Translate this page into:
Effect of Mentha arvensis enriched diet to promote the growth and immune response of Clarias batrachus against Aeromonas hydrophila challenge
⁎Corresponding authors. thiruna.ps@gmail.com (Periyasamy Thirunavukkarasu), inhokim@dankook.ac.kr (In Ho Kim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
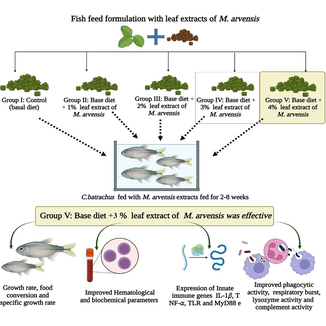
The study was conducted to investigate the effects of fish fed diet Mentha arvensis extract on growth performance, non-specific immunity and expression of some immune-related genes and resistance to Aeromonas hydrophila in Clarias batrachus. Five diets were formulated with 0, 1, 2, 3, and 4% of M. arvensis leaf extract. The results indicated that, compared to the control groups, 2-4% dietary inclusion increased growth and feed consumption. In the dietary inclusion of 3-4% M. arvensis extract groups were increased relative on weight gain, specific growth rate, RBC, WBC, total hemocyte counts, total protein, globulin than control. Fed diet supplements with 3% mint-extract increased the total protein, WBC and globulin and phagocytic indexes and lysozyme activity increased at the 2, 3 and 4% of mint groups relative to the control. The PCR analysis showed that TNF, IL-1, MyD88, and TLRs were increased in the 2-4% fed diet M. arvensis extract groups than the control. These results suggest that 3% of M. arvensis extract significantly influences the immunomodulatory activity and immune-specific genes of C. batrachus.
Keywords
Growth performance
Wild mint
Walking cat fish
Serum biochemical parameters
Innate immune-related genes
1 Introduction
The walking catfish, Clarias batrachus, is a bottom dweller, freshwater and omnivorous fish, among Asia’s most prominent cultivated fish species (Narra, 2016). Aeromonas hydrophila is utmost commonly come upon bacterial pathogen. To reduce mortalities, chemicals and antibiotics are used to prevent and treat infection, increasing environmental pollution and enhancing antibiotic-resistant pathogens. Natural immunostimulants could be a potential alternative to other detrimental chemical supplements and allow sustainable aquaculture (Baba et al., 2018; Sattanathan et al., 2020a). Mint extracts (Mentha piperita) showed positive effects on growth, hemato-biochemical factors, and increased resistance to various bacterial infections in tambaqui (Colossoma macropomum) (Ribeiro et al., 2016), Caspian brown trout (Adel et al., 2015a), Yersinia ruckeri (Adel et al.,2016), Labeo rohita (Sattanathan et al., 2020b), Rachycentron canadum (Wu et al., 2016), Rutilus kutum (Adel et al., 2015b) and Lates calcarifer (Talpur,2014). As a result, it is critical to reduce inflammation and boost immunity in animals by balancing pro- and anti-inflammatory cytokine production by sufficient nutrition and/or supplements (Baba et al.,2018). The effects of some feed additives, such herbs, prebiotics, probiotics, and synbiotics, on the immune system have been examined (Hoseinifar et al., 2016; Khalil et al., 2017). However, to the best of our knowledge there is no information on the effects of A. hydrophila infection on the fish expression of innate immune gene profiles in C. batrachus. Thus, the work was to investigate the effects of dietary inclusion of M. arvensis on growth performance, mortality rate, hematological parameters and immune responses in the C. batrachus, challenged with A. hydrophila.
2 Materials and methods
2.1 Herbal collection and diets
The experimental sample of the M. arvensis herbal was collected from a local market and washed in distilled water. The herbal extract was air-dried, and then the dark was made into powder with nylon mesh. M. arvensis powder was mixed in a 1:5 ratio with 90% ethanol for 48 h using a shaker. The M. arvensis powder was shaken for 48 h while being combined in a 1:5 ratio with 90% ethanol. The filter paper was used to separate the ethanol from the powder mixes, which was done in a rotary evaporator at 30–45°C; the extract of M. arvensis was stored at -20 °C till usage (Adel et al., 2015b). The commercial fish-fed diet (Growel-feeds, India, containing 8% ash, 40% protein, 6% fat, 3% fiber, and 12% moisture) was ground into a powder and combined to the appropriate concentration to produce five experimental diets with varying compositions. The nutritional source of fish was pleased by the diets according to NRC (2011). The obtained pathogenic bacteria, A. hydrophila (MTCC1739; IMTECH, Chandigarh, India), followed by the culturing duration of 24 h in the tryptone soya broth (TSB) at 37°C and the glycerol-containing culture was kept at −20°C until required.
2.2 Experimental design and pathogen
Healthy C. batrachus (average weight 9±2.4 g) were purchased from local fish farm in Tamil Nadu, India. The fish were kept in a 500-L rectangle fibre tank a 24°C for two weeks to get used to the lab environment. Three hundred fishes were randomly split into five groups in each triplicate after two weeks of acclimatization to commercial fish-fed diets, as follows: 0%: Control (basal diet), 1%: Diet + 1 g/kg M. arvensis, 2%: Diet + 2 g/kg M. arvensis, 3%:Diet + 3 g/kg M. arvensis, and 4%: Diet + 4 g/kg M. arvensis for 8-weeks feeding trial.
The A. hydrophila were cultured overnight in LB broth under shaker with 200 rpm. The bacterial culture medium was centrifuged to the pellet cells and the cells were washed thrice with 1XPBS. The cell counting was done and suspension was diluted to get a cell density of 1×107 cells/mL. Fish was given anesthesia in a 150 mg/L buffered MS-222 solution (Sigma), experimental groups 1 to 4% were challenged intraperitoneally (IP) injected with 100 µL of PBS containing A. hydrophila (1x107cells/mL) on the zero day. The control group fishes were injected with 100 µL PBS. The respective pellets diets were provided twice a day throughout the experimental time. During the experiment, water parameters were measured, including dissolved oxygen level of 5.2 ± 0.14 to 6.5 ± 0.43 mg/L, temperature level at 25 to 30°C, and pH range of 6.95 to 7.80, ammonia 0.12 ± 0.01 to 0.15 ± 0.02 mg/L and nitrate 0.022 ± 0.01 to 0.024 ± 0.02 mg/L.
2.3 Sampling collection
Each experimental sample group was randomly selected at weeks 2, 4, 6, and 8 post challenge with A. hydrophila. The challenged fish were then given a 150 mg/L buffer solution while sedating (MS-222, Sigma-Aldrich, USA). Each sample's blood was collected from the caudal vein using a 24-gauge syringe needle. The collected blood was split between tubes that had been heparinized and non-heparinized. The non-heparinized blood samples were centrifuged at 3500xg (RCF) for 15 min to properly extract the serum after 2 h of incubation at room temperature. Before usage, the serum was separated and kept at −20°C. Tissue samples from the spleen and head of the kidney were collected, promptly added to the TRI® reagent (Invitrogen, USA), and then stored at −20°C until use.
2.4 Hematology
Giemsa staining was used to stain the collected blood cell, and its estimated value was based on its morphological characteristics. By using a hematocytometer, the diluted blood cells were utilized to calculate the erythrocytes (1:1000 in PBS) and leucocytes (1:100 in PBS) (Blaxhall and Daisley, 1973). Using a commercial estimate kit (Liquichem Total Protein/Albumin kit, Recorders and Medicare Systems Pvt. Ltd., India), the total protein and albumin components of the serum were determined using the BCG technique and the Biuret method (Lopez and Carl, 2015), respectively.
2.5 Growth performance
The following parameters were measured with being deprived of feeding for 24 h before weighing and sampling and at the end of the feeding trial.
Where Wi is the initial weight, Wf is the final weight, and T is the number of days in the feeding period.
2.6 Phagocytic activity
The phagocytic activity of leucocytes was analyzed by a modification of early procedure (Lopez and Carl, 2015). The phagocytosis index was determined to mean the number of yeast cells engulfed by positive phagocytes multiplied by the percentage of phagocytosis using the following formula (Sönmez et al., 2015). The microscopic method determined the phagocytic activity:
Phagocytic activity (%) = (number of phagocytic cells/Total number of cells) × 100.
2.7 Lysozyme activity
A turbidimetric test was used to measure the lysozyme activity (Mandalakis et al., 2021). In a 0.5 M sodium phosphate buffer, 10–50 µL of test serum and 0.5–1 mL of micrococcus lysodeikticus suspension (Sigma) was added and mixed. Using an OD at 530 nm spectrophotometer, the samples were measured. As a benchmark, egg white lysozyme was employed.
2.8 Respiratory burst activity and complement activity
The respiratory burst activity of blood leucocytes was confirmed by nitro blue tetrazolium reagent (Mandalakis et al., 2021). The complement activity (Mandalakis et al., 2021) was measured by hemolysis unit mL−1 using sheep red blood cells (HiMedia,India) as targets. In the complement activity, the volume of complement producing 50% hemolysis (ACH50 unit/mL) was determined.
2.9 RNA extraction and cDNA synthesis
Following the manufacturer's instructions, total RNA was extracted from each group's spleen samples (n = 6) using the TRI® reagent (Sigma, India). About 0.2 U of DNase was used to separate RNA samples. On a Nano-drop system, the quantitative and qualitative analysis of RNA was determined at 260 and 280 nm (A260/A280). In accordance with the instructions provided by the manufacturer (Bangalore Genei, Karnataka, India), 1 ug of RNA sample was added to a 20L reaction of the Prime ScriptTM RT Reagent Kit for cDNA synthesis.
2.10 Real-time PCR analysis
Quantitative real-time PCR (ABI 7500, Applied Biosystem, USA) analysis was used to amplify gene expression profiles using the pairs of carp-specific primers for GAPDH, IL-1β, TNF- α, TLR-2, MyD88 (Table 1) in specific genes obtained from NCBI. All the primers were commercially purchased from Bangalore Genei, Karnatka, India. A total of 20 µL of the real-time PCR reaction were used, consisting of 2 µL of cDNA, 0.5 µL of forward and reverse primers (both at 100 mM), 10 µL of 2x SYBR Green qPCR Master Mix, and 5 µL of water. The thermal analyzer cycle at 95°C for 5 mins, followed by 40 cycles at 95°C for 10 s, 56°C for 15 s, and 72°C for 15 s. The relative gene expression levels target gene was normalized to GADPH and analyzed using the 2-ΔΔCT method (Schmittgen and Livak, 2008).
Primers
Nucleotide Sequences (5′-3′)
Size (bp)
TM
Optimum Annealing Temperature (◦C)
Primer Efficiency (%)
Slope
R2
Pearson's coefficient
Acc. No.
GAPDH
F-TGTCCCAACTCCCAATGTGT
R-CTGCAGCCTTAACCACCTTC95
74.5905
59
110
−1.195
0.981
0.991
KC414932.1
IL-1β
F-TGAGAATGTGATTGAAGAGACCA
R-AAGACAAGGTTGTGCAGTGC88
68.1894
61
103
−3.166
0.994
0.953
JQ309137.1
TNF-α
F-CGCTGGTTTCCAACAGTTCT
R-CTCGTTGCCCTCCAGTTTTA83
72.5533
60
97
−3.384
0.998
0.974
KM593875.1
TLR-2
F-GCGAAGAGGACACACCTAGA
R-AGATGCTTCAACAGGAACGC113
67.2567
58
95
−3.031
0.981
0.980
KC907861.1
MyD88
F-GATGGTCAAACGCCAGAGAC
R-CGCACAGCTTCAGGTTGTAA118
73.2412
60
108
−3.144
0.992
0.951
JQ990986.1
2.11 Statistical analysis
All the data were statistically analyzed by one-way analysis of variance, using SPSS (version 19). Tukey's tests were used to compute a significant comparison between the treated groups. The mean and standard deviation data (n = 6) are presented as follows, and P values under < 0.05 were regarded as significant.
3 Results
The present study evaluated the survival, growth rate, and weight gain of infected fish that were increased with M. arvensis extracts fed diet compared with control groups (Table 2). The survival rate (96.2, 96.8 and 97.6 %) was seen in all fish groups with a fed diet. The weight gain and growth rate were noted in 3 and 4% of M. arvensis-enriched diets. The feed conversion rate was noted as 3 and 4% with M. arvensis concentrated diets treated fish group. In the control groups, 1% M. arvensis fed diet; fish showed a similar effect of feed conversion rate. a,b,cMeans within the same row with different superscript letters are significantly different (P < 0.05). Data are presented as mean±standard deviation. 1Means 0,1,2,3 and 4% were commercial fish-fed diet with infection plus 0,1,2,3 and 4 g/kg of dry matter of M. arvensis extract, respectively.
Parameters
Diets1
0 %
1 %
2 %
3 %
4 %
Initial Weight (g)
9.27±0.00
9.25±0.03
9.24±0.02
9.26±0.03
9.27±0.02
Final Weight (g)
9.41±0.17a
10.25±1.05ab
10.41±0.51ab
11.68±0.08c
11.47±0.81c
Weight gain (g)
13.66±17
100.33±10ab
117±53ab
242.16±60b
219.33±80b
Specific growth rate (%)
1.48±0.22a
1.71±0.34ab
1.85±0.11ab
1.92±0.08b
1.98±0.24b
Feed conversion ratio
2.18±0.19a
2.13±0.39a
1.97±0.20a
1.97±0.28a
1.92±0.15a
Survival rate (%)
66.66±5.77a
73.33±5.77ab
73.33±5.77ab
80±0.00b
76.66±5.77ab
The experimental results revealed that the total number of RBC and WBC were increased by M. arvensis extracted fed diet in a dose-dependent manner (Table 3). The RBC was increased with 3% of fed diet M. arvensis supplement compared with control on weeks 6 and 8. The WBC level progressively increased after 6–8 weeks in C. batrachus fish-fed diet supplementation with 3 and 4% of M. arvensis extracts. Similarly, globulin increased in 3 and 4% M. arvensis supplemented fed diets, which was not found in the 1 and 2% supplementation diet compared to the control group. The administration of supplemented-fed diets at 1–2% treatment did not increase the total protein and globulin for 2–4 weeks. The total protein was significantly increased in fish fed with 3–4% M. arvensis fed diets (Table 3), 3 and 4 g/kg of dry matter of M. arvensis extract, respectively.
Parameter
Weeks1
Diets2
0 %
1 %
2 %
3 %
4 %
RBC (million/m3)
0
2.12±0.03abc
2.12±0.03abc
2.14±0.05abc
2.18±0.01abc
2.18±0.21abc
2
2.22±0.12bcd
2.22±0.12abc
2.25±0.16abc
2.22±0.06abc
2.26±0.10abc
4
2.16±0.08abc
2.16±0.0 abc
2.23±0.07abc
2.74±0.17abcde
2.75±0.18abcd
6
2.14±0.08abc
2.14±0.08abc
2.54±0.26abcd
2.85±0.01abc
2.90±0.05cde
8
1.98±0.27abc
1.98±0.27abc
2.53±0.12abcd
3.67±0.19 g
3.33±0.49de
WBC (Per uL)
0
4320.03±5.64 a
4327.60±10.10 bc
4325.77±9.86 abc
4322.90±7.57 ab
4321.93±3.02ab
2
4324.50±0.61 abc
4329.60±7.28 bcd
4332.53±1.68 cde
4342.03±2.37 efg
4339.50±2.56efg
4
4326.60±6.98 abc
4331.97±0.75 cde
4332.07±2.00 cde
4343.47±1.58 efg
4342.10±0.00efg
6
4326.47±3.72 abc
4332.40±7.75 cde
4340.10±4.06 efg
4349.50±3.22 ghi
4343.77±1.32efg 4345.53±4.71efg
8
4327.87±3.02 bc
4335.97±4. 69 def
4345.57±2.70 efg
4353.53±2.55 g
Hematocrit (%)
0
31.10±0.00ab
31.24±1.16ab
31.13±0.61ab
31.51±0.32abc
31.25±0.74ab
2
30.87±1.45ab
31.26±0.21ab
31.68±0.19abc
32.54±0.16bcd
31.50±0.19ab
4
30.50±1.22a
31.49±0.19ab
31.66±0.26abc
33.56±0.21def
32.59±0.40bcd
6
29.67±0.15a
31.63±0.56abc
32.58±0.21bcd
34.38±0.30de
34.57±0.52de
8
30.17±1.25a
32.57±1.18bcd
33.79±0.02de
34.90±1.00e
34.67±0.33e
Hemoglobin (g/dL)
0
8.23±0.07ab
8.69±0.22ab
8.79±0.06ab
9.99±0.53abc
8.15±0.24ab
2
8.34±0.07ab
8.79±0.26ab
9.49±0.47abc
8.67±0.22bcd
8.45±0.20ab
4
8.28±0.16a
8.69±0.08ab
9.98±0.01abc
9.97±0.43cdf
8.97±0.62bcd
6
8.25±0.03a
9.04±0.17abc
9.57±0.40bcd
11.06±0.39df
9.96±0.06df
8
8.21±0.03a
9.57±0.23bcd
9.80±0.12df
10.87±0.51d
10.64±0.40d
Total protein (mg/dL)
0
1.26±0.14a
1.27±0.10a
1.27±0.07a
1.29±0.08 a
1.27±0.09a
2
1.20±0.15a
1.27±0.01a
1.26±0.03a 1.27±0.15a
1.36±0.09 a
1.25±0.13a
4
1.25±0.18a
1.25±0.21a
2.26±0.15b
2.65±0.26bc
3.06±0.10c
6
1.27±0.13a
2.21±0.17b
2.28±0.06b
3.74±0.17d
3.75±0.17d
8
1.27±0.15a
2.24±0.24b
3.95±0.35d
3.78±0.25d
Albumin (mg/dL)
0
1.21±0.10a
1.23±0.15b
1.27±0.22abc
1.29±0.17abcd
1.25±0.11abc
2
1.25±0.15abcd
1.28±0.15abc
1.68±0.20abcdef
1.64±0.14abcdef
1.67±0.16abcdef
4
1.36±0.19abcde
1.67±0.20abcde
1.81±0.17cdef
1.76±0.13abcdef
1.77±0.12abcdef
6
1.57±0.22abcdef
1.79±0.47abcde
1.82±0.15def
2.58±0.26 g
1.91±0.11f
8
1.45±0.20abcde
1.89±0.30abcde
1.86±0.15def
2.99±0.04 g
2.46±0.10 g
Globulin (mg/dL)
0
1.27±0.06ab
1.29±0.07abc
1.24±0.16a
1.23±0.09a
1.26±0.10ab
2
1.32±0.11abcd
1.32±0.07abcd
1.33±0.11abcd
1.37±0.11abcde
1.39±0.03abcde
4
1.36±0.21abcd
1.37±0.19abcd
1.37±0.10abcde
1.61±0.19abcde
1.60±0.13abcde
6
1.40±0.14abcde
1.39±0.06abcd
1.39±0.07abcde
1.73±0.11e
1.69±0.15de
8
1.41±0.17abcde
1.40±0.09abcde
1.45±0.16abcdef
1.67±0.09cde
1.66±0.14bcde
The phagocytic activity in M. arvensis supplemented fed diets gradually increased (Fig. 1A), and a significant difference was observed in the phagocytic activity after 2 weeks. Significantly phagocytic activity was observed in infected fish fed diet with 3 and 4% of M. arvensis extract treated experiments after 2 weeks. In the beginning, the treated and control group indicated the normal level. The respiratory burst was increased in all treatment groups of M. arvensis supplemented fed diets evaluated against the control group (Fig. 1B). The greatest respiratory burst was observed in the fish-fed diet with 3 and 4% of M. arvensis extracts. All four-dose treatment groups increased the respiratory bust in 6–8 weeks compared to the control group. The overall experimental results of the lysozyme activity in serum showed a significant enhancement compared with a control group (Fig. 1C). The enzyme production reached statistically significant with 3 and 4% of M. arvensis extract formulated diet from 6 to 8 weeks. M. arvensis extract-supplemented fish group showed a significant increase in the complement activity throughout the experiment than the control fish group (Fig. 1D). It was significantly higher with 3 and 4% of the fed diet on 6–8 weeks. However, no prominent differences were found in the complement activity between 1 and 2% of the fish and the control groups.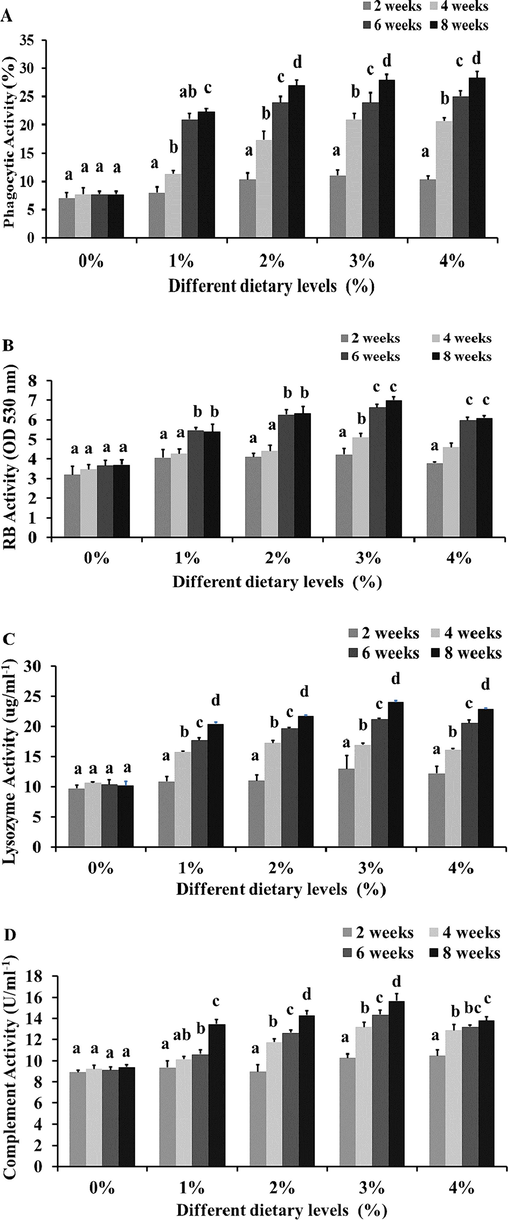
The (A) phagocytic activity, (B) respiratory burst (RB), (C) lysozyme activity and (D) complement activity in C. batrachus after treatment of M. arvensis extracts. Data are represented as mean±standard deviation (n = 6). Significant differences are indicated by different letters on treated and control groups (p < 0.05). Means 0,1,2,3 and 4 % were basal diet with infection plus 0, 1, 2, 3 and 4 g/kg of dry matter of M. arvensis extract, respectively.
The relative mRNA expression of IL-1β, TNF-α, TLR2 and MyD88 of an immune gene in the spleen were analyzed by the quantitative-PCR method and significantly expressed on 2–8 weeks (Fig. 2). The level of IL-1 transcription was significantly greater after the second week of mint extract fed diets (3 and 4%) treated fish (Fig. 2A). Expression of TNF-α in fish mint treated 2 and 3% was significantly higher at 8 weeks post treatment as compared to the control (Fig. 2B-D). Expression of TLR and MyD88 in fish mint treated 3% was significantly higher at 6 to 8 weeks post treatment as compared to the control (Fig. 2C and D). Mint fed diet of fish to showed a time-depend induction of TNF-α, TLR and MyD88 gene transcription was up-regulated than the control group (Fig. 2B-D).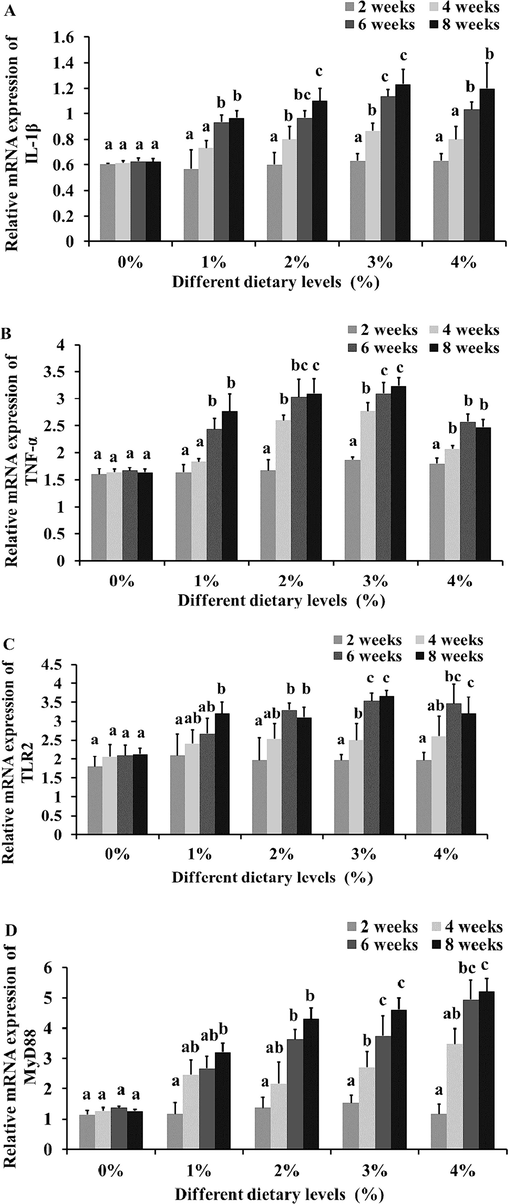
Innate immune genes of (A) IL-1β, (B) TNF-α, (C) TLR and (D) MyD88 expressions in C. batrachus after treatment of M. arvensis extracts. Data are represented as mean±standard deviation (n = 6). Significant differences are indicated by different letters on treated and control groups (P < 0.05). Means 0 %, 1 %, 2 %, 3 % and 4 % were basal diet with infection plus 0, 1, 2, 3 and 4 g/kg of dry matter of M. arvensis extract, respectively.
4 Discussion
The main compounds of M. Arvensis were discovered to be alkaloids, flavonoids, polyphenols, tannins, cardiac glycosides and eugenol when it was analyzed (Malik et al., 2012). The recent study showed that the 34 compounds and other minor substances were examined, such as piperitone (1.32 %), methone (5 %), neomenthyl acetate (5.18 %) and isomenthone (5.24 %) and menthol 77.94 % (Makkar et al., 2018). Among them, menthol, p-menthone, isomenthone and neo-menthol were major and commercially valued. Also, the study demonstrated that the mint and its ingredients have diverse biotic activities and anti-inflammatory properties (Makkar et al., 2018).
The immunological response to infections is now being studied and broadly beneficial when used as dietary additive of marine polysaccharides from seaweed (Liu et al., 2020). Usage of natural goods or newly created natural chemicals has improved fish's immune defense and survival against infections. Recently, many plant extracts like, sage, mint and thyme oils, Allium stipitatum powder, Taraxacum officinale, flower extract and Berberis vulgaris fruit extract have been discovered and provided in fish feed (Sönmez et al., 2015; Shekarabi et al., 2022a; Shekarabi et al., 2022b). The growth act, survival proportion, and immune function against V. harveyi disease resistant increased with the supplementation of peppermint feed (Adel et al., 2015a). On the other hand, M. spicata did not respond to growth level and antioxidant activity in juveniles fish (Sönmez et al., 2015). The experiment assay analyzed the effect of M. arvensis extract on the growth performance, existence, non-specific/specific immune response, and disease resistance of C. batrachus against A. hydrophila. The results of M. arvensis extract-treated fish survival and growth performance were at high-level (Adel et al., 2015b).
In the immune system, blood parameters are a basic tool to indicate the physiological condition, health status and disease tracking, the feed supplement or anti-nutritional elements concentration based on enhance the hematological and biochemical profiles (Abasali and Mohamad, 2010). The WBC is one of the important factors in preserving against chemicals and pathogens (Abasali and Mohamad, 2010). In C. batrachus, the hematological parameters such as WBC, RBC, cumulative protein and globulin were slightly greater in the infected fish-fed diet at 3 and 4 % M. arvensis extract treatment groups, which was similar to other case studies (Adel et al., 2015a; Adel et al.,2016; Abasali and Mohamad,2010). The WBC number was increased with a 3% fed diet of the M. arvensis fish group at 6 weeks. The RBC response was observed in M. arvensis extract-treated fish group after 4th week. Similar results reported that increased RBC and Hb parameters in other studies (Paknejad et al.,2020). The ability of the fish immune system to better inhibit the bacterial population in fish bodies (Adel et al., 2015a). The levels of globulin and albumin in the fish serum total protein were thought to be associated with a more active innate immune response. Diverse humoral components in serum proteins engage phagocytosis activity and initiate the host natural immune response to fish infection (Adel et al., 2015a).
The peptide levels of globulin indicate the potential innate immune function in the blood. In addition, M. arvensis extract enhanced the other immunomodulatory factors such as phagocytic index and respiratory burst during infection periods. Fishes were given a diet containing a variety of herbal extracts, the fish's rudimentary innate immune systems' phagocytic and respiratory burst responses were boosted (Sattanathan et al., 2020b). Likewise, a study reported that the C. auratus against A. hydrophila supplemented with azadirachtin (Kumar et al., 2013). The superoxide anion is generated during respiratory bursts by phagocytes in banana shrimp (Liu et al., 2020), a toxic form of oxygen, and also observed in herbal-treated fish (Jian and Wu, 2004). In the fish immune system, phagocytosis is one of the essential cellular responses confirmed by different kinds of herbal extracts (Chi et al., 2016).
The potential non-specific immune mechanism of alternative complement activity is preserved against microorganisms such as bacterial, fungal, viral and parasitic in fish (Jian and Wu, 2004). Lysozyme is a significant part of the non-specific immune system in fish, which can hydrolyze bacterial cell walls (Adel et al., 2015a; Adel et al., 2016). The rise in lysozyme enzyme level suggests promoting various humoral factors which can protect against the host infection (Chi et al., 2016). In this study, the herbal extract significantly increased the lysozyme activity after 2 weeks. This experiment's results agree with the prior research with some know antimicrobial activities (Chi et al., 2016; Liao et al., 2021). The obtained results suggest that M. arvensis taken as a supplement may activate the antimicrobial defenses of E. malabaricus, which may affect phagocytic activity, the production of reactive oxygen species, and serum lysozyme activity. One of the main sterilizing strategies for removing bacteria in teleost’s has been discovered as a complement's bacterial activity (Liao et al., 2021). Our experiment result showed that supplementing the fed 1–4% M. arvensis diet after 2 weeks increases the complement activity. Although complement activation is often beneficial for fish, extended activation may have negative consequences, including immunosuppression (Awad et al., 2015). In these hypothesis parameters, evidence in fish with mint treatment might be elevated against pathogens and increase non-specific immunity.
However, the immune-related gene expression against bacterial infection is limited in C. batrachus. In the present study, M. arvensis extract was responsible for the genes related to the immune system, such as TNF-α, IL-1β, and MyD88, in the infected C. batrachus treated with 3 and 4% of fed diets after 4 weeks. Our results correlated with previous studies showed that the fish feed diet supplemented with Olea Europea L. (Baba et al., 2018), S. platensis (Ragap et al., 2012), Trigonella foenum-graceum (Awad et al., 2015) extracts will exhibit IL-1β, IL-8. The M. arvensis extracts enhanced cytokines, and also found that the plant extracts improved TLR2 and MyD88. Similarly, previous study has noted that the translation of immune responses (Adel et al., 2015a) fish by mint extracts. Thus, the study hypothesized and the result suggested that the M. arvensis extracts can enhance the immune response of C. batrachus during bacterial infection.
5 Conclusions
In conclusion, our reports suggested that the suitable dose of fed diet supplementation of Mentha arvensis extract leads to increased survival rates during bacterial infection. Slightly increased levels of hematological factors such as WBC, RBC, globulin, and complementary revealed the hematological effect of Mentha arvensis extract. The genes responsible for immunity, such as TNF-α, IL-1β, and MyD88, could be used as potential indicators and increase the level of gene expression. Our results indicated the possible immune-stimulatory and pro-inflammatory role was noted 3% of Mentha arvensis enriched diet in Clarias batrachus. Further research on the specific fraction of Mentha arvensis should be conducted to understand better the effect of immunomodulatory activity and immune-specific genes of Clarias batrachus.
Author Contributions: This research article was produced through collaboration between the authors. Conceptualization, P.T., I.H.K., and B.B.; Writing original manuscript, P.T. and B.B.; Methodology, data curation, and formal analysis, P.T., D.A.D., M.P., A.M.; Review and editing, B.B., S.S., J.W.L., and W.C.L.; Organization of the working groups, M.P., A.M., and B.B. Interpretation, and review/revision, J.W.L., S.S., I.H.K., B.B., and J.W.L. All authors have read and agreed to the published version of the manuscript.
Ethics Statement: The animal experimental study was conducted by following the institutional IACUC guide (CU/AN/2456/2020.11.13] at Biotechnology Department, Nehru Arts and Science College, Coimbatore, Tamil Nadu, India.
Acknowledgement
All the authors are thankful to their respective Universities and Institutes for their support. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-RS-2023-00275307).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Immune response of common carp (Cyprinus carpio) fed with herbal immunostimulants diet. J Anim Vet Adv.. 2010;9(13):1839-1847.
- [Google Scholar]
- 2015a Dietary peppermint (Mentha piperita) extracts promote growth performance and increase the main humoral immune parameters (both at mucosal and systemic level) of Caspian brown trout (Salmo trutta caspius kessler, 1877) Fish Shellfish Immunol. 2015;47(1):623-629.
- [CrossRef] [Google Scholar]
- Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilus frisii kutum) Fish Shellfish Immunol.. 2015;45(2):841-847.
- [CrossRef] [Google Scholar]
- Immunological and biochemical parameters, skin antibacterial activity, and survival in rainbow trout (Oncorhynchus mykiss) following the diet supplemented with Mentha piperita against Yersinia ruckeri. Fish Shellfish Immunol.. 2016;55:267-273.
- [CrossRef] [Google Scholar]
- Effects of fenugreek (trigonella foenum graecum) on gilthead seabream (Sparus aurata L.) immune status and growth performance. Fish Shellfish Immunol.. 2015;45(2):454-464.
- [CrossRef] [Google Scholar]
- Dietary olive leaf (olea europea L.) extract alters some immune gene expression levels and disease resistance to Yersinia ruckeri infection in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol.. 2018;79:28-33.
- [CrossRef] [Google Scholar]
- Routine haematological methods for use with fish blood. J. Fish Biol.. 1973;5:771-781.
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of a bioactive compound isolated from Dryopteris crassirhizoma on the grass Carp Ctenopharyngodon idella. J Immunol Res.. 2016;2016:3068913.
- [CrossRef] [Google Scholar]
- Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: a review. Reviews in Aquac.. 2016;8(1):89-102.
- [CrossRef] [Google Scholar]
- Influences of traditional chinese medicine on non-specific immunity of Jian Carp (Cyprinus carpio var. Jian) Fish Shellfish Immunol.. 2004;16(2):185-191.
- [CrossRef] [Google Scholar]
- Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish Shellfish Immunol.. 2017;67:119-128.
- [CrossRef] [Google Scholar]
- Kumar, S., Raman, R.P., Pandey, P.K., Mohanty, S., Kumar, A., Kumar, K.,2013. Effect of orally administered azadirachtin on non-specific immune parameters of goldfish Carassius auratus (Linn. 1758) and resistance against Aeromonas hydrophila, Fish Shellfish Immunol. 34(2),564-73. Doi: 10.1016/j.fsi.2012.11.038. M.A.
- Antimicrobial resistance of Escherichia coli from aquaculture Farms and their environment in Zhanjiang. China, Front Vet Sci.. 2021;24(8):806653
- [Google Scholar]
- Dietary seaweed (enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol.. 2020;10:202-212.
- [Google Scholar]
- Lopez, J., Carl, A., 2015. Burtis and David E. Bruns: Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 7th ed: Elsevier, Amsterdam. 1075 pp, ISBN 978-1-4557-4165-6, Indian J Clin Biochem, Doi: 10.1007/s12291-014-0474-9.
- Evaluation of Mentha arvensis essential oil and its major constituents for fungitoxicity. J Food Sci Technol.. 2018;55(9):3840-3844.
- [Google Scholar]
- Phyto-chemical analysis, anti-allergic and anti-inflammatory activity of Mentha arvensis in animals. Afr J Pharm Pharmaco.. 2012;6(9):613-619.
- [Google Scholar]
- Antibacterial effects of essential oils of seven medicinal-aromatic plants against the fish pathogen aeromonas veronii bv. sobria: to blend or not to blend? Molecules. 2021;26(9):2731.
- [Google Scholar]
- Haematological and immune upshots in Clarias batrachus exposed to dimethoate and defying response of dietary ascorbic acid. Chemosphere. 2016;168:988-995.
- [Google Scholar]
- NRC, 2011. Nutrient Requirements of Fish and Shrimp. The National Academies Press, Washington, DC. Doi: 10.17226/13039.
- Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus) Fish Physiol Biochem.. 2020;46(5):1883-1895.
- [Google Scholar]
- Immunostimulant effects of dietary Spirulina platensis on tilapia Oreochromis niloticus. J. Appl. Pharm. Sci.. 2012;2(2):26-31.
- [Google Scholar]
- Hematological responses of tambaqui Colossoma macropomum (serrassalmidae) fed with diets supplemented with essential oil from Mentha piperita (lamiaceae) and challenged with Aeromonas hydrophila. Acta Amazonica.. 2016;46(1):99-106.
- [Google Scholar]
- Effect of green algae Chaetomorpha antennina Extract on growth, modulate immunity, and defenses against Edwardsiella tarda infection in Labeo rohita. Animals (basel). 2020;10(11):2033.
- [Google Scholar]
- Influences of dietary inclusion of algae chaetomporpha aerea enhanced growth performance, immunity, haematological response and disease resistance of Labeo rohita challenged with Aeromonas hydrophila. Aquac. Rep.. 2020;17:100353
- [Google Scholar]
- Analyzing real-time PCR data by the comparative CT method. Nature Protocols. 2008;3(6):1101-1108.
- [Google Scholar]
- Growth performance, blood biochemistry profile, and immune response of rainbow trout (Oncorhynchus mykiss) fed dietary persian shallot (Allium stipitatum) powder. Aquac.. 2022;548:737627
- [Google Scholar]
- Effect of dietary barberry fruit (Berberis vulgaris) extract on immune function, antioxidant capacity, antibacterial activity, and stress-related gene expression of siberian sturgeon (Acipenser baerii) Aquac. Rep.. 2022;23:101041
- [Google Scholar]
- Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiol Biochem.. 2015;41(1):165-175.
- [Google Scholar]
- Mentha piperita (peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquac.. 2014;420:71-78.
- [CrossRef] [Google Scholar]
- Effects of medicinal herbs “Plantago asiatica”, “Houttuynia cordata” and “Mentha haplocalyx” on non-specific immune responses of cobia (Rachycentron canadum) Fish Shellfish Immunol.. 2016;58:406-414.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103170.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







