023).
For the systematic investigation of the effects of agitation on pectinase synthesis from A. niger HFD5A-1, pectinase activity increased with an enhancement of agitation speed, reaching the maximum (1.56 U/mL) at 150 rpm. However, beyond 150 rpm, mycelial degeneration caused by shear stress resulted in lower pectinase production. Similarly, decreased enzyme activity at lesser agitation speed could be due to lower dissolved oxygen (Darah et al., 2013).
Pectinase treatment not only enhances the juice quality by reducing the bitterness and cloudiness but also enhances the overall appearance by preventing the darkening of juice and providing the characteristic color, all of which are important in terms of the market acceptability of the juices (Hosseini et al., 2021).
In the present study, grape juice treated with A. terreus FP6 pectinase appeared clearer since the enzyme lysed the pectin polysaccharide into negatively charged galacturonic acid (i.e., small monomers). Eventually, the negatively charged monomers and the positively charged proteins produced the protein-galacturonic acid complex. Finally, due to the lysis of larger pectin complexes in the juice (leading to a reduction in residual weight) coupled with the electrostatic agglomeration of the oppositely charged particles, a decrease in turbidity or improvement in the clarification percentage of grape juice was achieved (Larsen et al., 2021).
The present study's findings are in corroboration with earlier literature, where A. niger SH-2 pectinase treatment to orange, apple and grape juices showed 19.2-fold, 7.3-fold and 3.8-fold increase in light transmittance respectively (He et al., 2018). Pectinases produced by A. niger LB-02-SF, when applied to apple and grape juices, were almost similar in terms of the performances of two high-quality commercial enzyme formulations, namely Novozym 33095 and Pectinex Ultra SP-L in reducing the turbidity and improving the clarity of juices (Poletto et al., 2015).
The results of the present research indicated that the extractability of phenolic compounds from grape juice increased with the usage of pectinase and is associated with improved color. The documented TPC values in the present work are higher than those reported for any grape juice treated with commercial pectinases. Moreover, the A. terreus FP6 pectinase seems to be more efficient than the commercial enzymes, with less reaction time.
A previous study indicated Thermomucor indicae-seudaticae-N31 pectinase (PEP-N31) and commercial pectinase, i.e., Vinozym Vintage FCE (Novozyme, Germany) for a four-hour treatment to obtain higher phenolic levels in the pre-heated grape juice. The TPC in the enzyme-treated juice was 1677.25 and 1682.10 mg GAE/L, respectively, for PEP-N31 and Vinozym Vintage FCE. For the 'control', the TPC was 1422.59 mg GAE/mL (de Carvalho Tavares et al., 2020). When pectinolytic and cellulosic enzymes were used, the improvement in the extractability of phenolic compounds from Concord grape juice was evident. Compared to the ‘control’, the enzymatic treatments provided an increase of 20.55 %, and 15.75 %, 6.43 % in total phenolic acids, respectively for Pectinex Ultra Clear, respectively, Lallzyme Beta, and both (Magro et al., 2016).
5 Conclusion
The present work, for the first time, reveals the potential of A. terreus FP6 to produce pectinase from valorised Citrus limetta peel and the utilisation of the enzyme in reducing the turbidity of grape juice. Besides the ubiquitous nature of the fungal source, its non-fastidious nutritional requirement would help formulate cost-effective pectinase fermentation. Furthermore, the increase in TPC and decrease in turbidity of the grape juice following the A. terreus FP6 pectinase treatment was impressive. A 2.38- and 2.76-fold increase in TPC and 34.21 % and 69.54 % of grape juice clarification was recorded after 1 h and 3 h of enzyme treatment, respectively. This indicated that the catalytic potential of the enzyme was significantly superior to the existing commercial pectinase formulations. The result advocates the strong candidature of the A. terreus FP6 pectinase application in large-scale enzyme-assisted fruit juice clarification. However, with the intent to improve the enzyme production and catalytic properties, future research must focus on the genetic recombination based fermentation approaches and in silico methods of protein modification.
Accession number
The GenBank accession number (MZ068227) for the Aspergillus terreus isolate FP6 registered in the NCBI database is provided in the manuscript. The link for the same is https://www.ncbi.nlm.nih.gov/nuccore/MZ068227.
Code availability
SeqScape software (version 5.2), Molecular Evolutionary Genetic Analysis software (version 10.1.7), GraphPad Prism software (version 9.4.0).
Consent for publication
All the authors mutually agreed for submitting the manuscript. The manuscript has not been published elsewhere, either wholly, in part, or another form. The manuscript has not been submitted to another journal and will not be published elsewhere.
Funding
The authors extend their appreciation to the Researchers Supporting Project Number (RSP2024R419), King Saud University, Riyadh, Saudi Arabia.
CRediT authorship contribution statement
Dibbyangana Mukhopadhyay: Conceptualization, Data curation, Methodology, Writing – original draft, Formal analysis. Rajrupa Bhattacharyya: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Sourav Bhattacharya: Conceptualization, Validation, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Methodology, Project administration, Supervision. Bassam Khalid Alnafisi: Funding acquisition.
Acknowledgments
The authors are grateful to the management of JAIN (Deemed-to-be University) for providing the research facilities. The Researchers Supporting Project Number (RSP2024R419), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Pectinase production from Aspergillus niger IBT-7 using solid state fermentation. Bangladesh J. Bot.. 2018;47:473-478.
- [CrossRef] [Google Scholar]
- Optimization of pectinase production from Geotrichum candidum AA15 using response surface methodology. Pak. J. Bot.. 2019;51:743-750.
- [CrossRef] [Google Scholar]
- Prospects of Stenotrophomonas pavanii DB1 in diesel utilization and reduction of its phytotoxicity on Vigna radiata. Int. J. Environ. Sci. Technol.. 2020;17:445-454.
- [CrossRef] [Google Scholar]
- Thermostable and organic solvent-tolerant acid pectinase from aspergillus terreus FP6: purification, characterization and evaluation of its phytopigment extraction potential. 3 Biotech.. 2021;11:487.
- [CrossRef] [Google Scholar]
- Lignocellulosic agricultural waste valorization to obtain valuable products: An overview. Recycling.. 2023;8:61.
- [CrossRef] [Google Scholar]
- Bioprospecting of the agaricomycete Ganoderma australe GPC191 as novel source for L-asparaginase production. Sci. Rep.. 2021;11:6192.
- [CrossRef] [Google Scholar]
- Effects of pectinase pre-treatment on the physicochemical properties, bioactive compounds, and volatile components of juices from different cultivars of guava. Foods.. 2023;12:330.
- [CrossRef] [Google Scholar]
- Involvement of physiochemical parameters on pectinase production by Aspergillus niger HFDFA-1. J. Pure. Appl. Microbiol.. 2013;7:2541-2549.
- [Google Scholar]
- Assessment of pectinase-producing fungi isolated from soil and the use of orange waste as a substrate for pectinase production. Rev. De Ciênc. Farm. Básica. Apl.. 2019;40:e635.
- [Google Scholar]
- The improvement of grape juice quality using Thermomucor indicae-seudaticae pectinase. J. Food Sci. Technol.. 2020;57:1565-1573.
- [CrossRef] [Google Scholar]
- Studies on the utilization of citrus peel for pectinase production using fungus Aspergillus niger. Int. J. Environ. Stud.. 2004;61:199-210.
- [CrossRef] [Google Scholar]
- Growth productivity as a determinant of the inoculum effect for bactericidal antibiotics. Sci. Adv.. 2022;8:eadd0924.
- [CrossRef] [Google Scholar]
- A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind's challenges. J. Fungi (Basel). 2022;8:23.
- [CrossRef] [Google Scholar]
- Microbial growth under limiting conditions-future perspectives. Microorganisms.. 2023;11:1641.
- [CrossRef] [Google Scholar]
- Pectinase from microorganisms and its industrial applications. ScientificWorldJournal. 2022;2022:1881305.
- [CrossRef] [Google Scholar]
- Efficient over-expression and application of high-performance pectin lyase by screening Aspergillus niger pectin lyase gene family. Biotechnol. Bioprocess Eng.. 2018;23:662-669.
- [CrossRef] [Google Scholar]
- Continuous clarification of barberry juice with pectinase immobilised by oxidized polysaccharides. Food Technol. Biotechnol.. 2021;59:174-184.
- [CrossRef] [Google Scholar]
- Production of bioethanol from sweet lime peel via a statistically optimized simultaneous saccharification and fermentation process using isolated enzymes. Energy Sources A: Recovery. Util. Environ. Eff.. 2019;44:1-9.
- [CrossRef] [Google Scholar]
- Pectinases as promising green biocatalysts having broad-spectrum applications: Recent trends, scope, and relevance. Biotechnol. Appl. Biochem.. 2023;70:1663-1678.
- [CrossRef] [Google Scholar]
- Effects of ultrasound on the enzymatic degradation of pectin. Ultrason. Sonochem.. 2021;72:105465
- [CrossRef] [Google Scholar]
- Oxygen and hydrocarbon volumetric transfer coefficients in the production of an oil-degrading bacterial consortium: emulsifying activity and surface tension in a bioreactor. 3 Biotech.. 2023;13:146.
- [CrossRef] [Google Scholar]
- Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol.. 2020;11:556140
- [CrossRef] [Google Scholar]
- Synergistic effects of Pectinex Ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. LWT Food Sci. Technol.. 2016;72:157-165.
- [CrossRef] [Google Scholar]
- Assessment of cultivation parameters influencing pectinase production by Aspergillus niger LFP-1 in submerged fermentation. J. Genet Eng. Biotechnol.. 2023;21:45.
- [CrossRef] [Google Scholar]
- Spectrophotometric estimation of total phenolic content and antioxidant capacity of molasses and vinasses generated from the sugarcane industry. Waste Biomass Valor.. 2020;11:3453-3463.
- [CrossRef] [Google Scholar]
- Optimization of fermentation condition for pectinase production from Aspergillus flavus using African star cherry pectin as a carbon source. Int. J. Ecol. Environ. Sci.. 2019;1:15-19.
- [Google Scholar]
- Purification and biochemical characterization of pectinase produced by Aspergillus fumigatus isolated from soil of decomposing plant materials. J. Appl. Biol. Biotech.. 2019;7:1-8.
- [CrossRef] [Google Scholar]
- Pectinolytic enzymes-solid state fermentation, assay methods and applications in fruit juice industries: a review. 3 Biotech.. 2018;8:199
- [CrossRef] [Google Scholar]
- Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels. Braz. J. Microbiol.. 2005;36:63-69.
- [CrossRef] [Google Scholar]
- Downstream processing of pectinase produced by Aspergillus niger in solid state cultivation and its application to fruit juices clarification. Food Sci. Technol.. 2015;35:391-397.
- [CrossRef] [Google Scholar]
- Tunning pectinase activity under the effects of electric fields in the enhanced clarification of wine must. Front. Sustain. Food Syst.. 2023;7:1053013.
- [CrossRef] [Google Scholar]
- Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci. Technol.. 2021;108:1-10.
- [CrossRef] [Google Scholar]
- A study on pectinases from Aspergillus tamarii: toward greener approach for cotton bioscouring and phytopigments processing. Biocatal. Agric. Biotechnol.. 2018;15:295-303.
- [CrossRef] [Google Scholar]
- Optimized production of Aspergillus aculeatus URM4953 polygalacturonases for pectin hydrolysis in hog plum (Spondias mombin L.) juice. Process Biochem.. 2019;79:18-27.
- [CrossRef] [Google Scholar]
- Production, characterization, and industrial application of pectinase enzyme isolated from fungal strains. Fermentation.. 2020;6:59.
- [CrossRef] [Google Scholar]
- Agitation role (dissolved oxygen) in production of laccase from newly identified Ganoderma multistipitatum sp. nov. and its effect on mycelium morphology. BMC Microbiol. 2023;23:280.
- [CrossRef] [Google Scholar]
- Renovation of agro-waste for sustainable food packaging: A review. Polymers.. 2023;15:648.
- [CrossRef] [Google Scholar]
- Effect of addition of mosambi (Citrus limetta) peel powder on textural and sensory properties of papaya jam. Cogent. Food Agric.. 2015;1:1023675.
- [CrossRef] [Google Scholar]







