Translate this page into:
Deciphering the effect of Potentilla fulgens root extract against healthy HUVEC cell line and cancer cell lines (A549 and SKOV-3)
⁎Corresponding authors. m.firatbaran@gmail.com (Mehmet Fırat Baran), hrovshan@hotmail.com (Rovshan Khalilov)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Background
Potentilla fulgens, a highly valued indigenous medicinal herb grown in high altitudes of the Himalayan region with anticancer, hypoglycaemic, antibacterial, anti-inflammatory, and antiulcerogenic properties, are used in traditional systems of medicine. This study was aimed to investigate the effect of P. fulgens root extract, as one of the natural alternatives to chemotherapeutic drugs used in cancer treatment, on proliferation, apoptosis, and autophagy of human non-small cell lung cancer cell line (A549), human ovarian cancer cell line (SKOV-3), and healthy human umbilical vein endothelial cell line (HUVEC).
Methods
Anti-proliferative effect was assessed by MTT assay. The expression of autophagy and apoptosis-related proteins was evaluated by western blotting. Total oxidant status (TOS) and total antioxidant capacity (TAC) test were determined using standard kit methods.
Results
Our results showed that the extract inhibited proliferation of HUVEC, A549, and SKOV-3 cells in a dose-dependent manner. MTT assay analysis revealed that the extract significantly (P<0.05) induced mortality in HUVEC, A549, and SKOV-3 cells. Western blot results revealed increased expression of NF-κB after the extract treatment but led to the down-regulation in Beclin-1, Bax, extracellular-signal-related kinase 1 and 2, Sequestosome-1, and cleaved Casp-3 levels. Treatment groups showed an increase in TOS and TAC values in A549 and SKOV-3 cell lines, while HUVEC cell line showed an increase in TAC and a decrease in TOS values, compared to the control group.
Conclusions
Our findings indicated that P. fulgens root extract inhibited the proliferation of healthy cells and cancer cells through cell cycle arrest, representing its limited application as therapeutic agent in cancer treatment.
Keywords
P. fulgens
Cancer
Autophagy
Apoptosis
Beclin
Bax
1 Introduction
Lung cancer is one of the most prevalent cancer types in the world. Unfortunately, less than 15 % of patients survive for few years, despite new treatment approaches. (Mir et al., 2013). Ovarian cancer is the deadliest of all gynecologic cancers and the fifth most common cancer in women. When this cancer is detected in the Phase I stage, the patient's survival rate exceeds 90 %, but when the disease reaches the Phase III or Phase IV stage, this rate drops below 20 % (Bunn Jr and Franklin, 2002).
The activation of the cellular apoptotic mechanism is a promising target for cancer treatment. In this regard, the suppression of the apoptotic pathway during cancer can occur through the over-expression of anti-apoptotic proteins or the under-expression of pro-apoptotic proteins (Pfeffer and Singh, 2018). Additionally, tumor cells can potentially avoid apoptosis by increasing the expression of caspase inhibitors and reducing the expression of Bax (Erdoğan and Uzaslan, 1998).
Autophagy prolongs cell survival in tumor cells with a damaged apoptosis mechanism (Hashemi et al., 2023). Autophagy is the cell's self-destructing process to clean up damaged or unnecessary proteins and organelles and balance energy sources in response to nutritional stress (Hashemi et al., 2023). Autophagy is a process that helps cells to survive under stress by breaking down and recycling cellular components. When autophagy is compromised, cells and tissues may be exposed to the toxic effects of excessive accumulation of autophagy substrates (Aman et al., 2021). Tumor cells, however, have high basal levels of autophagy and may depend on it for their survival (Aman et al., 2021). In hypoxic regions, autophagy is induced in tumor cells, which gives them a survival advantage (Kabakov et al., 2021). Therefore, inhibiting autophagy may be a new way to target cancer therapy by preventing tumor cells from surviving (Kartlaşmış et al., 2018, Sutton et al., 2018).
Chemotherapy and radiotherapy are accompanied by different side effects, and some tumors develop resistance to chemotherapy (Liu et al., 2021). To solve these problems, there is a push towards the use of natural substances that stimulate apoptosis in cancerous cells but do not harm normal tissues (Limtrakul et al., 1997). Potentilla fulgens is an annual plant belonging to the Rosaceae family which grows in temperate and high altitude (1800–4350 m) regions in the Northern and Northeastern regions of India, with a thick woody stem, long thin leaves, and yellow flowers (Laloo, 2013). The roots of P. fulgens and the whole plant have traditionally been used to treat gum and dental disorders (pus discharge, toothache, and tooth decay), diarrhea, stomach problems (peptic ulcers), coughs, colds, diabetes, and cancer (Kumar et al., 2013). Phytochemical analysis of P. fulgens root extract identified important bioactive compounds such as alkaloids, tannins, polyphenols, terpenoids, and flavonoid (Tomczyk and Latté, 2009). The extract's rich content of therapeutically important bioactive compounds has made it a suitable candidate in terms of the use of natural substance in cancer treatment (Anal et al., 2014; Ozukum et al., 2023). In vitro studies on various cancer cell lines showed that it had anti-proliferative effects (Anal et al., 2014; Ozukum et al., 2023). The current study was designed to investigate the effects of P. fulgens root extract on cell proliferation, apoptosis, and autophagy of human “non-small cell” lung cancer cell line (A549), human ovarian cancer cell line (SKOV-3), and normal human umbilical vein endothelial cell line (HUVEC).
2 Materials and methods
2.1 Preparation of P. fulgens root extract
The aqueous extract of P. fulgens roots used in the study was supplied in dry powder form from Xi'an Yuensun Biological Technology Company (China Spe:20:1 Batch No:YS-EM-131030A). It was dissolved in 2 % ethanol and heated in a boiling water bath for 10 min before being cooled. The solution was centrifuged at 2000 rpm for 10 min in glass tubes, and the resulting supernatant was stored at 4 °C for further use (İpek et al., 2019).
2.2 Cytotoxic activities of extract
Cell viability tests were performed against cancer cell lines (A5459 and SKOV-3) and the healthy HUVEC cell line using MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (Mosmann, 1983). The cell lines were obtained from the American Type Culture Collection (ATCC). A549, SKOV-3, and healthy HUVEC cell lines were grown in a T75 flask using RPMI-1640 medium (Sigma-Aldrich R8758, USA) with 10 % FBS, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin. The flask was incubated at 37 °C with 5 % CO2. Cells were counted using the hemocytometric method after reaching 80–90 % confluency. At regular time interval (24, 48, and 72 h), cells (5 × 103 cells) were seeded into 96-well plates in three replications. The cells were left to adhere before further experimentation. Then, the cells were washed with phosphate buffered saline and the extract was added at different concentrations (100, 80, 60, 40, 20, 10, and 5 µg/mL). Ethanol (2 %) was used as negative control. At 24, 48, and 72 h, the medium was aspirated and 25 µL of MTT solution was added into each well. After 4 h of incubation at room temperature, 100 µL of dimethyl sulphoxide was added to each well and left undisturbed for 1 h. The absorbance was read at 570 nm using micro plate reader. The control wells’ average absorbance value was considered 100 % viable cell. The absorbance values obtained from the wells that were treated with the extract were compared to the control absorbance value and represented as % viability.
2.3 Total oxidant status (TOS) and total antioxidant capacity (TAC) level
The logIC50 values obtained from the MTT results were used to determine the TOS and TAC. After 24 h, the cell suspensions were removed using Trypsin-EDTA, and then centrifuged at 1000 × g (2–8 °C) for 20 min to collect supernatant. The commercially available TOS Assay Kit and TAC Assay Kit (Rel Assay Diagnostic-Turkey) were used for analyzing TOS and TAC, respectively using the collected supernatant (Öztoprak et al., 2022). Further, oxidative stress index (OSI) was calculated as:
2.4 Western blot
Cells were cultured and exposed to the extract at effective doses determined by MTT, with 2 % ethanol control group. Cell lysates were made with RIPA lysis buffer and a protease-phosphatase inhibitor. Total cellular protein concentration was measured with a BCA protein assay kit (Kandemir and Ipek, 2023).
Protein samples were adjusted to 20 µg and mixed with 2X Laemmli sample buffer with 5 % p-mercaptoethanol. After heating at 95 °C for 5 min, samples were separated on an SDS-polyacrylamide gel. The proteins were transferred to a PVDF membrane and blocked in PBS-T using 5 % skim milk for 1 h. Samples were incubated with primary antibodies overnight at 4 °C, followed by the addition of secondary antibodies for 1 h at room temperature. After washing, the signal was visualized and analyzed using software. Antibodies used included Beclin, cleaved caspase, Bax, ERK ½, p-ERK, NF-κB, p-NF-κB, SQSTM1, and β-actin as the internal control (Kandemir and Ipek, 2023).
2.5 Statistical analysis
The GraphPad Prism 8 program calculates the value of extract's inhibitory concentration (IC50). The data were analyzed using Statistical Package for the Social Sciences (SPSS) Inc Chicago, IL, USA, software version 21. Data were compared between groups using unpaired t-test and among multiple groups by one-way ANOVA, followed by Tukey's post hoc tests. P<0.05 was considered statistically significant (Cui et al., 2023).
3 Results and discussion
3.1 Cytotoxicity assay
Figs. 1 and 2 represent the cytotoxic activities of extract against A549, SKOV-3, and healthy HUVEC cells. The logIC50 values were estimated as 1.740, 1.436, and 1.177 μg/mL for A549 cells; 2.268, 1.970, and 1.487 μg/mL for SKOV-3 cells; and 1.561, 1.214, and 1.051 μg/mL for HUVEC cells at 24, 48, and 72 h, respectively. No significant (P>0.05) difference at 48 and 72 h was observed for A549 and HUVEC cells, while significant (P<0.05) differences were observed for SKOV-3 at 24–72 h.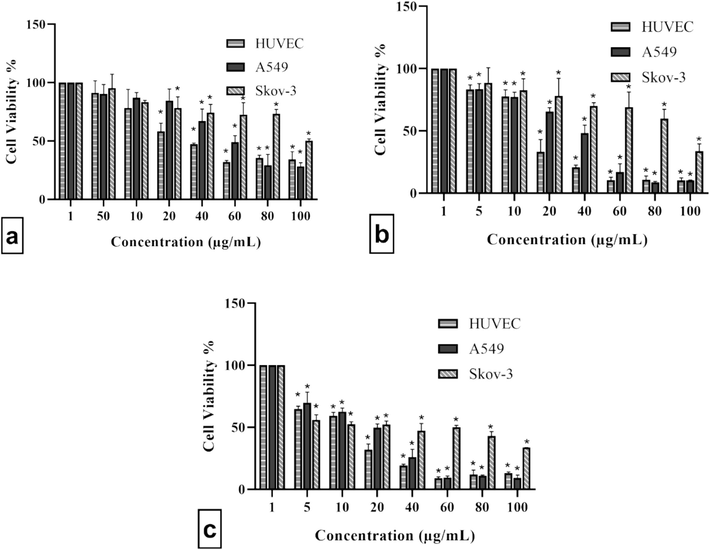
Treatment of HUVEC, A549, and SKOV-3 cells with different concentrations of extract for (a) 24, (b) 48, and (c) 72 h. The extract suppressed HUVEC, A549, and SKOV-3 cells viability in a dose–dependent manner. Data were presented as mean ± standard deviation with significance level of *P<0.05.
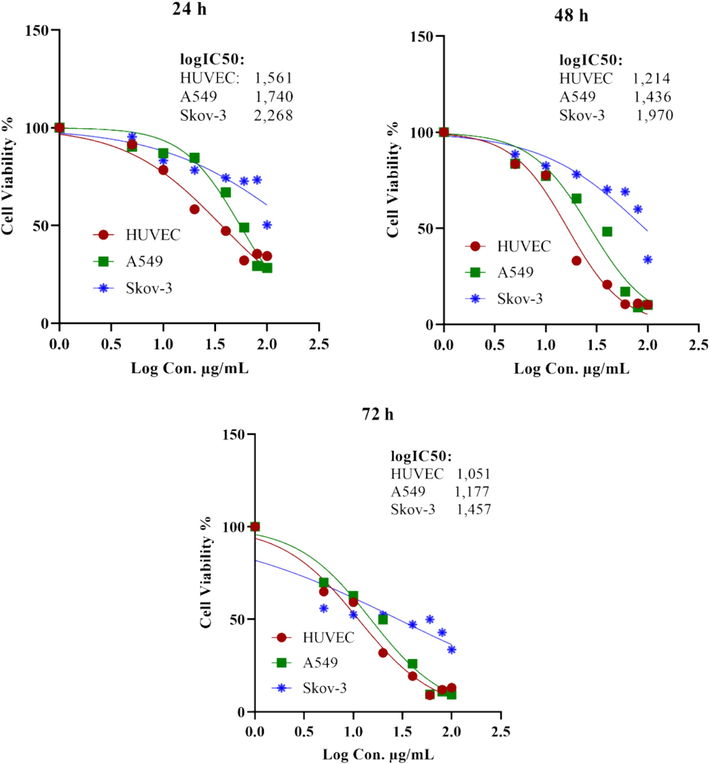
LogIC50 values for HUVEC, A549, and SKOV-3 cell lines after extract exposure for 24, 48, and 72 h.
P. fulgens root extract (methanolic, butanolic, and dichloromethane) have been found to be cytotoxic against glioblastoma cancer cell lines (U87, U118, and T98G) (Kandemir and Ipek, 2023). In another study, the methanolic extract also increased the survival rate of mice with Ehrlich ascites cells and inhibited the growth of MCF-7 cells in a dosage-dependent manner (Radhika et al., 2012). In the present study, P. fulgens root extract inhibited the growth of A549 and SKOV-3 cells, which was consistent with the findings of previous studies. However, it has been determined that the growth inhibitory effect in healthy cell line is similar to the cancer cell lines (Kandemir and Ipek, 2023).
3.2 TOS and TAC analyses
Treatment groups showed an increase in TOS and TAC values in A549 and SKOV-3 cell lines, while HUVEC cell line showed an increase in TAC and a decrease in TOS values compared to the control group (Fig. 3). However, OSI values were significantly decreased in all three cell lines.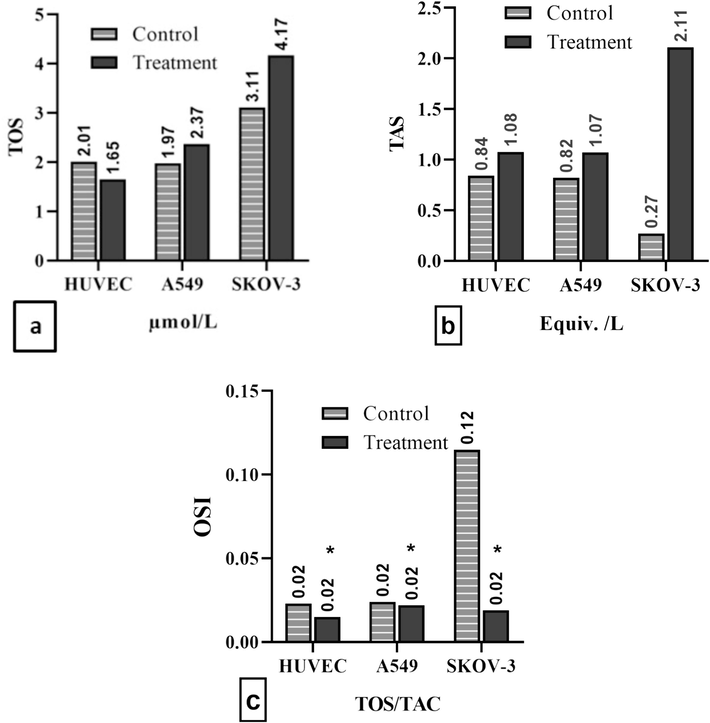
(a) TOS, (b) TAC, and (c) OSI in cell lines. Data were presented as mean ± standard deviation with significance level of *P<0.05.
Polyphenols in P. fulgens root extract has potent antioxidant capacity and protective effects on human health, including neoplastic diseases, as they contribute to radical scavenging and antioxidant activity. Other antioxidant compounds such as carotenoids and vitamins also contribute to radical scavenging activities (Kumar et al., 2013). Our study demonstrated the antioxidant properties of P. fulgens root extract by causing an increase in the total antioxidant status of the extract in cancer cells and healthy cells in vitro. However, despite the decrease in total oxidant saturation in healthy cells, the increase in TOS levels in cancer cells suggests that different mechanisms of the extract come into play in these cells. The decrease in OSI values in all cell lines suggests that the extract may cause a decrease in the formation of oxidant molecules or the release of reactive oxygen species in the cell.
3.3 Bax and Beclin-1 expression
In A549, SKOV-3, and HUVEC cells, the level of Bax protein was significantly (P<0.05) lower than the control groups. Fig. 4 displays the relative amounts of Bax protein expression. Bax can be found either in the cytoplasm or weakly bound to the membrane when inactive. However, exposure to a death signal can activate Bax in the mitochondria. Higher levels of Bax may suggest that apoptosis is being induced (Perego et al., 1996). Our findings showed that P. fulgens root extract exerted anti-proliferative effects and caused apoptosis in all tested cell lines, thereby indicating cell death by the extract through a Bax-independent pathway.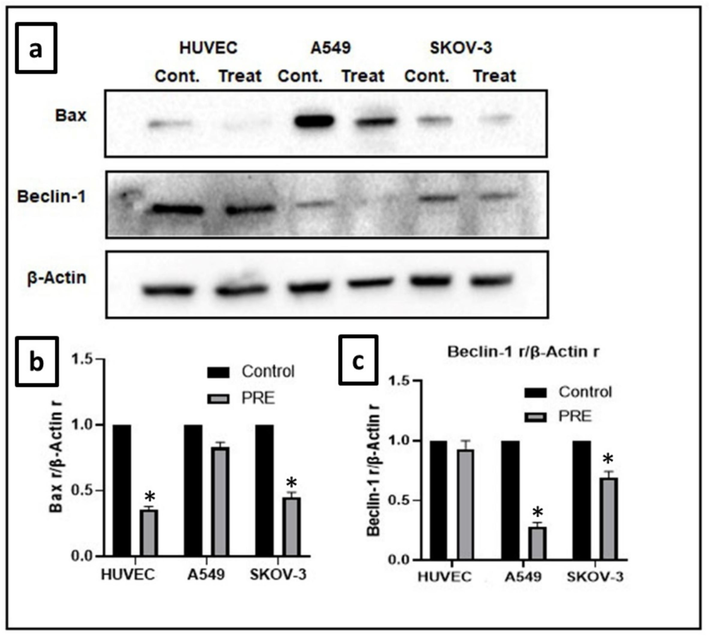
Effect of extract on expression of Bax and Beclin-1 protein in cell lines. (a) In A549, SKOV-3, and HUVEC cell lines, the Bax levels and the autophagy marker Beclin-1 levels were decreased compared to the control groups. The reduction in Bax was significant (P<0.05) in both HUVEC and SKOV-3 cell lines, while the reduction in Beclin-1 was significant (P<0.05) in both A549 and SKOV-3 cell lines. (β-actin is the loading control). (b) The intensity of Bax was analyzed through densitometry and presented as a ratio to the total level of β-actin. (c) The intensity of Beclin-1 was analyzed through densitometry and presented as a ratio to the total level of β-actin. (Control, arbitrarily set to 100 %; *P<0.05). PRE − P. fulgens root extract.
Fig. 4 shows the relative amounts of Beclin-1 protein expression. Our results showed that the extract substantially plummeted Beclin-1 expression. Autophagy is a crucial process that helps maintain the balance within cells by breaking down and recycling damaged cytoplasmic components, such as organelles and macromolecules. Without autophagy, survival against stress is compromised, and cells and tissues are exposed to the toxic effects of excessive accumulation of autophagy substrates. Cancer cells can use autophagy to recover from the stresses of adverse conditions, and this protective mechanism can also make them resistant to anticancer therapy. Inhibiting autophagy can enhance the therapeutic response and increase the effectiveness of cancer treatment. Beclin-1 is one of the first autophagy genes reported in association with cancer. This protein plays a central role in autophagy. In association with Beclin-1, autophagy can occur in both tumor and normal tissue. Autophagy in tumor cells increases tumor cell viability (Sahni et al., 2014, Liu et al., 2017). Inhibition of autophagy may be a new target for cancer therapy, as it could prevent the survival of tumor cells. (Schweichel and Merker, 1973). In this study, the expression of Beclin-1 was plunged in treatment groups, which has been associated with the tumor-suppressive property of autophagy (Yue et al., 2003).
3.4 Nuclear factor-kappa B (NF-kB) expression
Western blotting was used to evaluate the phosphorylation level and expression of NF-kB protein. The results showed that the phosphorylation level decreased (p-NF-kB) by inducing NF-kB protein expression compared to the control groups. Statistical analyses showed that this decrement was significant (P<0.05) for HUVEC and A549 cell lines. The relative amounts of NF-kB protein expression are shown in Fig. 5.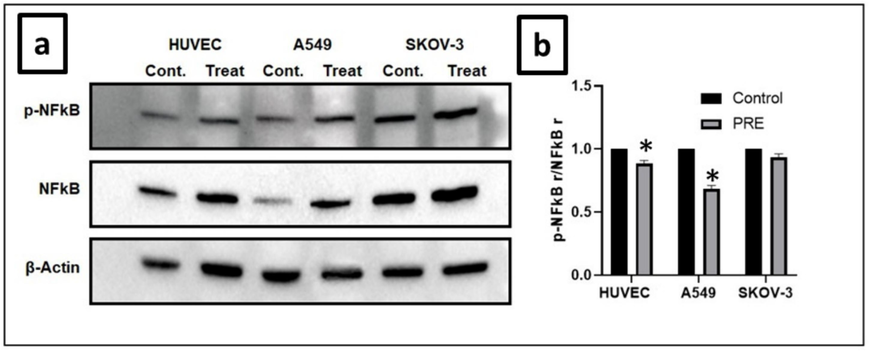
Effect of extract on NF-kB expression in cell lines. (a) In A549, SKOV-3, and HUVEC cells, the extract caused an increase in phosphorylation by promoting the expression of NF-kB protein. This increase was significant in the HUVEC and A549 cell lines. (b) The intensity of p-NF-kB was analyzed through densitometry and presented as a ratio to the total level of NF-kB. (Control, arbitrarily set to 100 %). (*P<0.05). PRE − P. fulgens root extract.
NF-kB prevents cell death by activating genes that produce proteins inhibiting apoptosis, such as Bcl-2 family members, c-Flip, and IAPs. It also increases the production of antioxidant proteins that prevent reactive oxygen species-induced apoptosis and necrosis. However, the effect of NF-kB on apoptosis depends on the type of death stimulus present, and in unfavorable conditions, it can activate proteins that resist apoptosis (Karin, 2006). NF-kB probably exerts both anti- and pro-apoptotic functions, determined by the state of death stimulus rather than tissue origin. In inappropriate physiological conditions, NF-kB causes resistance to the apoptotic stimulus through the activation of a host of complex proteins. However, NF-kB activation in response to certain stimuli can lead to the induction of apoptosis. Activating some pro-apoptotic proteins such as caspases, interferon-regulated factor-1, c-myc, and p53 can explain this issue (Ghobrial et al., 2005). The function of NF-kB in apoptosis is bidirectional. Previous findings reported that NF-kB mediated the apoptosis, while few studies reported the anti-apoptotic effect of NF-kB. Continuous suppression of NF-kB in tumors prevents cell proliferation, inhibits the cell cycle, and causes apoptosis. The NF-kB pathway may have different effects in various types of cancer (Shishodia and Aggarwal, 2002). In our study, it is thought that the anti-proliferative effect of the extract may be due to the suppression of the NF-kB pathway.
3.5 ERK ½ expression
Fig. 6 displays the relative amounts of ERK ½ protein expression. Our findings indicated that the extract decreased ERK ½ expression and caused a significant reduction in phosphorylation levels of A549 cells. The ERK ½ pathway is responsible for regulating various cellular processes, including cell proliferation. It has been reported that interfering with the ERK signal cascade can interrupt DNA repair processes (Mi et al., 2023). Additionally, ERK ½ plays a crucial role in signal transduction, stimulating cell proliferation, growth, and differentiation. Studies have revealed that the activation of the ERK pathway is associated with the pathology, progression, and oncogenic behavior of various types of human cancers, including non-small lung cell cancer, breast cancer, head and neck squamous cell cancer, and colorectal cancer (De Luca et al., 2012). ERK ½ activates p90RSK, which, in turn, activates CREB, inducing the expression of Bcl-xL and Bcl-2. These Bcl-2 family members play a critical role in preserving the integrity of mitochondria by preventing cytochrome c release and subsequent caspase-9 activation (Liu et al., 2015).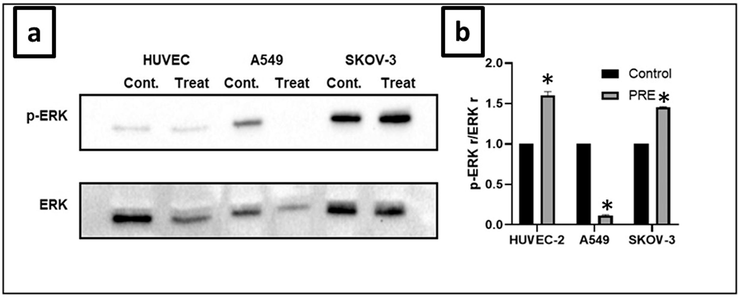
Effect of extract on ERK ½ protein expression in cell lines. (a) The extract inhibited ERK ½ protein expression in A549, SKOV-3, and HUVEC cells, reducing phosphorylation. (b) The intensity of p-ERK was analyzed through densitometry and presented as a ratio to the total level of ERK ½ (Control, arbitrarily set to 100 %). (*P<0.05). PRE − P. fulgens root extract.
Ras mutations are common oncogenic mutations in many human tumors. Oncogenic Ras continuously activates the ERK-1 and ERK-2 pathways, which allow tumor cells to proliferate. Therefore, inhibitors of the ERK ½ pathway are considered potential anticancer agents. In our current study, P. fulgens root extract might have inhibited the proliferation of lung cancer cells by inhibiting DNA damage repair via ERK ½.
3.6 Cleaved caspase-3 and SQSTM1 expression
The analysis of the cleaved caspase-3 protein expression was done through the western blot method, with β-actin protein serving as a loading control. The results of the western blot analysis indicated a significant reduction in the expression of the cleaved caspase-3 protein (Fig. 7). Caspases are considered central regulators of apoptosis, and their expression is a marker of the process of various types of cancer (Coutinho‐Camillo et al., 2011). Caspases involved in apoptosis have two types, initiator caspases (caspase-8 and −9) and executioner caspases (caspase-3, −6, and −7), which was classified according to their mechanism of action. For apoptosis to occur, caspase-3 must be fully activated, which involves cleaving it to an aspartate residue to form cleaved caspase-3. This activated form of caspase-3 breaks down multiple cellular proteins and causes morphological changes and DNA fragmentation in cells (Liu et al., 2017; D’arcy, 2019; Hu et al., 2020).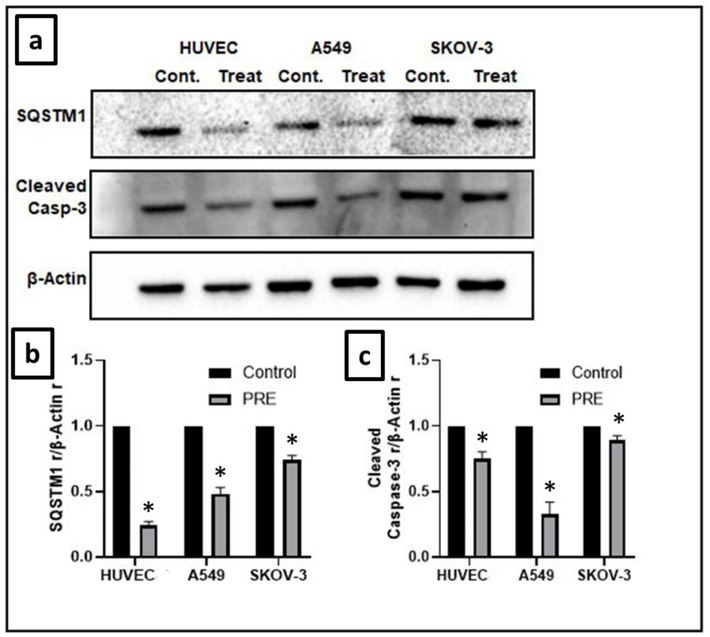
Effect of extract on cleaved caspase-3 and SQSTM1 expression in cell lines. (a) Levels of cleaved caspase-3 and the autophagy marker SQSTM1 were notably lowered in HUVEC, A549, and SKOV-3 cell lines as compared to the control groups. The loading control was β-actin. (b) The intensity of cleaved caspase-3 was measured by densitometry and presented as a ratio to the total level of β-actin. (c) The intensity of SQSTM1 was analyzed through densitometry and presented as a ratio to the total level of β-actin. (Control, arbitrarily set to 100 %). (*P<0.05). PRE − P. fulgens root extract.
In this investigation, the expression of cleaved caspase-3 decreased. This reduction indicates that the caspase-dependent apoptosis pathway did not activate, and therefore, the extract did not cause cell death through this pathway. It is possible that the extract suppressed cell viability through alternative pathways, such as a caspase-independent apoptosis pathway or other cell death pathways.
Fig. 7 illustrates the relative amounts of Sequestosome 1 protein (p62/SQSTM1) expression. There was a significant (P<0.05) reduction in the expression of p62/SQSTM1. Autophagy is a process of programmed release of toxic components through the lysosomal system, and p62/SQSTM1 is one of the receptors involved in selective autophagy (Kumar et al., 2022). According to Li et al. (2021), the excessive production of p62 leads to the spread of cancer to the bone by stimulating migration rather than the growth of lung adenocarcinoma cells. Autophagy can prevent the accumulation of p62 and suppress tumor formation. Additionally, specific proteins that regulate autophagy, including p62 (SQSTM1), have been found to influence the activity of NF-kB (Li et al., 2021). Our findings revealed that the groups that received the extract treatment had weaker expression of SQSTM1 expression, compared to the control groups. Lower levels of SQSTM1 expression have been associated with the tumor suppressor function of autophagy.
4 Conclusions
Our findings demonstrated that P. fulgens root extract had the ability to inhibit the ERK½, NF-kB, p62/SQSTM1, and Beclin-1 signaling pathway, leading to a decrease in the cell viability in lung (A549) and ovarian (SKOV-3) cancer cells. This anti-proliferative activity of the extract indicated its potential to be used as a new chemotherapeutic agent for the treatment of lung and ovarian cancers. However, the toxicity of extract against healthy HUVEC cell line confines its application as anticancer agent. In addition, the extract, which has high antioxidant properties, reduced the formation of oxidant molecules or the release of reactive oxygen species in healthy cells, suggesting that P. fulgens root may offer a new approach for cancer treatment but its effect on healthy cells should not be ignored.
CRediT authorship contribution statement
Polat İpek: Conceptualization, Investigation, Methodology, Writing – original draft. Ayşe Baran: Conceptualization, Investigation, Methodology, Writing – original draft. Mehmet Fırat Baran: Conceptualization, Investigation, Data curation. Aziz Eftekhari: Project administration, Supervision, Visualization, Writing – review & editing. Ameer Khusro: Writing – review & editing, Methodology, Visualization, Conceptualization, Validation. Mohammad Mehdi Ommati: Resources, Visualization, Writing – review & editing. Elvin Aliyev: Data curation, Visualization. Rovshan Khalilov: Visualization, Supervision, Formal analysis. D. Esther Lydia: Conceptualization, Validation. Mohamed Farouk Elsadek: Formal analysis, Funding acquisition. Saeedah Musaed Almutairi: Visualization, Funding acquisition.
Acknowledgements
This research was funded by Dicle University Scientific Research Projects Unit (Project Number: VETERINER.21.002). The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R470), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro cytotoxicity of the polar extracts of Potentilla fulgens L. against human cancer cell lines: Detection and isolation of bioactive phenolics. J. Chem. Pharm. Res.. 2014;6(9):89-95.
- [Google Scholar]
- Epidermal growth factor receptor expression, signal pathway, and inhibitors in non–small cell lung cancer. Semin. Oncol.. 2002;29(5):38-44.
- [CrossRef] [Google Scholar]
- Caspase expression in oral squamous cell carcinoma. Head Neck. 2011;33(8):1191-1198.
- [CrossRef] [Google Scholar]
- HDAC inhibitor ITF2357 reduces resistance of mutant-KRAS non-small cell lung cancer to pemetrexed through a HDAC2/miR-130a-3p-dependent mechanism. J. Transl. Med.. 2023;21(1):125.
- [CrossRef] [Google Scholar]
- Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int.. 2019;43(6):582-592.
- [Google Scholar]
- The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets. 2012;16(sup2):S17-S27.
- [CrossRef] [Google Scholar]
- Erdoğan, B. and E. Uzaslan, 1998. Apoptozis Mekanizmaları: Fas-FasL Bağımlı Apoptozis, Uludağ Üniversitesi Tıp Fakültesi Göğüs Hastalıkları ve Tüberküloz Anabilim Dalı (2003). Evan, G., Littlewood, TA, Matter of life and cell death. Science. 281 1317-1321.
- Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin.. 2005;55(3):178-194.
- [CrossRef] [Google Scholar]
- Towards dual function of autophagy in breast cancer: A potent regulator of tumor progression and therapy response. Biomed. Pharmacother.. 2023;161:114546.
- [Google Scholar]
- Hu, Y.-J., J.-T. Zhong, L. Gong, et al., 2020. Autophagy-related beclin 1 and head and neck cancers. OncoTargets and therapy. 6213-6227. Doi: 10.2147/OTT.S256072.
- Investigation of possible antidiabetic effects of Potentilla fulgens in diabetic rats and comparison with other antidiabetics. Haydarpasa Numune Med. J.. 2019;59(1):1-7.
- [CrossRef] [Google Scholar]
- Hypoxia-induced cancer cell responses driving radioresistance of hypoxic tumors: approaches to targeting and radiosensitizing. Cancers. 2021;13(5):1102.
- [Google Scholar]
- Antiproliferative effect of Potentilla fulgens on glioblastoma cancer cells through downregulation of Akt/mTOR signaling pathway. J. Cancer Res. Ther.. 2023;19(7):1818-1824.
- [CrossRef] [Google Scholar]
- Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431-436.
- [CrossRef] [Google Scholar]
- Cancer metabolism and autophagy. Archives Med. Rev. J.. 2018;27(4):388-396.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitory terpenoids from Potentilla fulgens and their quantitative estimation by validated HPLC method. J. Funct. Foods. 2013;5(3):1135-1141.
- [CrossRef] [Google Scholar]
- Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front. Cell Dev. Biol.. 2022;10:793328
- [CrossRef] [Google Scholar]
- Quality control standardization of the roots of Potentilla fulgens Wall.: A potent medicinal plant of the Western Himalayas and North-eastern India. Pharmacogn. J.. 2013;5(3):97-103.
- [Google Scholar]
- p62 overexpression promotes bone metastasis of lung adenocarcinoma out of LC3-dependent autophagy. Front. Oncol.. 2021;11:609548
- [CrossRef] [Google Scholar]
- Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett.. 1997;116(2):197-203.
- [CrossRef] [Google Scholar]
- Clematichinenoside serves as a neuroprotective agent against ischemic stroke: the synergistic action of ERK1/2 and cPKC pathways. Front. Cell. Neurosci.. 2015;9
- [CrossRef] [Google Scholar]
- Expression levels of cleaved caspase-3 and caspase-3 in tumorigenesis and prognosis of oral tongue squamous cell carcinoma. PLoS One. 2017;12(7):e0180620
- [CrossRef] [Google Scholar]
- Molecular mechanisms of chemo‐and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021;2(3):315-340.
- [Google Scholar]
- Chinese medicine formula ‘Baipuhuang Keli’ inhibits triple-negative breast cancer by hindering DNA damage repair via MAPK/ERK pathway. J. Ethnopharmacol.. 2023;304:116077
- [CrossRef] [Google Scholar]
- A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomed. Nanotechnol. Biol. Med.. 2013;9(7):1114-1122.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [Google Scholar]
- Assessment of lead, mercury, cadmium, chromium and total antioxidant capacity levels of employees exposed to exhaust gases in closed parking lots. Int. J. Environ. Anal. Chem.. 2022;102(12):2841-2853.
- [CrossRef] [Google Scholar]
- Methanolic root peel extract of Potentilla fulgens L. shows anti-proliferative activity on root meristematic cells of Lathyrus sativus L. and antiamoebic activity on trophozoites of Entamoeba histolytica. S. Afr. J. Bot.. 2023;163:523-530.
- [Google Scholar]
- Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res.. 1996;56(3):556-562.
- [Google Scholar]
- Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci.. 2018;19(2)
- [CrossRef] [Google Scholar]
- Comparison of effectiveness in antitumor activity between flavonoids and polyphenols of the methanolic extract of roots of Potentilla fulgens in breast cancer cells. J. Complement. Integr. Med.. 2012;9(1)
- [CrossRef] [Google Scholar]
- The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7(3):253-266.
- [CrossRef] [Google Scholar]
- Nuclear factor-κB activation: A question of life or death. BMB Rep.. 2002;35(1):28-40.
- [CrossRef] [Google Scholar]
- RAS-related GTPases DIRAS1 and DIRAS2 induce autophagic cancer cell death and are required for autophagy in murine ovarian cancer cells. Autophagy. 2018;14(4):637-653.
- [CrossRef] [Google Scholar]
- Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol.. 2009;122(2):184-204.
- [CrossRef] [Google Scholar]
- Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. U.S.A.. 2003;100(25):15077-15082.
- [CrossRef] [Google Scholar]







