High fructose Corn Syrup recast glucose transporter-5, Wnt, NF-κB signalling and mitochondrial apoptosis in an animal model of oral oncogenesis
⁎Corresponding author at: Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, Annamalai Nagar-608002, Chidambaram, Tamil Nadu, India. ganapathysindhu@gmail.com (Sindhu Ganapathy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Wnt signalling pathway, is mediated by members of T-cell factor (TCF) transcription factors family, is essential for the control of epithelial cell proliferation and death. Glucose transporter-5 (GLUT5), fructose-specific transporter, is also important in allowing transcellular fructose uptake. The goal of this work to determine how the High fructose Corn Syrup (HFCS) affected Wingless-related integration site (Wnt) and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling in the 7,12 –dimethylbenzaanthracene (DMBA)-induced hamster buccal pouch carcinogenesis (HBPCs) model.
Methods
Four groups of hamsters were created. For 12 weeks, 0.5 % DMBA was applied 3 times/week to the left side buccal pouches of the hamsters in groups (2 & 4). Additionally, the animals in groups (3 & 4) were given through drinking water of HFCS 25 %. The control animals were from group 1. By using western blot analysis, signalling network markers of the GLUT-5, Wnt, TCF-4, GSK-3β and NF-κB as well as mitochondrial apoptotic pathway marker expression B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) and cyclooxygenase – 2 (COX-2) was assessed.
Results
Drinking water uptake of 25% HFCS encouraged progress of HBP carcinomas by constitutive stimulating of the Wnt pathway via GSK-3β overexpression. HFCS suppressed Wnt signalling which contributed the NF-κB attenuation and changes the signalling markers in apoptotic network.
Conclusions
Our hypothesis suggests a mechanically crosstalk between Wnt and NF-κB signalling pathways in HBP carcinomas that is developed by HFCS. HFCS that targets the Wnt pathway and its downstream signalling mediators could be additive reason for cancer development.
Keywords
HFCS
DMBA
Wnt signalling
Mitochondrial apoptosis
GLUT-5
1 Introduction
Now a days high intake dietary fructose causes risk of obesity and metabolic syndrome by distorted fructose metabolism (Marshall et al., 1957; Rizkalla, 2010). Fructose is sweeter than other carbohydrates and is commonly utilized in processed foods and beverages (Santhekadur, 2019; White et al., 2015). Since 1970, fructose employed in the form of HFCS has causing a hike in fructose intake in the human diet (White, 2008; Meyers et al., 2017). HFCS-55 is a liquid composed of 55 % fructose, 42 % glucose, and 3 % other saccharides. Changes in cellular metabolism may aid tumor development and survival (Malik and Hu, 2015).Obesity and metabolic syndrome show increased vulnerability to fructose-containing sugars and increased risk for chronic kidney disease (CKD) and mortality (Andres-Hernando et al., 2023). While both fructose and glucose promoted unfavorable effects on muscular insulin sensitivity, impacts of the fraction of fructose and glucose in all beverages revealed that only fructose impaired hepatic insulin sensitivity, (Hieronimus et al., 2024).
Over expression of glucose transporter (GLUT) is important in cancer promotion. GLUT-5 for intestinal fructose absorption and metabolism, a fructose transporter is necessary. Fructose promotes lung adenocarcinoma via GLUT-5, stimulates fructose consumption, and its up regulations has been associated in several cancers. However, bioenergetics changes remain poorly understood (Suwannakul et al., 2021).
Wnt signalling pathway mediates epithelial cell proliferation, apoptosis and invasion (Wang et al., 2010). Wnt signalling is triggered via combination between Wnt and transcription factor members of the TCF family. Wnt signalling plays a part in cell functions proliferation, and motility. Some recent evidence suggests Wnt signalling dysregulation delivers to the development of a variety of malignancies in humans. The accepted Wnt signalling pathway activates by altering the multiprotein complex encompassing glycogen synthase kinase 3 (GSK-3), inhibition transfer into prolonged stability of free cytosolic beta-catenin (MacDonald et al., 2009).
Wnt action is also crucial for directing the apoptotic machinery. The Bcl-2 family of protein attenuate mitochondrial apoptosis. Bcl-2 comprises the apoptosis regulator Bcl-2 and its homologs (Hardwick and Soane, 2013). They govern the permeability of the outer membrane mitochondria and can be either pro-apoptotic (Bax) or anti-apoptotic (Bcl-2). Apoptotic mechanism, a type of programmed apoptotsis, is tightly checked by pro, anti-apoptotic Bcl-2 family members, and inhibitor of apoptosis proteins (Elmore, 2007). Bax are Pro-apoptotic Bcl-2 proteins advertise mitochondrial membrane permeabilization, which triggers the release of cytochrome C and activation of the caspase cascade, while anti-apoptotic members Bcl-2 block mitochondrial death, supporting cell survival (Shimizu et al., 1999; Hussar, 2022). However, the effect of HFCS on The Wnt signalling pathway and its downstream signalling circuits have not been studied. The objective of this study was to take a look at the way HFCS affects Wnt signalling and accumulated networks NF-κB and mitochondrial cell death in the DMBA-induced HBPCs model. A set of Wnt signalling markers, GSK-3β, NF-κB signalling, GLUT 5, TCF-4 and mitochondrial apoptosis (Bcl-2, Bax and COX-2) was evaluated in HFCS with DMBA hamster model.
2 Materials and methods
2.1 Chemicals
Sigma Chemical Company, St. Louis, MO, USA, Sodium dodecylsulfate (SDS), N,N,N0,N0-tetramethylenediamine (TEMED), and Trizol. Santa Cruz Biotechnology, USA, gave the primary antibodies (Wnt, NF-kB, GLUT 5 & TCF-4 mouse monoclonal antibodies, GSK-3 rabbit polyclonal antibodies and secondary antibodies. Thermo Fisher Scientific, Bangaluru, India, produces an ELISA assay kit for Bax, Bcl-2, and COX-2.
2.2 Experimental animals
The Animal Source were Mesocricetus auratus (Golden Syrian Hamsters) 8–10 weeks old (80–120 g) Male, 48 numbers purchase from Central Animal House, K M College of Pharmacy, Madurai, and Biogen, Animal Laboratory House, Bangaluru. The animals were acclimatized for one week before the experiment, after being divided into four groups of six animals each. The Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA-Proposal no. IAEC/K.KAVITHA/AU/Ph.D/KMCP/161/2022–23), Ministry of Environment, Forests, and Climate Change, Government of India has approved the study design. The animals were housed in polypropylene cages, which regulate temperature and humidity through a light–dark cycle.
2.3 Experimental design
24 hamsters were separated into four groups (n = 6), Group 1: vehicle control; Group 2 (0.5 % DMBA); Group 3 (HFCS 25 %); and Group 4 (0.5 % DMBA + HFCS 25 %) for 12 weeks. Tumor morphology and buccal histology were evaluated for 14th week. Hamsters' left buccal pouches were treated with 0.5 % DMBA in liquid paraffin using a no. 4 brush three times per week for 12 weeks. HFCS was delivered via drinking water. The experimental configuration is shown in Fig. 1.
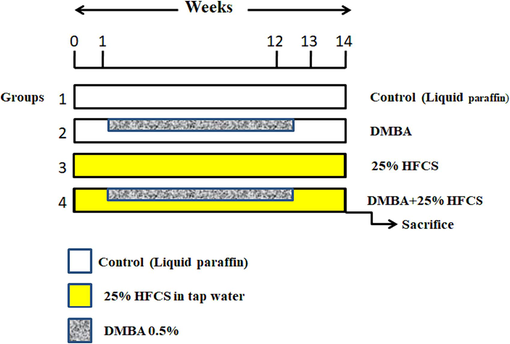
- Schematic illustraction of the experimental design. DMBA; 7,12-dimethylbenz (a) anthracene, HFCS-High Fructose Core Syrup.
Animals were killed 24 h after treatment finished by injecting an overdose of ketamine (60 mg/kg bw). The initial and final weights of the animal were subtracted to calculate its body weight. The overall amount of tumors in the HBP was determined. The volume of the tumor was obtained using the formula V= (4/3 π) (D1/2), (D2/2), (D3/2), where D1, D2, and D3 are the tumor’s three diameters (mm3) (Manoharan et al., 2012). The tumor burden was established via dividing the number of lesions per hamster by the tumor volume and Gross appearance of tumour were photographed.
2.4 Western blot
Westernblot determinations were assessed for detection of protein expression levels. Buccal tissues were lysed on ice for 5 min in RIPA buffer contains 1 mM phenylmethylsulfonyl fluoride (PMSF). The liquefied extract was segregated after cool centrifugation, and sample containing protein concentrations has been read out at 595 nm using the Coomassie Protein Assay Kit (Thermo Fisher Scientific). During electrophoresis on a 5 %–20 % polyacrylamide gradient gel (Wako), ratio amounts of protein (20–30 mg) were transferred to a 0.2 mMPVDF membrane (Merck). In room temperature upon that, 30 min went on blocking in 5 % skim milk. Work proceeded with probe the membranes and Primary antibodies at overnight. Following three rinses with Tris-buffered saline-Tween20, secondary antibodies were incubated on the membranes for 1 h. The immunoreactive bands have been generated with GE Healthcare’s enhanced chemiluminescence detection kit reagents and recorded with a Fujifilm LAS4000mini (Tokyo, Japan). For the quantitative study of relative protein expression of targets and β-actin, ImageJ software was utilized. All of the goals have been assessed multiple times.
2.5 Enzyme-linked immunosorbent assay (ELISA) estimation of apoptotic marker protein
The activity of Bax and Bcl-2 was assay in the buccal mucosa using Human BAX & Bcl-2 Human ELISA Kit, Thermo Fisher Scientific Inc India. The buccal mucosa, dissected and excised, from control and experimental hamsters was rinsed in a 1X wash buffer and added the 1X Biotynylated detection solution, Ag binding (100 μl), HRP conjugate (100 μl), substrate (90 μl) and finally add the stop solution (50 μl) then read OD value at 450 nm after15 minutes. COX-2 peroxidase activity was measured colorimetrically in a microtiter plate reader by looking for oxidized N,N,N',N'-tetramethyl-P-phenylenediamine (TMPD) at 590 nm. Cayman’s ELISA test kit was used to measure COX-2 in the buccal mucosa. The buccal mucosa was dissected and excised from control and experimental hamsters and centrifuged in 5–10 ml of cold buffer. The supernatant was used for analysis after it had been centrifuged at 10,000g for 15 min at 4 °C. COX-2 peroxidase activity was measured colorimetrically in a microtiter plate reader at 590 nm.
2.6 Statistical analysis
The data were compared using one-way ANOVA and Duncan’s Multiple Range Test (DMRT) in SPSS version 17.0 for Windows. The data is presented in the form of mean standard deviation. SPSS Inc. is based in Chicago, Illinois.
3 Result
3.1 Effect of HFCS on body weight alterations in oral cancer hamsters
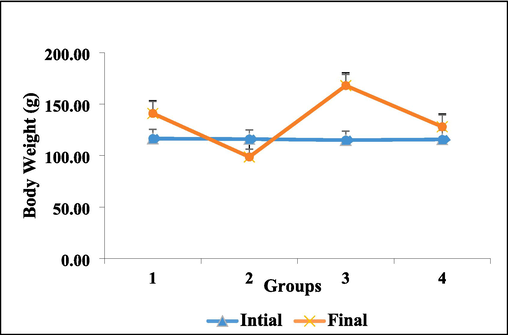
Fig. 2 illustrates the discrepancies in body weight (BW) measurements recorded in control and experimental hamsters at the beginning and end of each stage. BW levels in group 2 hamsters were significantly (p ≤ 0.05) below those in group 1 hamsters. In group 4, DMBA painted plus HFCS hamsters at dosages of 25 %/kg b.w. resulted in a significant throw in BW (p ≤ 0.05). Group 3 hamsters with HFCS 25 %/kg b.w water intake showed an immense rise in BW.
-
Untreated control and experimental hamster body weights. For each set of six hamsters, values are reported as mean SD. At p < 0.05 (DMRT), values that do not share a similar superscript letter differ considerably.
3.2 Tumor burden, incidence, and volume
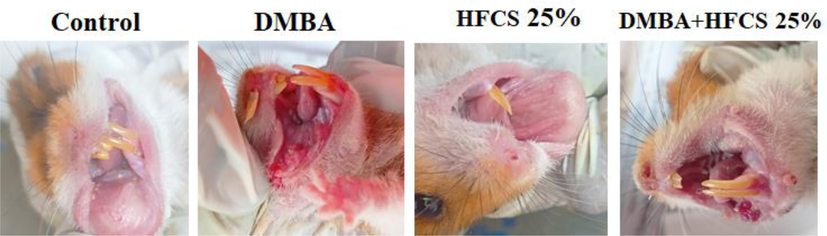
Table 1 reveals that in hamsters painted with DMBA alone, we observed 100 % tumor formation, with a mean tumor volume of 178.05 mm3 and a tumor burden of 1958.55 mm3 (group 2). DMBA plus HFCS 25 % fed hamsters in groups 4 showed significant (p ≤ 0.05) increases in tumor occurrence, volume (182.05 mm3), and burden (2730 mm3). No tumours were found in the control group of hamsters fed HFCS (25 %/kg b.w). At week 6, all DMBA painted mucosa surfaces were noticeably roughened and grainy, with the occasional white plaque-like lesion. Hamsters in the vehicle-treated and HFCS (25 %/kg b.w) only treated groups carried similar cheek pouches. When compared to DMBA alone, HFCS and DMBA treated group emerge earlier tumor formation, between weeks 8 and 10. However, tumor growth in DMBA-induced hamsters took ten to twelve weeks to appear (Fig. 3).
| Parameters | Control | DMBA | 25 % HFCS | DMBA + 25 % HFCS |
|---|---|---|---|---|
| Tumor incidence | 0 | 100 % | 0 | 100 % |
| Total number of tumor/hamsters | 0 | 11 ± 0.69b | 0 | 15 ± 0.69b |
| Total volume (mm3)/hamsters | 0 | 178.05 ± 7.59b | 0 | 182.05 ± 10.35b |
| Tumor burden (mm3)/hamsters | 0 | 1958.55 ± 136.51b | 0 | 2730.75 ± 201.15b |
The mean SD for each batch of six hamsters is provided. At p < 0.05, there is a substantial difference between values with various superscript letters.
-
Photograph showing the gross appearance of oral tumors. Buccal pouch mucosa of experimental and control animals (n = 6): gross appearance. Group 2&4 Exophytic well-defined tumor mass in the hamster’s buccal pouch at 12 weeks after being painted with DMBA and DMBA + HFCS (25 %). Group 1 control &3HFCS (25 %)alone have shown the normal buccal pouch.
3.3 GLUT5 expression boosted up in oral tumor of hamsters
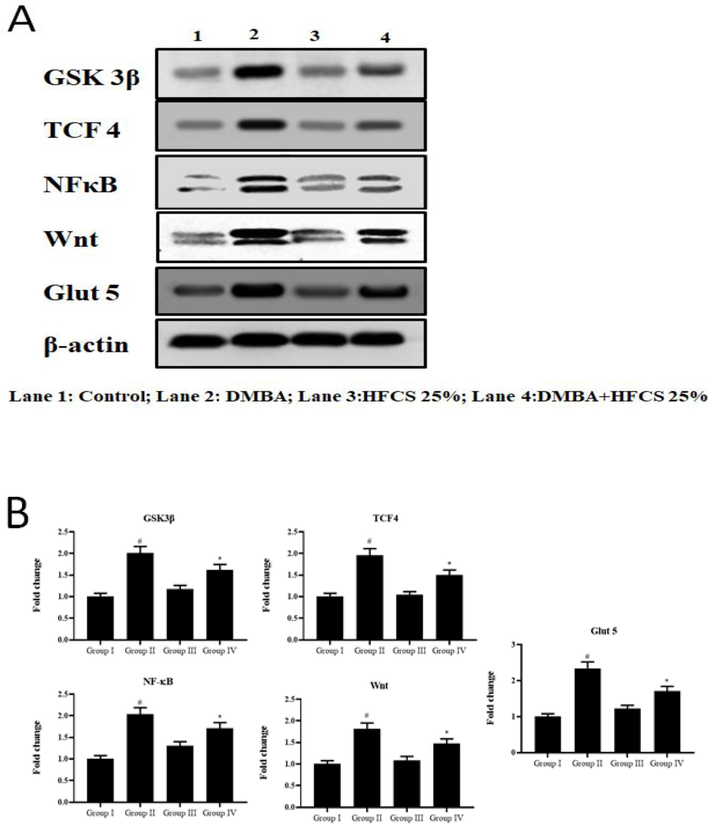
GLUT5 mediated tumour growth were analysed in control and experimental hamster, among water drinking HFCS, higher body weight was observed compared to control (Fig. 4). In comparison to DMBA, HFCS consumption significantly boosted tumor development, with larger tumor volume and burden in the HFCS + DMBA groups. GLUT5 greatly increased tumor volume and weight as compared to the control group of HFCS-fed hamsters. GLUT5 was significantly higher in DMBA alone and HFCS + DMBA groups compared to the control and alone HFCS groups, based on Western blotting analysis.
-
Effect of HFCS on GSK-3β, TCF-4, NF-κB, Wnt and GLUT 5 proteins modulation by western blot investigation. In Fig. 4 A: Lane 1 & Lane 3- There was no statistically significant difference between the control (Group 1) and the HFCS25% alone (Group 3) (p < 0.01). DMBA alone (Groups 2) resulted in considerably increased expression (p < 0.05) than the control (Group 1). HFCS 25 % treated hamsters (Groups 3) had considerably lower expression (p < 0.05) than DMBA alone (Group 2). In Fig. 4B. The data provided are the means and standard deviations of independent experiments.The intensity was measured with a densitometer, normalized to beta-actin expression, and expressed as a fold change with respect to the control. The data provided are the means and standard deviations of independent experiments. At p < 0.05, values that do not share a common superscript differ significantly (DMRT).
3.4 Effect of HFCS administration on Wnt signalling and GSK-3β
We first studied the effect of HFCS by drinking water expression of Wnt signalling pathway members. As DMBA-painted animals were compared to control, the expression of Wnt, TCF-4, and GSK-3 was significantly increased. Drinking HFCS water, significantly higher the expression of these markers in DMBA-painted animals. HFCS alone given animals did not affected with any substantial changes in the expression of Wnt family members (Fig. 4).
3.5 Effect of HFCS administration on mitochondrial apoptosis
Because the control of abnormal Wnt and NF-κB signalling by additive food is usually by the induction of apoptosis, we hypothesised the effect of HFCS on cell death. In this work, we found that enhanced Bcl-2 expression was related with Bax down regulation in DMBA-painted hamsters (group 2) and HFCS with DMBA (group 4) compared to controls. Although control and HFCS 25 % absorption by drinking water not demonstrate any significant variations in the expression of the above-mentioned proteins compared to DMBA with 25 % HFCS (Fig. 4).
3.6 COX-2 and NF-κB contribution in HFCS induced tumour growth
Western blot analyses of NF-κB (Fig. 4) and COX-2 ELISA results were showed in (Fig. 5). Analysis of NF-κB and COX-2 revealed increased expression associated with in DMBA painted animals. HFCS uptake by drinking water significantly upregulated NF-κB and COX-2 expression compared to group 1 animals. COX-2 has been shown to be a target of the Wnt pathway. Expression of NF-κB, which is responsible for COX-2 rise, was found to be crucial to TCF-4 activation in the present investigation. As a consequence, HFCS is a COX-2-dependent mediator. As the result, Wnt accumulates in the cytoplasm that transfers into the nucleus, eventually ending in TCF target gene triggering, which is important for tumorigenesis. The following Wnt increase resulted in TCF target gene transcription, growth development, and apoptosis suppression. From these findings, we suggested HFCS encouraged the tumor development pathway through multiple targets.
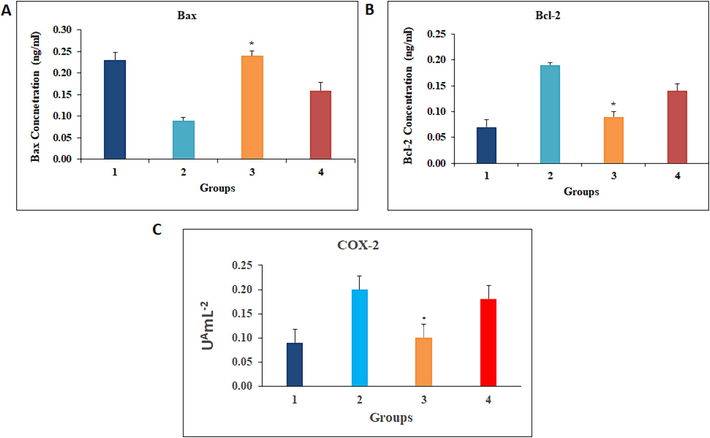
-
The effect of HFCS on the expression of Bax, Bcl-2, and COX-2. The data provided are the means and standard deviations of independent experiments. Per minute, A - nmol of TMPD is oxidized. At p < 0.05, values that do not share a common superscript differ significantly (DMRT).
4 Discussion
Gathering information from in vitro, in vivo, and preclinical human research has proven the important function on Wnt signalling pathway in the creation and multiplication of different types of cancer in humans (Chiariniet al., 2020). Aberrant Wnt signalling activation leads to the sequential growth of HBPcs examined in the current research. We show that HFCS targets in the Wnt signalling its downstream effectors in the HBPCs model. Altering the expression of the multifunctional proteins GSK-3β, which inhibit Wnt signals were transduced network, has broad implications in hindering tumour development (VidyaPriyadarsini et al., 2012).
Fructose intake has lately high and processed foods and beverages include HFCS, a commercial sugar component known for its intense sweetening power and low cost (White et al., 2015). A decrease in physical action and a rise in BW are major risk factors for a variety of diseases (Booth et al., 2012). Fructose consumption may be linked to metabolic syndrome and obesity. Goncalves et al. (2019) reported that HFCS raised fructose dose in the intestinal lumen and serum, respectively, and that tumors transported both sugars. Fructose was transformed to fructose-1-phosphate within the tumors, activating glycolysis and increasing fatty acid production, all of which promote tumor growth. As a result, we discovered that the addition HFCS may cause or support the tumor in the current investigation.
The effect of HFCS on BW is unknown and subject to controversy. Maximum reports were evidenced that the sweeteners HFCS effects affected on body weight by fructose intake and research duration (Pereira et al., 2017). According to (Ross et al., 2009) Male Wistar rats were fed 15 % fructose or corn starch for 15 months, and the changes in body weight were not statistically significant. After two weeks of feeding golden Syrian hamsters 60 % fructose, obesity and subsequent weight growth were found. Studies on males, females, and middle-aged males showed that body weight increased as a result of varied food amounts and study time (Dalbøge et al., 2015).
According to Rizkalla (2010), those weight gain never get practiced any bodily alterations such as of intake fructose at normal levels (Rippe and Angelopoulos, 2013) found no changes between the glucose, fructose, and HFCS groups. According to (Bremer et al., 2009) Changes in BW with the HFCS and sucrose, and Environmental studies associated to HFCS usage are unclear. (Forshee et al., 2007) stated that male rats fed 8 % HFCS for 12 h had more BW than sucrose drinking groups after 2 months, never changed in glucose level was seen between groups and animals feeding ad libitum HFCS. The volume and duration of research were different from theirs, and we had greater body weight, belly fat, and TG after 6 or 7 months comparing to the chow group (Bocarsly et al., 2010). In these findings, hamsters that drank only DMBA group had lower BWs than groups 4, however hamsters who consumed HFCS at varying doses of 25 %/kg b.w. had high bodies. Control group, hamsters given water with 25 % HFCS/kg b.w. gained significant BW. Similarly our findings corroborated past publications (Martínez et al., 2020).
Fructose acts as a fuel for cancer cells, stimulating their proliferation. Advanced research on the part of GLUT5 in cancer formation is essential, as the obvious relationship remains unknown. GLUT5, a sole fructose transporter, plays a vital component in the normal transcellular uptake of fructose from the gut lumen into the enterocyte via facilitated diffusion, and its overexpression reported in malignancies including lung, renal, ovarian, and myeloma (Su et al., 2018). (Goncalves et al., 2019) found that mice fed HFCS had a substantial increase in tumor size and grade, even in the absence of obesity and metabolic syndrome.
HFCS increased glucose & fructose dose in the intestinal and tumors transferred both sugars thanks to increased synthesis of the transporter proteins GLUT 5. In present study, which is evidenced by GLUT 5 elevated expression HFCS taking in higher in oral buccal tissues by stimulating the growth of cancer cells and ATP production, but not in normal control tissues. Our results support the idea that HFCS may promote the proliferation of DMBA-induced oral tissues.
Then we wanted to figure out the manner in which HFCS produces cancer in stages. GSK-3 has been shown to drive β-catenin degradation, which results in β-catenin ubiquitination and its subsequent shipment to the proteosome for degradation, in addition to normal Wnt signalling pathways. As a result, we speculated that HFCS likely down-regulated beta-catenin not only through the Wnt pathway, which may affect the phosphorylation of GSK-3β, but also by directly impacting β-catenin. Our results suggested that HFCS digestion may reduce the phosphorylation of GSK3 and TCF triggering Wnt production in the cytoplasm, and thereby blocking β-catenin nuclear transfer.
Wnt signalling has recently been shown to activate cell survival and death, including the classical NF-κB pathway by boosting the phosphorylation and degradation of IκB-a by IκB kinase (Liuet al., 2022). As a result, free cytosolic NF-κB translocate to the nucleus, binds to DNA κB elements, and controls the oncogenic phenotype (Park and Hong, 2016). In the present study showed promoted expression of NF-κB in HFCS with DMBA treated hamsters when compared to DMBA alone hamsters tissue protein. But in control and HFCS 25 % alone hamster tissue NF-κB proteins expression were reduced. Increased NF-κB protein expression were help to development tumour growth between DMBA and HFCS with DMBA groups.
(Savran et al., 2019) determined that Melatonin (MLT)’s have the chemopreventive effect in rats model against HFCS-induced ROS formation and inflammation. Further they investigated Bax expressions were suppressed but Bcl-2 increased in HFCS induced rats (He et al., 2017). Similarly, the apoptotic markers enzyme Bax reduced but Bcl-2 increased in DMBA and HFCS + DMBA administered rats. The status significantly altered in the opposite direction of their normal range. The 25 % concentration of HFCS indicated reduced Bax expression but greater Bcl-2 expression. It describes the effects of HFCS on hamsters. In the overarching premise of the current investigation, we analyze HFCS with DMBA gives more effect in tumour growth and apoptotic protein expression in experimental hamsters.
While many gene factors have an important effect on tumor creation, dietary choices can also have an impact on the condition is being treated by altering systemic and micro environmental signalling pathways. In addition to the genetic abnormalities associated with tumor formation, metabolic reprogramming occurs to meet the unique needs of actively replicating cells. Overall, cancer’s metabolic reprogramming results in uncontrolled growth of cells. This is accomplished by enhancing developmental pathways while repressing the inhibition supplied by apoptotic signals (Strober and Brady, 2019).
5 Conclusion
In summary, our findings showed the relationship between HFCS stimulation of Wnt signalling and its downstream effectors molecules in the development of HBP cancer. We believe that abnormal Wnt signalling during DMBA-induced HBPCs is a hallmark of malignancy progression. Inhibiting Wnt signalling could be a probable target for OSCC inhibition. Consumption of HFCS in the HBP model would be a critical link in the effective inducer of oral cancer.
CRediT authorship contribution statement
Kavitha Kalimuthu: Writing – original draft, Investigation, Formal analysis. Sindhu Ganapathy: Conceptualization and Supervision. Mohammad Ahmad Wadaan: Funding acquisition and Visualization. Vennila Lakshmanan: Data curation. Balasubramani Ravindran: Software and Validation. Vijayalakshmi Annamalai: Writing – review & editing and Methodology.
Acknowledgement
The authors express their sincere appreciation to the Researchers Supporting Project Number (RSP2024R466) King Saud University, Riyadh 11451, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- High fructose corn syrup accelerates kidney disease and mortality in obese mice with metabolic syndrome. Biomolecules. 2023;13:780.
- [CrossRef] [Google Scholar]
- High-fructose corn syrup causes characteristics of obesity in rats: increased body weight, body fat and triglyceride levels. Pharmacol. Biochem. Behav.. 2010;97(1):101-106. Epub 2010 Feb 26. PMID: 20219526; PMCID: PMC3522469
- [CrossRef] [Google Scholar]
- Lack of exercise is a major cause of chronic diseases. Compr. Physiol.. 2012;2(2):1143-1211. PMID: 23798298; PMCID: PMC4241367
- [CrossRef] [Google Scholar]
- Relationship between insulin resistance-associated metabolic parameters and anthropometric measurements with sugar-sweetened beverage intake and physical activity levels in US adolescents: Findings from the 1999-2004 National Health and Nutrition Examination Survey. Arch. Pediatr. Adolesc. Med.. 2009;163(4):328-335. PMID: 19349561; PMCID: PMC4264593
- [CrossRef] [Google Scholar]
- The role played by Wnt/β-Catenin Signaling Pathway in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci.. 2020;21(3):1098. PMID: 32046053; PMCID: PMC7037748
- [CrossRef] [Google Scholar]
- Hamster model of diet-induced obesity for preclinical evaluation of anti-obesity, anti-diabetic and lipid modulating agents. PLoS One. 2015;10(8):e0135634. PMID: 26266945; PMCID: PMC4534139
- [CrossRef] [Google Scholar]
- Apoptosis: A review of programmed cell death. Toxicol. Pathol.. 2007;35(4):495-516. PMID: 17562483; PMCID: PMC2117903
- [CrossRef] [Google Scholar]
- A critical examination of the evidence relating high fructose corn syrup and weight gain. Crit. Rev. Food Sci. Nutr.. 2007;47(6):561-582. PMID: 17653981
- [CrossRef] [Google Scholar]
- High-fructose corn syrup enhances intestinal tumor growth in mice. Science. 2019;363(6433):1345-1349. PMID: 30898933; PMCID: PMC6487857
- [CrossRef] [Google Scholar]
- Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect Biol.. 2013;5(2):a008722. PMID: 23378584; PMCID: PMC3552500
- [CrossRef] [Google Scholar]
- Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med.. 2017;15(1):873-877. Epub Nov 3. PMID: 29399092; PMCID: PMC5772899
- [CrossRef] [Google Scholar]
- Effects of consuming beverages sweetened with fructose, glucose, high-fructose corn syrup, sucrose, or Aspartame on OGTT-derived indices of insulin sensitivity in young adults. Nutrients.. 2024;16(1):151.
- [CrossRef] [Google Scholar]
- Apoptosis regulators Bcl-2 and Caspase-3. Encyclopedia. 2022;2:1624-1636.
- [CrossRef] [Google Scholar]
- Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Sig. Transduct. Target Ther.. 2022;7:3.
- [CrossRef] [Google Scholar]
- Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell.. 2009;17(1):9-26. PMID: 19619488; PMCID: PMC2861485
- [CrossRef] [Google Scholar]
- Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J Am CollCardiol.. 2015;66(14):1615-1624. PMID: 26429086; PMCID: PMC4592517
- [CrossRef] [Google Scholar]
- Berberine prevents 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis: A biochemical approach. Eur. J. Cancer Prev.. 2012;21(2):182-192. PMID: 21968688
- [CrossRef] [Google Scholar]
- Enzymatic conversion of d-glucose to d-fructose. Science. 1957;125:648-649.
- [CrossRef] [Google Scholar]
- DMBA-Induced Oral Carcinoma in Syrian Hamster: Increased Carcinogenic Effect by Dexamethasone Coexposition. Biomed. Res. Int. 2020:1470868. PMID: 32149076; PMCID: PMC7042540
- [CrossRef] [Google Scholar]
- High fructose corn syrup induces metabolic dysregulation and altered dopamine signaling in the absence of obesity. PLoS One. 2017;12(12):e0190206. PMID: 29287121; PMCID: PMC5747444
- [CrossRef] [Google Scholar]
- Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):15. PMID: 27043634; PMCID: PMC4931664
- [CrossRef] [Google Scholar]
- Fructose consumption in the development of obesity and the effects of different protocols of physical exercise on the hepatic metabolism. Nutrients. 2017;9(4):405. PMID: 28425939; PMCID: PMC5409744
- [CrossRef] [Google Scholar]
- Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: What do we really know? Adv. Nutr.. 2013;4(2):236-245. PMID: 23493540; PMCID: PMC3649104
- [CrossRef] [Google Scholar]
- Health implications of fructose consumption: A review of recent data. Nutr. Metab. (Lond). (7):82. PMID: 21050460; PMCID: PMC2991323
- [CrossRef] [Google Scholar]
- A high fructose diet impairs spatial memory in male rats. Neurobiol. Learn. Mem.. 2009;92(3):410-416. Epub 2009 Jun 12. PMID: 19500683; PMCID: PMC2737072
- [CrossRef] [Google Scholar]
- The dark face of fructose as a tumor promoter. Genes Dis.. 2019;7(2):163-165. PMID: 32215285; PMCID: PMC7083712
- [CrossRef] [Google Scholar]
- Melatonin protects the heart and endothelium against high fructose corn syrup consumption-induced cardiovascular toxicity via SIRT-1 signaling. Hum. Exp. Toxicol.. 2019;38(10):1212-1223. Epub 2019 Jun 30. PMID: 31256681
- [CrossRef] [Google Scholar]
- Shimizu, S., Narita, M., Tsujimoto, Y., 1999. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC Nature. Jun 3 399(6735), 483-7. doi: 10.1038/20959. Erratum in: Nature 2000 Oct 12;407(6805):767. PMID: 10365962.
- Dietary fructose consumption and triple-negative breast cancer incidence. Front. Endocrinol. (Lausanne). 2019;10:367. PMID: 31244777; PMCID: PMC6581676
- [CrossRef] [Google Scholar]
- GLUT5 increases fructose utilization and promotes tumor progression in glioma. Biochem. Biophys. Res. Commun.. 2018;500(2):462-469. Epub 2018 Apr 19. PMID: 29660339
- [CrossRef] [Google Scholar]
- Targeting fructose metabolism by glucose transporter 5 regulation in human cholangiocarcinoma. Genes Dis.. 2021;9(6):1727-1741. PMID: 36157482; PMCID: PMC9485202
- [CrossRef] [Google Scholar]
- Aberrant activation of Wnt/β-catenin signaling pathway contributes to the sequential progression of DMBA-induced HBP carcinomas. Oral Oncol.. 2012;48(1):33-39. Epub 2011 Sep 15. PMID: 21924667
- [CrossRef] [Google Scholar]
- The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr. Drug Targets. 2010;11(6):745-751. PMID: 20041844; PMCID: PMC3084452
- [CrossRef] [Google Scholar]
- Straight talk about high-fructose corn syrup: What it is and what it ain’t. Am. J. Clin. Nutr.. 2008;88:1716S-S1721.
- [CrossRef] [Google Scholar]
- Fructose content and composition of commercial HFCS-sweetened carbonated beverages. Int. J. Obes. (Lond).. 2015;39(1):176-182. Epub 2014 May 6. PMID: 24798032; PMCID: PMC4285619
- [CrossRef] [Google Scholar]







