Translate this page into:
Effects of heat treatment on the chemical composition, antioxidant activity, and toxicity of agarwood oil
⁎Corresponding authors. raccha@kku.ac.th (Arunrat Chaveerach), shiouyih.lee@newinti.edu.my (Shiou Yih Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The fragrant compound in agarwood has been a valuable ingredient in perfumery. The agarwood oil that is extracted using hydro-distillation technique undergoes a certain heat treatment to blend well with other compounds. As it is highly prized and proprietary, information on the effects of heat treatment on the agarwood oil is limited, hindering its potential as a bioresource in other pharmaceutical and cosmeceutical applications. In this study, we reveal the effects of heat treatment on agarwood oil, with a special focus on terpene compounds – an important chemical component found in agarwood. We found that agarwood oil was thermally stable at the temperatures of 80 °C, 120 °C, and 180 °C and time points 5 min, 10 min, and 20 min, respectively, without causing major loss in its terpene compounds, including monoterpenes, diterpenes sesquiterpenes, and sesquiterpenoids. Further analysis using the DPPH (2,2-diphenyl-1-picrylhydrazyl) antioxidant assay showed an overall increase in free radical scavenging activity for these heated agarwood oil. Additionally, the heat-treated agarwood oil did not return with any toxic effects on mononuclear blood cells. The findings of this study would be beneficial to the downstream applications of agarwood oil in perfumery and pharmaceutical product development.
Keywords
Aquilaria malaccensis
DPPH
GCMS
GC-FID
Red List species
1 Introduction
Agarwood, a valuable product from Thymelaeaceae resinous heartwood trees, is primarily found in Aquilaria and Gyrinops species (Lee and Mohamed, 2016). Its formation is a self-defense mechanism against damage to the tree. However, the number of trees with agarwood is limited due to the low number of natural injuries in the wild. Locals mechanically wound the trees to induce agarwood formation (Azren et al., 2019), but harvesting is disastrous due to the agarwood's formation in the xylem of the tree trunk (Barden et al., 2000). Due to being heavily exploited in the wild, Aquilaria and Gyrinops species are now listed under the Convention for International Trade of Endangered Species (CITES) Appendix II (UNEP-WCMC, 2023), and are categorized as “Critically endangered”, “Data deficient”, “Endangered”, and “Vulnerable” by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (IUCN, 2023).
Agarwood oil, a volatile aromatic compound, is produced from agarwood sawdust distillation. Sustainable production is achieved through plantation cultivation and artificial inducement techniques (Azren et al., 2019. The yield of agarwood oil is affected by pre-treatment and extraction processes (Pornpunyapat et al., 2011). Despite its small extract amount and laborious extraction, agarwood oil remains in high demand due to its use in religious ceremonies, perfume production, and traditional medicine (Naef, 2011). The quality of agarwood oil is crucial (Pornpunyapat et al., 2011), and analytical methods like gas chromatography-mass spectrophotometry (GC/MS) and gas chromatography with flame ionization detection (GC-FID) are used to examine its main chemical constituents (Shaian et al., 2021).
Research on agarwood's phytochemical properties has identified numerous compounds, including sesquiterpenes, sesquiterpenoids, aldehydes, carboxylic acids, chromones, and ketones (Tajuddin et al., 2016). The scent of agarwood is crucial in evaluating its quality (Yang et al., 2021). However, due to its rarity and high cost, little study has been conducted on agarwood oil. This study aims to describe the thermal behavior of agarwood oil heated at different points, examining its phytochemical compounds, antioxidant properties, and toxicity reactions. The findings could be useful in pharmaceutical and cosmeceutical product development.
2 Material and methods
2.1 Sample preparation
Agarwood oil was purchased from a bio essential oil distillation factory in Nilai, Negeri Sembilan, Malaysia. The agarwood oil was extracted from eight-year-old Aquilaria malaccensis grown on a plantation in Lanchang, Pahang, in July 2022. Species identification was conducted by one of the corresponding authors, Prof. Dr. Lee Shiou Yih. The agarwood wood chips are sourced from mature trees inoculated with fungal inoculum, while the species of the fungus are not disclosed due to trade secrets. The inoculated tree was left for two-and-a-half years prior to being harvested for oil extraction. Hydro distillation was conducted on a commercial hydro distiller (see Appendix A), in which approximately 70 kg of the wood chips containing the agarwood were grinded into powder form and submerged in tap water, then heated at a temperature between 90 °C and 100 °C for six days. About 800 mL of the oil extract was collected along the heating process; however, only 24 mL were used in this study and were kept in the dark at room temperature prior to further analysis. The agarwood oil was then distributed into ten clear 2-mL screw-cap flat-base glass vials, in which each glass vial contained 0.5 g of the agarwood oil. All the oil samples, including the glass vial without its screw cap, were weighed prior to analysis to obtain the initial sample weight.
2.2 Heat treatment
Three different temperature points, 80 °C, 120 °C, and 180 °C, were selected, while three different time points, 5 min, 10 min, and 20 min, were used to observe the time-related behavior of agarwood oil and to prevent excessive vaporization of limited agarwood oil samples. These heating points (temperature and time points) were chosen to observe the volatility of the chemical compounds within the oil, which would classify them as aldehyde, ketone, sesquiterpene, and sesquiterpenoid compounds with boiling points of 80 °C, 120 °C, and 180 °C, and compounds with boiling points of greater than 180 °C, as described by Alavi et al. (2011). All heating points were carried out under two oil sample replicates. Non-heated agarwood oil samples were used as a control. The heating procedure was carried out in a Thermoline SOV70B oven (Thermoline, Australia), with the oil-filled glass vials placed on an aluminum dispensing tray. At the end of each time point, the glass vials were removed from the oven using tongs. The samples were left to cool down for 15 min before proceeding to the next analysis.
2.3 Calculation of mass loss
The samples were weighed on a Setra EL-410S precision balance (Setra, USA) and the mass loss of each sample in percentage was calculated using the following equation:
2.4 DPPH assay
A total of 2.4 mL of ethanolic DPPH (0.2 mM) was prepared, and 0.1 mL of an agarwood oil sample was added to it, which was then labelled as A1. For the blank, the agarwood oil sample was replaced with 0.1 mL of absolute ethanol, which was then labelled as A0. For A2, the 2.4 mL of ethanolic DPPH was replaced with absolute ethanol, and 0.1 mL of an agarwood oil sample was added to it. The mixture was incubated in the dark at room temperature for 20 min. The absorbance value was quantified at 517 nm using a Libra S12 UV–Vis spectrophotometer (Biochrom, UK). The percentage of DPPH free radical scavenging activity for each sample was calculated using the following formula:
2.5 GC–MS and GC-FID analysis
GC–MS analysis was carried out using an Agilent 7890A gas chromatograph that was equipped with a mass spectrophotometer and a DB-1 (100 % dimethylpolysiloxane) (30 m × 250 µm, film thickness 0.25 µm), and a selective mass detector was used for GC–MS and GC-FID. The oven was equipped with a detector that was operated in full scan mode under an electron impact ionization (EI) of 70 eV. The oven temperature was set at 230 °C with a rate of 3 °C/min. The oven was programmed to run at an initial temperature of 70 °C, hold for 3 min, increase by 3 °C per min until 230 °C, and finally hold for 3 min. The flow rate of carrier gas (helium) was maintained at 1.0 mL per min. Compounds were identified by comparing the retention indices (RIs) and mass spectra with literature data and National Institute of Standards Technology (NIST) libraries. Retention indices were calculated using a homologous series of n-alkanes (C7-C20) (Tajuddin and Yusoff, 2010).
2.6 Preparation of working solutions for toxicity experiments
Two samples were selected for the toxicity experiments, which included the heated agarwood oil sample with the highest DPPH value and a non-heated agarwood oil sample as the control group. Both samples were diluted in 10-fold serial dilutions with 10 % dimethyl sulfoxide (DMSO) at five different concentration levels as a working solution for cytotoxicity treatments. All working solutions were kept at −20 °C until further analysis.
2.6.1 Isolation and preparation of human peripheral blood mononuclear cells (PBMCs)
PBMCs were obtained from venous blood collected with sodium heparin anticoagulant from the blood bank of Srinagarind Hospital. The isolation process involved utilizing Ficoll-Paque™ Plus (Cytiva, Sweden) according to the prescribed procedure. The approach is slightly changed from Freshney’s (2010) method. The PBMCs, which were recently obtained and had a viability of at least 98 %, were mixed with modified RPMI-1640 medium containing L-glutamine. The medium was supplemented with 10 % Fetal Bovine Serum (FBS) (Cytiva, USA), as well as 100 µg/mL streptomycin and 100 U/mL penicillin (Thermo Fisher Scientific, Waltham, MA, USA). The cells were calculated as a concentration of 106 cells/mL for toxicity testing using a hemocytometer.
2.6.2 Cytotoxicity test using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
The PBMCs, produced at a concentration of 1 × 106 cells per well, were distributed into 96-well plates with 125 µL per well. An additional 12.5 µL of the non-heated agarwood oil and heated agarwood oil working solution were added to the respective wells at five different concentration levels. The plates were placed in a CO2 incubator with controlled humidity at a temperature of 37 °C and a CO2 concentration of 5 % for a duration of 24 h. A 10 % solution of dimethyl sulfoxide (DMSO) was utilized as the control for the vehicle. The plates were subjected to centrifugation at 1,500 rpm for 10 min, after which the supernatant was discarded. A 10 µL volume of MTT solution was applied to each well, resulting in a final concentration of 0.5 mg/mL. Subsequently, the plates were incubated for 4 h at 37 °C. The formazan crystals were dissolved with the addition of 100 µL of DMSO into each well and quantified at a wavelength of 570 nm using a fluorescence microplate reader (SpectraMax M5 series, Molecular Devices, CA, USA). The experiment was conducted three times for each treatment. The percentage of cell viability was determined using the following formula: % cell viability = [(average viable of treated cells/average viable of untreated cells negative control) × 100]. The viable cells underwent cellular reduction of MTT, resulting in the formation of a violet crystal formazan. This reduction was facilitated by mitochondrial succinate dehydrogenase activity. The quantification of the violet crystal formazan was performed using Freshney’s (2010) approach. In addition, if the cell viability result is less than 50 %, the 50 % inhibition of cell viability (IC50) is determined using nonlinear regression analysis in GraphPad Prism version 8.0.2 (GraphPad Software, Boston, USA). This analysis involves assessing the relationship between extract concentrations and cell viability. The IC50 value is further employed in the LD50 calculation (Ekwall et al., 1998) to determine the values at which the substance becomes harmful.
2.6.3 Genotoxicity testing
The comet assay was conducted using the method outlined by Singh et al. (1988), with some minor adjustments. To confirm the outcome, all slides were examined using a fluorescent microscope (Nikon, Minato-ku, Japan) at a magnification of 200. The microscope was fitted with a 560 nm excitation filter, a 590 nm barrier filter, and a CCD video camera PCO (Kelheim, Germany). A minimum of 150 cells per experiment were taken using ImageJ. The olive tail moment (OTM), which is the measurement of the amount of DNA in the tail of the comet multiplied by the median migration distance, was analyzed using CASP software version 1.2.3 (CASPlab, Wroclaw, Poland). The untreated cells were used as a negative control, whereas the positive control was exposed to UVC radiation. The values were represented as the mean ± standard deviation (S.D.). To determine statistical significance, the non-parametrical Mann Whitney U test was utilized to compare data between groups (p < 0.05) using GraphPad Prism version 8.0.2 (GraphPad Software, Boston, USA).
3 Results
3.1 Mass loss
At the 80 °C heating point, the average mass loss of agarwood oil was observed to remain constant at 2 % for the time points of 5- and 10-min, while it showed an increased value of 3 % at the longest heating point of this temperature at the time point of 20-min (Fig. 1). At 120 °C, the average mass loss of agarwood oil displayed a consistent increase of mass loss at 2 %, 3 %, and 4 % for time points of 5-, 10-, and 20 min, respectively (Fig. 1). At 180 °C, the average mass loss of agarwood oil showed an increasing trend from 2 % to 7 % loss from a time point of 5-min to a time point of 20-min (Fig. 1). Among all temperatures and time points that were used, the greatest mass loss (7 %) was obtained at the heating point of 180 °C during the 20-min time point. Overall, the mass loss of agarwood oil was positively related to the increasing heating temperature and the duration of heating.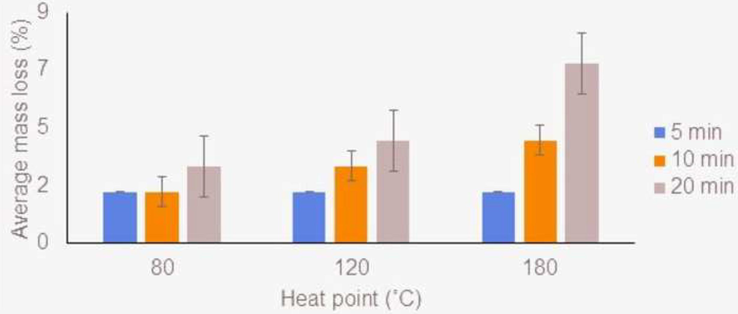
Bar graph of average mass loss (%) of agarwood oil for three different heating points.
3.2 DPPH assay
At the 80 °C heating point, the DPPH scavenging activity of agarwood oil showed an increasing pattern at 47.25 %, 53.64 %, and 55.43 % from the time points of 5-, 10-, and 20-min, respectively (Fig. 2). At the 120 °C heating point, the DPPH scavenging activity decreased from 62.67 % to 33.49 % at the time points of 5-min and 10-min, respectively, and the activity increased to 58.21 % at the time point of 20-min. At the 180 °C heating point, the DPPH scavenging activity followed an increasing trend, 68.1–74.44 %, from the time point of 5–10 min followed by a decrease in free radical scavenging activity (69.57 %) at the highest time point of 20-min. Among all temperatures and time points that were used, the highest DPPH scavenging activity (74.44 %) was obtained at 180 °C heating temperature at a time point of 10-min. Almost all agarwood oil samples displayed higher scavenging activity than the control (50.39 %). Apart from the scavenging activities, 47.25 % were observed at 80 °C with a time point of 5-min and 33.49 % at 120 °C with a time point of 10-min, respectively.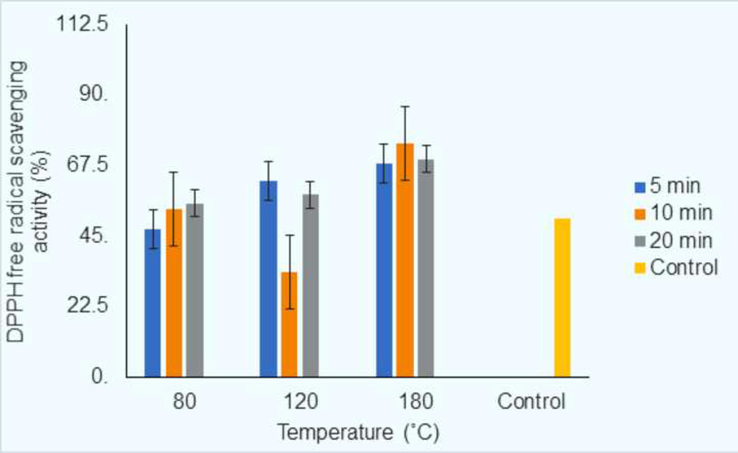
Bar graph of DPPH free radical scavenging activity (%) of agarwood oil for three different heating points (80 °C, 120 °C, and 180 °C) and at three different time points (5 min, 10 min, and 20 min) and the most right is control (of non-heat treated) agarwood oil of DPPH free radical scavenging activity.
3.3 Identification of compound through GC analyses
The number of compounds identified by GC-FID was higher than GC–MS. The GC-FID and GC–MS analysis of the essential oils resulted in the identification of 45 different compounds (Table 1, see Appendix A) and are subsequently classified into four groups, namely aldehyde, ketone, sesquiterpene, and sesquiterpenoid. a = GC-FID, b = GC–MS.
Compounds
RI
Percentage area (%)
Ident.*
Control
80 °C
80 °C
80 °C
120 °C
120 °C
120 °C
180 °C
180 °C
180 °C
5 min
10 min
20 min
5 min
10 min
20 min
5 min
10 min
20 min
Aldehyde
Benzaldehyde
933
0.54
0.55
0.54
0.48
0.54
0.51
0.49
0.55
0.49
0.43
a,b
Hexadecanal
1794
0.24
0.2
0.2
0.18
0.18
0.2
0.26
0.18
0.2
0.2
a
Total of aldehyde
0.78
0.75
0.74
0.66
0.72
0.71
0.75
0.73
0.69
0.63
Ketone
4-Phenyl-2-butanone
1207
6.36
6.39
6.49
6.49
6.53
6.36
6.39
6.13
6.25
6.13
a,b
1,5-Diphenyl-3-pentanone
1964
0.29
0.28
0.3
0.31
0.3
0.28
0.28
0.29
0.29
0.3
a,b
Total of ketone
6.65
6.67
6.79
6.80
6.83
6.64
6.67
6.42
6.54
6.43
Sesquiterpene
α-Gurjunene
1408
0.2
0.2
0.2
0.2
0.2
0.2
0.2
0.2
0.2
0.2
a
β-Caryophyllene
1432
0.14
0.15
0.14
0.14
0.15
0.15
0.15
0.19
0.14
0.14
a
Aromadendrene
1439
0.19
0.2
0.19
0.19
0.19
0.2
0.2
0.23
0.17
0.17
a
α-Caryophyllene
1447
0.13
0.13
0.13
0.13
0.14
0.13
0.13
0.25
0.13
0.13
a,b
allo-Aromadendrene
1464
17.8
17.9
18.04
18.03
18.12
17.91
18.16
17.55
17.7
17.68
a
γ-Selinene
1474
0.13
0.14
0.14
0.14
0.14
0.14
0.14
0.19
0.15
0.15
a,b
γ-Muurolene
1477
0.15
0.15
0.15
0.15
0.15
0.15
0.15
0.17
0.16
0.16
a
β-Vetispirene
1480
0.12
0.12
0.12
0.12
0.13
0.12
0.13
0.13
0.13
0.13
a,b
Valencene
1487
0.23
0.23
0.23
0.23
0.23
0.24
0.24
0.28
0.3
0.3
a,b
δ-Guaiene
1494
0.6
0.6
0.6
0.6
0.6
0.6
0.6
0.58
0.6
0.6
a,b
γ-Cadinene
1501
0.4
0.39
0.42
0.36
0.4
0.42
0.42
0.4
0.4
0.34
a
Selina-3,7(11)-diene
1524
0.28
0.27
0.27
0.27
0.28
0.28
0.28
0.27
0.28
0.28
a
α-Calacorene
1529
0.43
0.44
0.44
0.43
0.43
0.44
0.44
0.42
0.42
0.44
a
Dehydro-aromadendrene
1533
1.04
1.02
1
1
1.03
1.03
1.05
1.1
1.26
1.22
a
Total of sesquiterpene
21.84
21.94
22.07
21.99
22.19
22.01
22.29
21.96
22.04
21.94
Sesquiterpenoid
Dihydro-β-agarofuran
1490
0.69
0.69
0.7
0.7
0.7
0.7
0.7
0.65
0.67
0.67
a,b
Kessane
1521
0.2
0.2
0.18
0.18
0.2
0.2
0.2
0.19
0.16
0.19
a,b
α-Agarofuran
1537
0.52
0.52
0.53
0.52
0.52
0.52
0.53
0.51
0.53
0.54
a
cis-Nerolidol
1548
2.62
2.47
2.37
2.4
2.56
2.56
2.66
2.75
2.89
2.68
a
nor-Ketoagarofuran
1560
1.41
1.47
1.42
1.41
1.41
1.48
1.5
1.4
1.44
1.45
a
Epoxybulnesene
1572
1.32
1.3
1.28
1.29
1.31
1.3
1.34
1.23
1.14
1.09
a
Caryophyllene oxide
1583
1.56
1.57
1.58
1.57
1.68
1.57
1.6
1.52
1.56
1.58
a
Guaiol
1598
1.11
1.12
1.12
1.11
1.09
1.12
1.15
1.11
1.09
1.1
a
10-epi-γ-Eudesmol
1603
3.77
3.78
3.72
3.78
3.66
3.79
3.76
3.63
3.73
3.73
a,b
γ-Eudesmol
1613
0.41
0.39
0.41
0.45
0.43
0.38
0.33
0.41
0.38
0.36
a,b
Agarospirol
1619
3.95
3.96
3.97
4
3.97
3.95
3.95
5.99
3.88
3.91
a,b
τ-Muurolol
1628
6.04
6.02
6.11
6.15
6.08
6
5.91
6.78
5.98
5.93
a
α-Eudesmol
1636
9.04
9.04
9.14
9.18
9.1
9
8.92
9.45
8.8
8.98
a
Jinkoh-eremol
1641
0.76
0.75
0.75
0.75
0.76
0.74
0.74
0.98
0.9
0.74
a
Kusunol
1653
10.27
10.2
10.2
10.09
10.17
10.23
10.34
9.97
10.36
10.34
a
Bulnesol
1657
1.07
1.07
1.07
1.06
1.06
1.08
1.08
1.02
1.12
1.13
a
Dehydrojinkoh-eremol
1667
4.53
4.55
4.57
4.53
4.49
4.53
4.85
4.52
4.52
4.8
a
α-Bisabolol
1685
0.6
0.6
0.61
0.53
0.6
0.54
0.33
0.32
0.58
0.59
a
Rotundone
1699
1.12
1.1
1.11
0.92
1.1
0.86
0.88
0.77
1.09
1.1
a
Valerenol
1709
2.35
2.34
2.35
2.28
2.31
2.31
2.34
2.21
2.31
2.29
a
Selina-4,11-dien-14-oic acid
1725
0.15
0.16
0.16
0.1
0.08
0.16
0.16
0.14
0.16
0.17
a
α-Costol
1729
0.45
0.45
0.45
0.45
0.44
0.44
0.44
0.42
0.43
0.43
a,b
Selina-3,11-dien-9-al
1737
1.15
1.15
1.16
1.13
1.13
1.14
1.15
1.1
1.1
1.09
a
Dehydrofukinone
1772
3.99
4.02
4.05
3.94
3.98
4.09
4.14
4.29
4.5
5.09
a,b
Guaia-1(10),11-dien-15-oic acid
1818
0.41
0.45
0.46
0.45
0.43
0.44
0.44
0.4
0.4
0.43
a
oxo-Agarospirol
1834
0.24
0.23
0.28
0.27
0.22
0.21
0.21
0.17
0.12
0.14
a
Dihydrocolumellarin
1839
0.18
0.18
0.18
0.18
0.18
0.17
0.18
0.17
0.17
0.17
a,b
Total of sesquiterpenoid
59.91
59.78
59.93
59.42
59.66
59.51
59.83
62.10
60.01
60.72
Based on the GC analysis, the agarwood oil samples are dominated by sesquiterpenoids (59.42–62.10 %), followed by other compounds (sesquiterpenes: 21.84–22.19 %, ketones: 6.42–6.83 %, aldehydes: 0.63–0.78 %). The highest percentage of total sesquiterpenoids (60.01–62.10 %) was achieved in the agarwood oil sample when being heat-treated at 180 °C, followed by the agarwood oil samples heated at 80 °C (59.42–59.93 %) and 120 °C (59.51–59.83 %). The percentage of total sesquiterpenoids in heat-treated agarwood oil samples is generally higher or similar than that of the control sample (59.91 %) (Table 1). However, the percentage area for the total sesquiterpene across agarwood oil samples at all heat points did not show any clear variation, except for the heat point 120 °C, which is recorded slightly higher when compared to that of the control sample (21.84 %); the total sesquiterpene percentages for the heat points 80 °C, 120 °C, and 180 °C were 21.94–22.07 %, 22.91–22.29 %, and 21.94–22.04 %, respectively.
The percentage area for the other compounds present in the heat-treated agarwood oil samples was similar or slightly less than that of the control sample for the total ketones but was clearly less than that of the control sample for the total aldehydes. The total ketone percentage in the control sample was 6.65 %; while the total ketone percentage in the heated agarwood oil samples was 6.67–6.80 %, 6.64–6.83 %, and 6.42–6.54 % for the heat points 80 °C, 120 °C, and 180 °C, respectively. In contrast, the total aldehyde percentage in the heated agarwood oil samples for all heat points was in the range of 0.63–0.75 %, which is clearly less than the total aldehyde percentage in the control sample (0.78 %).
3.4 Identification of cell viability and genotoxicity
Based on the DPPH analysis, the agarwood oil sample that was heat-treated at 180 °C for 10 min was selected for the cytotoxicity study. Both the non-heated and heated agarwood oils at the highest concentration revealed low cell viability rates of 53.59 ± 0.97 % and 51.71 ± 0.76 %, respectively (Fig. 3). A notable reduction in cell viability following exposure to agarwood oil was detected; the viability assay demonstrated a low percentage of surviving cells compared to the control group. The IC50 values were detected as 443.20 and 345.6 mg/mL for non-heated and heated agarwood oils, respectively. From the derived IC50 values, the predicted LD50 doses of non-heated and heated agarwood oils were 13,320.95 mg/50 kg and 12,143.66 mg/50 kg of rats, placing them under the category of class III-slightly hazardous.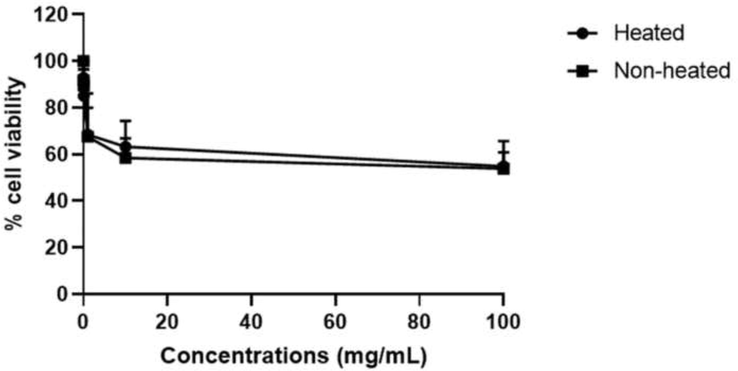
Cytotoxicity testing with the MTT assay of non-heated and heated agarwood oil on human peripheral blood mononuclear cells (PBMCs).
The agarwood oil samples (heated agarwood oil and control) with a concentration of 100 mg/mL were selected for genotoxicity evaluation on PBMCs using a comet assay. No clear DNA damage was detected with the treated PBMCs (p > 0.05) for both agarwood oil samples as specified by DNA condensing in cells, when compared to the two negative controls (Table 2). A comet tail in the test indicates that the mononuclear blood cells suffer no damage (Fig. 4); no genotoxic effect was detected on either of the agarwood oil samples.
Studied samples
OTM values (mean ± SD)
p-values
Negative control (-vg)
0.5109 ± 0.23a
–
0.0116 ± 0.03b
10 % DMSO
0.5091 ± 0.25a
0.8663
Heated agarwood oil (100 mg/mL)
0.4990 ± 0.35a
0.0763
Control (100 mg/mL)
0.0105 ± 0.02b
0.1123
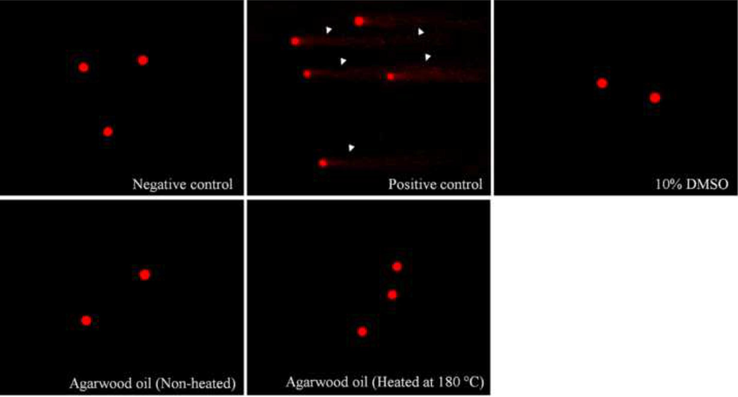
Genotoxicity evaluation of non-heated and heated agarwood oil at the concentration as 100 mg/mL on PBMCs through comet assay image (200×) when compared to negative control and positive control. The white arrow indicates the DNA damage fragments. The studied sample did not show toxic effect on PBMCs.
4 Discussion
This study explores the thermal behavior of agarwood oil, a topic previously limited to agarwood samples. The study characterized the thermal stability of agarwood oil through mass loss change at different temperatures and time points, allowing a better understanding of the oil's volatility. The total mass loss increased continuously at all three heating temperatures and time points, except for the temperature of 80 °C at 5-min and 10-min time points. No mass loss was detected at these two time points, possibly due to agarwood oil withstanding the temperature within the selected duration of incubation and remaining thermally stable. The thermal resistance of essential oils can be linked to their chemical composition, with most compounds, especially sesquiterpenes, having a boiling point of more than 180 °C due to not being vaporized when heated at 180 °C, i.e., δ-guaiene (boiling point = 274–275 °C; Nurjanah et al., 2020), and cadinene (boiling point = 274 °C; Ab Rahman, 2009). Agarwood oil, a plant essential oil without monoterpenes or diterpenes, has lower mass loss due to heat compared to other plant oils. Its color remains light-yellow, except for those heated at 180 °C, which turns darker. Heat-treated samples produce a woody, slightly bitter, and spicy aroma, with intensified aromas from 80 °C to 180 °C. Higher temperatures and extended heating periods increase mass loss in plant essential oil, as molecules convert into vapor due to increased kinetic energy, causing some to overcome intermolecular forces, such as dipole-16 dipole interaction, van der Waals force, and hydrogen bonding, and escape into the air as vapor (Zuhra et al., 2013).
Essential oil vapor contains low-molecular-weight chemical compounds and water (Syafiq et al., 2021), with monoterpenes, such as pinene, β-pinene, myrcene, and β-phellandrene, are temperature-sensitive and evaporating with water. However, sesquiterpenes have higher molecular weights (Hazrati et al., 2021). GC–MS studies on agarwood oil found sesquiterpenoids, monoterpenes, carboxylic acids, and chromones as major components (Shaian et al., 2021). Therefore, molecular weight could influence mass loss in agarwood oil, with minimal loss observed due to the greater amount of sesquiterpenes.
The antioxidant activity of agarwood oil, particularly sesquiterpenes and sesquiterpenoids, is enhanced over time due to prolonged heating. These compounds act individually or synergistically as antioxidants (Abd-ElGawad et al., 2020). For example, heat-treated nutmeg oil and Satureja hortensis oil show higher radical scavenging activity (Tomaino et al., 2005; Chambre et al., 2020). Long heating durations promote the accumulation of antioxidant products, which are parallel to the high inhibition rate of free radical scavenging activity (Chambre et al., 2020). The increased concentration of DPPH over time is due to the increase in denatured content and rapid dehydration (Nhi et al., 2020). Thus, heat-treated agarwood oil contains more antioxidant activities compared to untreated oil.
The study found that temperature changes did not alter the compounds of agarwood oil, with the aromatic compounds dominated by sesquiterpenoid group compounds (59.42–62.1 %) (Table 1). High-quality agarwood oil is often associated with its large amount of sesquiterpenoid, including kusunol, α-eudesmol, τ-muurolol, dehydrojinkoh-eremol, dehydrofukinone, and valerenol, contributing to its unique aroma (Ishihara et al., 1991). However, the amount of sesquiterpenoids in agarwood oil may vary between species, origins, and extraction methods, with no clear correlation between temperature and time points and sesquiterpenoid group rates (Nor Azah et al., 2008; Wetwiyaklung et al., 2009).
The comet assay is a sensitive method for assessing DNA damage in human PBMCs (Olive and Banáth, 2006). In this study, agarwood oil was found to decrease cell viability compared to the control group. However, the test showed no genotoxic effect on mononuclear blood cells, indicating no toxic effects (Fig. 4). The lack of IC50 values suggests no toxic effects on PBMCs (Table 2). The results suggest that A. malaccensis essential oil has a negative toxic effect under natural circumstances, with no significant DNA damage observed at a certain concentration (Gogoi et al., 2023). Therefore, further clinical validation is needed to determine appropriate dosage and administration methods.
5 Conclusion
This study reported mass loss of agarwood oil when heat-treated at three distinct high temperatures across three different time points. For the first time, the radical scavenging, chemical profile, and toxicity activities of the heat-treated agarwood oil were assessed. Based on the chemical composition and bioactivity results, we can confirm that agarwood oil subjected to heat treatment shows no apparent adverse effect. Agarwood oil can endure elevated temperatures while retaining the quality of its content, which is suitable for incorporation in perfumery and pharmaceutical product development.
CRediT authorship contribution statement
Nurul Aduka Syameera: Funding acquisition, Writing - original draft, Visualization, Investigation, Formal analysis, Project administration. Sanit Kaewdaungdee: Writing – original draft, Visualization, Investigation, Formal analysis. Saiful Nizam Tajuddin: Investigation, Methodology, Resources. Tawatchai Tanee: Data curation, Validation, Methodology. Runglawan Sudmoon: Formal analysis, Resources, Project administration. Arunrat Chaveerach: Conceptualization, Funding acquisition, Data curation, Resources, Writing – review & editing. Shiou Yih Lee: Conceptualization, Funding acquisition, Data curation, Writing - review & editing, Supervision, Resources.
Funding
This work was supported by the INTI International University Seeding Grant Scheme [grant number INTI-FHLS-01-19-2022] and the Research and Graduate Studies of Khon Kaen University, Thailand.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Analysis of agarwood oil composition via preparative thin layer chromatography. Malaysia: Universiti Malaysia Pahang; 2009. Doctoral dissertation
- Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem... 2020;13(2):4237-4245.
- [Google Scholar]
- The effect of heat treatment on chemical composition and antioxidant property of Lippia citriodora essential oil. J. Med. Plants. 2011;10(39):65-75.
- [Google Scholar]
- History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: a review. J. For. Res.. 2019;30:1-11.
- [Google Scholar]
- Heart of the matter: agarwood use and trade and CITES implementation for Aquilaria malaccensis. Cambridge, UK: Traffic International; 2000.
- Chemical composition, antioxidant capacity, and thermal behavior of Satureja hortensis essential oil. Sci. Rep.. 2020;10(1):21322.
- [Google Scholar]
- MEIC evaluation of acute systemic toxicity: Part VI. The prediction of human toxicity by rodent LD50 values and results from 61 in vitro methods. Altern. Lab. Anim.. 1998;26(2_suppl):617-658.
- [Google Scholar]
- Agarwood (Aquilaria malaccensis L.) a quality fragrant and medicinally significant plant based essential oil with pharmacological potentials and genotoxicity. Ind. Crop. Prod.. 2023;197:116535
- [Google Scholar]
- A comparative study: Influence of various drying methods on essential oil components and biological properties of Stachys lavandulifolia. Food Sci. Nutr.. 2021;9(5):2612-2619.
- [Google Scholar]
- IUCN 2023. The IUCN Red List of Threatened Species. Version 2022-2. https://www.iucnredlist.org.
- The origin and domestication of Aquilaria, an important agarwood-producing genus. In: Mohamed R., ed. Agarwood: Science Behind the Fragrance. Singapore: Springer; 2016. p. :1-20.
- [Google Scholar]
- The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: a review. Flavour Fragr. J.. 2011;26(2):73-87.
- [Google Scholar]
- Effects of vacuum concentration on color, polyphenol and flavonoid contents and antioxidant activity of pomelo Citrus maxima (Burm. f.) Merr. juice. IOP Conf. Ser. Mater. Sci. Eng.. 2020;991:012060
- [Google Scholar]
- Comparison of chemical profile of selected gaharu oils from Peninsular Malaysia. Malaysian J. Anal. Sci.. 2008;12(2):338-340.
- [Google Scholar]
- Isolation of guaiene from patchouli (Pogostemon cablin Benth.) oil using vacuum fractionation distillation. IOP Conf. Ser.: Earth Environ. Sci.. 2020;443:012094
- [Google Scholar]
- The comet assay: a method to measure DNA damage in individual cells. Nature Protocols (Electron. Ed.). 2006;1:1-23.
- [Google Scholar]
- Mathematical modeling for extraction of essential oil from Aquilaria crassna by hydrodistillation and quality of agarwood oil. Bangladesh J. Pharmacol.. 2011;6(1):18-24.
- [Google Scholar]
- Chemical composition of agarwood essential oil (Aquilaria malaccensis) upon exposure towards heat condition. Malaysian J. Chem.. 2021;23(2):1013.
- [Google Scholar]
- A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res.. 1988;175:184-191.
- [Google Scholar]
- Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol.. 2021;11:144-157.
- [Google Scholar]
- Resolution of complex sesquiterpene hydrocarbons in Aquilaria malaccensis volatile oils using gas chromatography technique. In: Mohamed R., ed. Agarwood: Science Behind the Fragrance. Singapore: Springer; 2016. p. :103-124.
- [Google Scholar]
- Chemical composition of volatile oils of Aquilaria malaccensis (Thymelaeaceae) from Malaysia. Nat. Prod. Commun.. 2010;5(12)
- [CrossRef] [Google Scholar]
- Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem.. 2005;89:549-554.
- [Google Scholar]
- UNEP-WCMC (Comps.)., 2023. The Checklist of CITES Species Website. CITES Secretariat, Geneva, Switzerland. Compiled by UNEP-WCMC, Cambridge, UK. http://checklist.cites.org.
- Chemical constituents and antimicrobial activity of essential oil and extracts of heartwood of Aquilaria crassna obtained from water distillation and supercritical fluid carbon dioxide extraction. Silpakorn Univ. Sci. Technol. J.. 2009;3(1):25-33.
- [Google Scholar]
- The Characteristic fragrant sesquiterpenes and 2-(2-phenylethyl) chromones in wild and cultivated “Qi-Nan” agarwood. Molecules. 2021;26(2):436.
- [Google Scholar]
- Effect of essential oil of Attarasa leaves (Litsea cubeba Lour. Pers) on physico-mechanical and microstructural properties of breadfruit starch-alginate edible film. Malaysian J. Anal. Sci.. 2013;17(3):370-375.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103141.
Appendix A
Supplementary material
The following are the Supplementary material to this article: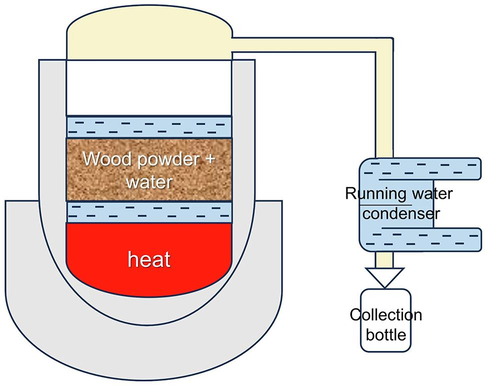
Illustration of hydro distillation used in this study.
Supplementary Fig. S2
Supplementary Fig. S2
Chromatogram of the analysis by gas chromatography for agarwood essential oil. (A) control, (B) 80 °C for 5 min, (C) 80 °C for 10 min, (D) 80 °C for 20 min, (E) 120 °C for 5 min, (F) 120 °C for 10 min, (G) 120 °C for 20 min, (H) 180 °C for 5 min, (I) 180 °C for 10 min, (J) 180 °C for 20 min.







