Translate this page into:
Evaluation of possible protective role of glabridin against gentamicin-instigated nephrotoxicity via attenuation of oxidative stress
⁎Corresponding author. umar.ijaz@uaf.edu.pk (Muhammad Umar Ijaz)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Glabridin (GB) is a natural dietary flavonoid of plant origin with excellent antioxidant properties. Gentamicin (GM) is an aminoglycoside antibiotic that can potentially cause renal toxicity. This investigation was intended to elucidate the attenuative effects of GB against GM-instigated nephrotoxicity. The entire experiment was carried out for a week on adult male albino rats (n = 24), which were randomly separated into 4 comparable groups that included Group I (0.9% physiological saline), group II (80 mg/kg of GM i.p.), group III (80 mg/kg; 50 mg/kg of GM + GB respectively), and group IV (50 mg/kg of GB orally). Biochemical analysis of antioxidant enzymes i.e., superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione reductase (GSR), Glutathione-S-transferase (GST), glutathione (GSH), reactive oxygen species (ROS), hydrogen peroxide (H2O2), thiobarbituric acid reactive substance (TBARS) level, renal serum neutrophil gelatinase-associated lipocalin (NGAL) as well as kidney injury molecule-1 (KIM-1) along with urinary profile (Albumin, creatinine, urea, urinary protein, urobilinogen, and creatinine clearance), inflammatory markers (nuclear factor (NF)-κB, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, interleukin (IL)-6 as well as cyclo-oxygenase (COX)-2), apoptotic markers (Bax, Caspase-3 as well as Caspase-9), anti-apoptotic markers (Bcl-2) as well as histopathology of renal tissues, were analyzed. GM significantly reduced antioxidant enzymes activity, on the other hand, GM treatment substantially elevated the concentration of ROS, H2O2, TBARS, as well as induced abnormality in renal serum markers and urine profile, inflammatory markers, pro-apoptotic markers whereas, declined the level of anti-apoptotic markers and induced substantial histopathological damages. However, GB treatment potentially alleviated damages caused by GM. Conclusively, the intriguing outcomes of the investigation propose that GB effectively protects against GM-prompted nephrotoxicity, which may indicate its anti-oxidant, anti-apoptotic as well as anti-inflammatory potential.
Keywords
Glabridin
Reactive oxygen species
Nephrotoxicity
Gentamicin
Antioxidant
1 Introduction
Gentamicin (GM) is an aminoglycoside antibiotic that is very useful to cure lethal gram-negative bacterial infections resistant to other antibiotics (Pillans et al., 2012). Although GM is a powerful antibacterial agent, its use is however restricted due to its tendency to cause nephrotoxicity as its side effect (Randjelovic et al., 2017). The maximum prescribed GM remedial dose is 160 mg to cure the urinary tract disorders (Labovitz et al., 1974). 30% of the cases administered with GM for more than a week present the signs of nephrotoxicity (Balakumar et al., 2010). The proposed pathological mechanisms include the generation of oxidative stress (OS), apoptosis as well as necrosis (Balakumar et al., 2010). GM-induced nephrotoxicity is a complicated state marked by an increment in serum creatinine as well as urea level along with tubular necrosis (Polat et al., 2006). Pathologically, the preferential site of GM-induced nephrotoxicity is direct tubular damage due to its excessive accumulation in the renal cortex (Wiland and Szechcinski, 2003). It has been reported that GM enters the tubular cells via endocytosis instated by cubilin/megalin complex (Sassen et al., 2006).
GM binds to membrane phospholipids, changes its function and lead to a condition known as phospholipidosis (Quiros et al., 2011). When the concentration of GM in endosomes attains a certain level, their membranes get disrupt and their content including GM is discharged into the cytoplasm. Inside the cytoplasm, GM acts on mitochondria directly as well as indirectly, breaks the respiratory chain, stimulates apoptosis pathway (intrinsic), lessens the adenosine triphosphate (ATP) formation as well as triggers OS by producing superoxide anion (O2−) along with hydroxyl radical (OH) that ultimately prompts cell death (Morales et al., 2010). Furthermore, it was stated earlier that GM directly enhances ROS generation in mitochondria (Morales et al., 2010). This ROS as feedback decreases the activity of enzymatic antioxidants as well as increases lipid peroxidation (LP) (Banday et al., 2008).
Glabridin (GB), a polyphenolic flavonoid extracted from the roots of a traditional Chinese medicinal plant, licorice (Glycyrrhiza glabra) with diverse pharmacological properties i.e., antioxidant (Li et al., 2021), anti-inflammatory (Parlar et al., 2020), anti-carcinogenic (Li et al., 2021), and antibacterial (Li et al., 2021). Moreover, GB showed neuroprotection, anti-osteoporosis, and phytoestrogen effects (Li et al., 2021). Despite the potential curative effects of GB, its remedies against GM-intoxicated renal damages were not elucidated yet. Keeping this critical aspect under consideration, the present investigation was intended to elucidate the remedial effects of GB against GM-provoked renal damage.
2 Materials and methods
2.1 Chemicals
GM was purchased from Gibco, Thermo Fisher Scientific, USA, while GB was from Sigma-Aldrich, Germany.
2.2 Animals
The whole trial was accomplished on twenty-four male rats (albino) with 170–200 g weight. They were housed in steel cages at 22–25 °C with photoperiod of 12-hour day/12-hour night in the animal research station of University of Agriculture, Faisalabad (UAF). Water (H2O) along with feed (Oxbow essential adult rat feed) were given ad libitum. Animals were handled in compliance with the institutional ethical committee approved number (18661-64/19-05-2022), by UAF, Pakistan.
2.3 Experimental design
Rats (n = 24) were separated into 4 equal groups (n = 6). The animals were administrated with subsequent doses: Group I or Control group; 0.9% physiological saline. Group II (80 mg/kg of GM); Group III (80 mg/kg of GM + 50 mg/kg of GB orally) and Group IV (50 mg/kg of GB through oral gavage). The whole trial was performed for 7 days. After a week, urine was collected using metabolic cages and stored at −70 °C for additional examination. Rats were anesthetized, decapitated, blood was composed in sterile tubes and renal tissues were separated. One kidney was put in liquefied nitrogen (N) for further biochemical analysis. For the assessment of histopathology, the 2nd kidney was fixed in formalin solution (10%) for further assessment.
2.4 Biochemical profile
CAT and POD activities were estimated by following the methods of Chance and Maehly (1955). SOD activity was determined by a procedure defined by Kakkar et al. (1984). GSR activity was ascertained by the methodology of Carlberg and Mannervik (1975). Younis et al.'s (2016) practice was adopted to estimate GST activity. The spectrophotometric experimental process presented by Jollow et al. (1974) was used to determine the GSH content. Hayashi et al.'s (2007) method was executed to assess the ROS concentration. Pick and Keisari's (1981) protocol was used to determine the concentration of H2O2. TBARS indicates the degree of the LP. The level of TBARS was estimated in accordance with the practice specified by Iqbal et al. (1996).
2.5 Biochemical analysis of renal serum and urine profile
Albumin, creatinine, urea, urinary protein, urobilinogen, and creatinine clearance were ascertained via approved indicative Medi-Screen kit France. KIM-1 as well as serum NGAL were estimated successively as per the company guidance using KIM-1 and NGAL Quantikine enzyme-linked immunosorbent assay (ELISA) Kits, R and D Systems, Co. Ltd., Changning, China.
2.6 Inflammation
TNF-α (CSB-E07379r), IL-1β (CSB-E08055r), NF-κB (CSB-E13148r) and IL-6 (CSB-E04640r) levels as well as the activity of COX-2 (CSB-E13399r) were measured using ELISA kit according to the company’s guidelines (Cusabio Technology Llc, Houston, TX, USA).
2.7 Apoptosis
Bcl-2 (CSB-E08854r), Caspase-3 (CSB-E08857r) as well as Bax (CSB-EL002573RA) along with Caspase-9 (CSB-E08863r) levels were estimated using ELISA kits obtained from Cusabio Technology Llc, Houston, TX, USA.
2.8 Histopathology
Firstly, specimens were fixed in a fixative solution, which was made of 10% anhydrous acetic acid, 20% formaldehyde, and 70% ethanol, while in the next step, specimens were encased in paraffin wax. Thin slices (3–4 μm) of these paraffin-encased tissues were pruned, stained using hematoxylin-eosin (H&E) stain, and eventually observed microscopically (400X).
2.9 Statistical analyses
The results that were displayed in the tables are in the form of Mean ± SEM. After applying ANOVA, the Tukey multiple comparison tests were employed to compare various groups at the significance level of p < 0.05 operating Minitab software.
3 Results
3.1 Effects of GB on the activity of antioxidant enzymes along with levels of ROS, H2O2, and TBARS
GM administration presented a considerable (p < 0.05) decreased in enzymatic antioxidants (CAT, POD, SOD, GST, GSR activities as well as GSH content), while substantially (p < 0.05) escalated TBARS, H2O2 as well as ROS levels in renal tissues when contrasted to Group I (control). Nonetheless, GB treatment alone and co-administration with GM considerably (p < 0.05) augmented antioxidant enzymes activity as well as levels of ROS, H2O2, and TBARS in comparison to Group II (GM-induced group) (Table 1). Dissimilar superscripts are considerably changed from other groups.
Parameters
Groups
Control
GM (80 mg/kg i.p.)
GM (80 mg/kg i.p.) + GB (50 mg/kg orally)
GB (50 mg/kg orally)
CAT (U/mg protein)
7.85 ± 0.15a
4.94 ± 0.11b
7.02 ± 0.10a
7.93 ± 0.20a
SOD (U/mg protein)
6.69 ± 0.09a
3.18 ± 0.10b
5.69 ± 0.15c
6.71 ± 0.12a
POD (U/mg protein)
7.03 ± 0.06a
4.14 ± 0.05b
6.08 ± 0.11c
6.99 ± 0.22a
GSR (nm NADPH oxidized/min/mg tissue)
4.47 ± 0.10a
2.62 ± 0.08b
3.85 ± 0.08c
4.41 ± 0.11a
GST (nM/min/mg protein)
23.73 ± 1.03a
12.95 ± 0.80b
20.71 ± 0.71c
23.07 ± 1.20a
GSH (μM/g tissue)
16.59 ± 0.31a
6.66 ± 0.33b
14.36 ± 0.26c
16.48 ± 0.32a
ROS (U/min)
0.84 ± 0.05a
2.29 ± 0.12b
1.23 ± 0.03c
0.99 ± 0.06a
H2O2 (nM/min/mg protein)
1.45 ± 0.08a
3.94 ± 0.12b
1.95 ± 0.04c
1.52 ± 0.09a
TBARS (nm TBARS/min/mg tissue)
13.8 ± 1.06a
25.99 ± 1.64b
16.5 ± 0.52c
13.8 ± 0.82a
3.2 Effects of GB on renal serum along with urine markers
Table 2 shows a potential increment in the urobilinogen, urea, creatinine, urinary proteins, urinary KIM-1, serum NGAL levels. However, urinary albumin as well as level of creatinine clearance were distinctly (p < 0.05) lowered down by GM intoxication in contrast to Group I. Co-treatment of GB recorded ameliorative function against GM-instigated damages by reviving these levels. In rats administered with GB alone, no adverse impacts were witnessed as the mean values were remarked adjacent to Group I. Dissimilar superscripts are considerably dissimilar from other groups.
Parameters
Groups
Control
GM (80 mg/kg i.p.)
GM (80 mg/kg i.p.) + GB (50 mg/kg orally)
GB (50 mg/kg orally)
Urea (mg/dl)
16.1 ± 1.36a
35.9 ± 0.73b
20.6 ± 0.77c
17.1 ± 1.68a
Creatinine (mg/dl)
1.45 ± 0.10a
4.51 ± 0.15b
2.18 ± 0.14c
1.57 ± 0.13a
Albumin (mg/dl)
7.70 ± 0.41a
4.67 ± 0.09b
6.45 ± 0.18c
7.41 ± 0.36a
Urobilinogen (mg/dl)
2.81 ± 0.17a
7.20 ± 0.23b
4.47 ± 0.08c
2.81 ± 0.16a
Urinary proteins (mg/dl)
6.95 ± 0.29a
31.9 ± 2.03b
14.9 ± 1.06c
7.46 ± 0.38a
Creatinine clearance (ml/min)
1.60 ± 0.07a
0.83 ± 0.05b
1.14 ± 0.04c
1.59 ± 0.10a
Urinary KIM-1 (mg/ml)
0.52 ± 0.16a
3.24 ± 0.21b
1.1 ± 0.13c
0.55 ± 0.09a
NGAL (ng/day)
0.85 ± 0.17a
3.82 ± 0.46b
1.58 ± 0.22c
0.84 ± 0.17a
3.3 Effect of GB on inflammatory indices
Table 3 depicts a substantial (p < 0.05) upsurge in COX-2 activity along with TNF-α, IL-1β, NF-κB as well as IL-6 levels upon exposure to GM when contrasted with group I. However, co-administration of GB with GM substantially (p < 0.05) alleviated the unusual rise in inflammatory markers levels compared to GM-intoxicated rats. There was no notable difference among the data values of GB alone treated and the group I rats. Dissimilar superscripts are considerably changed from other groups.
Parameters
Groups
Control
GM (80 mg/kg i.p.)
GM (80 mg/kg i.p.) + GB (50 mg/kg orally)
GB (50 mg/kg orally)
NF-κB (ng/g tissue)
13.95 ± 0.71a
66.46 ± 1.72b
21.22 ± 0.98a
13.60 ± 0.85a
TNF-α (ng/g tissue)
7.4 ± 0.58a
18.38 ± 0.84b
8.96 ± 0.82a
7.37 ± 0.42a
IL-1β (ng/g tissue
25.84 ± 1.13a
88.62 ± 2.00b
31.33 ± 2.33a
25.79 ± 0.88a
IL-6 (ng/g tissue)
5.83 ± 0.58a
27.51 ± 1.24b
7.20 ± 0.74a
5.72 ± 0.51a
COX-2 (ng/g tissue)
20.91 ± 1.02a
69.73 ± 1.42b
30.29 ± 2.36a
20.88 ± 0.94a
3.4 Effects of GB on anti-apoptotic and pro-apoptotic markers
GM generated damages in renal tissues include an escalated level of Caspase-3, Bax along with Caspase-9. However, Bcl-2 level was considerably (p < 0.05) decreased as contrasted to Group I. Co-administration of GB lowered the adverse effects of GM and returned Bcl-2, Caspase-3 as well as Bax and Caspase-9 close to Group I effectively as compared with the GM-induced group. GB alone treated group showed normal levels of pro-or-anti-apoptotic markers, remained near to group I (Table 4). Dissimilar superscripts are considerably changed from other groups.
Parameters
Groups
Control
GM (80 mg/kg i.p.)
GM (80 mg/kg i.p.) + GB (50 mg/kg orally)
GB (50 mg/kg orally)
Bcl-2
16.76 ± 0.75a
4.7 ± 0.48b
14.15 ± 0.89a
16.84 ± 0.76a
Bax
2.55 ± 0.11a
8.09 ± 0.40b
2.73 ± 0.18a
2.48 ± 0.12a
Caspase-3
1.51 ± 0.16a
8.47 ± 0.44b
2.62 ± 0.40a
1.46 ± 0.14a
Caspase-9
3.15 ± 0.35a
12.95 ± 0.79b
3.99 ± 0.71a
3.12 ± 0.33a
3.5 Effects of GB on renal histopathology
Analytic renal fragments from the group I and GB-treated rats exhibited typical tubules and glomerulus with normal histomorphometry (A and D respectively). Histological observation of the renal tissues from GM-intoxicated rats revealed the critical as well as widespread necrosis with dilatation of proximal tubules, direct impairment of tubular structure and tubular cell desquamation, vacuolization as well as intraluminal cast development (B). Histological analysis of the kidneys from GM-treated rats pretreated with GB or GM + GB co-administration presented reduced histopathological renal alterations (C) in Fig. 1.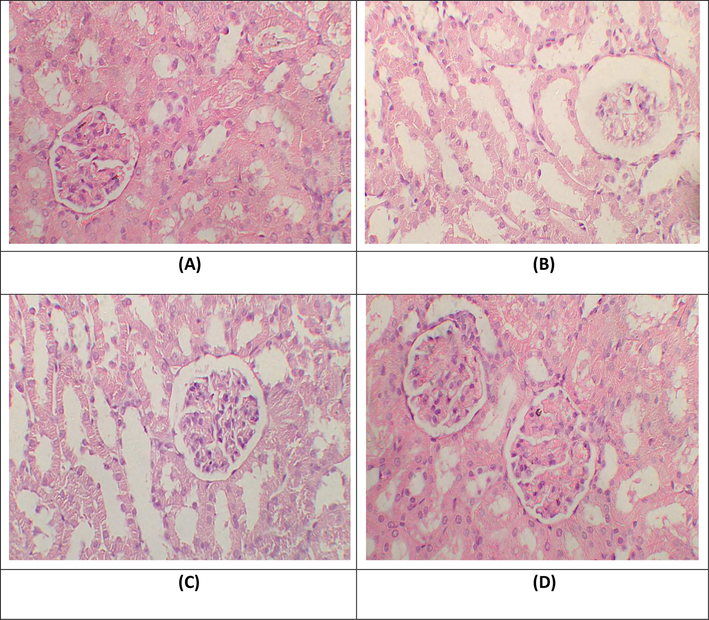
Histopathological observation of renal tissues. H&E stain; 40X (A) Group I; Normal glomeruli and tubules (B) Gentamicin treated group; necrosis in glomeruli, tubular dilation and vacuolization of tubular epithelial cells (C) Gentamicin + Glabridin treated group; normal glomeruli, rare atrophic tubules in cortex (D) Glabridin treated group; Glomeruli and tubules seem normal.
4 Discussion
The current research proposed to estimate the remedial potential of GB against GM-instigated nephrotoxicity. ROS has a critical role in the central biological processes in the human body. Nitric oxide (NO), H2O2, O2−, and OH are dominant reactive nitrogen and oxygen species (Davalli et al., 2016). CAT is a haem-containing enzyme that belongs to the oxidoreductase class of enzymes, defends cells from oxidative damage of OH and H2O2 (Safhi et al., 2016). POD, also referred to as catalases, catalyzes the breakdown of H2O2 into H2O as well as O2 (Padaki et al., 2015). SOD performs an ameliorative potential against free radicals because the dismutation of O2− to form H2O2 is linked with the action of SOD (Stinghen et al., 2014). GSR transforms glutathione disulfide (GSSG) into GSH. GSH keeps cells safe from OS via reducing the concentration of H2O2 along with other peroxides (Deponte, 2013). GST performs different functions, mostly intricate in detoxification processes (Allocati et al., 2018). Hence, a drop in the activity of enzymatic antioxidants raises the levels of ROS. Subsequently, when the body's antioxidant-defense capacity is unable to cope up with the increasing levels of ROS, OS occurs. This OS resultantly disturbs the selective permeability of the plasma membrane and leads to LP that harms basic levels of cellular organization (Ijaz et al., 2021). In this investigation, the outcomes of the biochemical assessment revealed that GM treatment substantially decreased the enzymes of the antioxidant system that included SOD, POD, CAT, GST, GSR as well as GSH content, while ROS, H2O2 and TBARS levels were raised in GM-intoxicated group when matched to control group. However, therapy of GB alleviated the damaging effects of GM by the bringing down the OS in renal tissues. The present research revealed that GB escalated the activities of enzymatic as well as non-enzymatic antioxidant defense. However, it lowered ROS, H2O2 and TBARS levels. The remedial effects of GB on the biochemical profile may be due to its anti-oxidant potency.
The urine profile analysis reveals the functional state of the kidneys. In normal conditions, urobilinogen is not part of urine (Younis et al., 2018), but its appearance in higher quantities in urine might be the result of GM-intoxicated renal toxicity in rats. GM-intoxication substantially elevated the levels of urobilinogen, urea along with creatinine, proteins of urine as well as decreased the level of albumin and creatinine clearance. Increased urinary creatinine, proteins of urine as well as reduced albumin and creatinine clearance are the indicators of intensive renal failure, which is further endorsed by the investigation of Khan et al. (Khan et al., 2010). Furthermore, a profound increment in urinary KIM-1 as well as serum NGAL levels was observed following the exposure to GM in the current investigation. KIM-1 as well as NGAL are considered as markers of acute renal damage (Lei et al., 2018). Typically KIM-1 does not exist in kidneys, but it appears during the earlier phases of renal injuries (Luo et al., 2016). NGAL is a 25 kDa protein present in cytosol, emitted to greater extents into the blood, renal proximal–distal tubule, and finally evacuated in urine followed by kidneys damage (Yim, 2015). However, this ailed urine and renal serum profile were restored to normal followed by GB treatment, which indicates nephroprotective potentials of GB.
GM treatment further showed a profound increase in the COX-2 activity along with the TNF-α, IL-1β, NF-κB as well as IL-6 levels. As evident, one of the pivotal inflammatory mediators is NF-κB, which triggers rapidly after perceiving the cellular stimulus that ultimately boosts TNF-α, IL-1β, COX-2 as well as IL-6 levels, which is related with ROS-linked ailments and other acute inflammatory responses (Kandemir et al., 2018). COX-2 is inductive form of COX that culminates as a fundamental part of inflammation (Gandhi et al., 2017). Elevated COX-2 activity was observed in the kidney of GM administrated rats that implies nephrotoxicity in GM-induced rats. However, in the current study, the supplementation of GB significantly declined the levels of inflammation markers, which authenticates the findings of Parlar et al. (2020).
GM exposure led to elevated Caspase-3, Bax along with Caspase-9 levels, known as pro-apoptotic proteins while lowering anti-apoptotic protein Bcl-2 level. The family of Bcl-2 proteins controls the mitochondrial-dependent pathway (apoptosis) by switching the opening of mitochondrial permeability transition pore (mPTP), the pivotal step for the stimulation of apoptosis (Mustafa et al., 2022). Furthermore, cysteine aspartic proteases, a member of the caspase family, perform an integral part in the instigation and accomplishing of cellular apoptosis and are considered markers for apoptotic cell death (Hassan et al., 2022). Bax plays its role as a pro-apoptotic protein as activated Bax in the outer mitochondria membrane modifies the actual membrane channel protein that directs the over ROS generation, inner mitochondrial membrane permeabilization, the opening of mPTP, excessive mitochondrial membrane permeabilization (MMP), limiting ATP production, and eventually, the liberation of cytochrome c from mitochondria into the cytosol takes place (Yu et al., 2007), besides Bcl-2 functions as an anti-apoptotic protein as it hinder mPTP opening (Maji et al., 2018). Subsequently, the discharged cytochrome c, as a result of Bax activation, triggers caspase-9 that prompts caspase-3 instigation to start cell apoptosis (Chipuk et al., 2009). However, GB co-treatment upregulated the levels of anti-apoptotic protein (Bcl-2). Furthermore, GB potentially downregulated the unusual rise in Caspase-9, Bax as well as Caspase-3 levels. Hence, these findings implicated the anti-apoptotic activity of GB against GM induction probably, due to the regulation of pro-apoptotic markers.
The outcomes of the histopathological analysis presented that GM induced renal impairment which was further verified with the abnormal urine profile. Extensive loss of cellular differentiation, immense inflammatory response, tubular, glomerular and interstitial damages, expanded tubules and tubular necrosis after GM treatment was noted. GM inebriation increases ROS production, evokes LP in renal tissues by reducing antioxidants (Banday et al., 2008), which results in the morphological damage such as marked tubular, glomerular as well as interstitial irregularities in the renal tissues of GM-injected rats (Alarifi et al., 2012). These critical injuries were mitigated by the treatment with GB. Renal tissues from the cotreated group showed decreased necrosis in the renal cells and displayed regular glomeruli size, free tubular lumen, maintained brush border, average medulla along with clear cytoplasm. Histological results exposed that GB administration inhibited the apoptotic actions in the renal tissues by lowering strong infiltration.
5 Conclusion
Conclusively the preliminary investigation proved that GB has an excellent defensive role against ROS, which is an actual mediator of GM-induced nephrotoxicity. GB administration substantially reformed the activity of enzymatic antioxidants and anti-apoptotic marker levels while decreasing the levels of LP, renal serum or urine profile, inflammatory markers, pro-apoptotic markers as well as histological abnormalities. The nephroprotective attribute of GB might be attributed to its anti-oxidant, anti-apoptotic along with anti-inflammatory nature.
Funding
This work was funded by Researchers Supporting Project number (RSP2023R414), King Saud University, Riyadh, Saudi Arabia.
Author contribution
MUI and SM designed the study, conceived the study, and analyzed the results. MUI, AH and SM conceived an initial part of the study, performed the experiment, histology and helped in compiling the results. BOA, MHA and MNR helped in writing the results. MUI, SM, AH, BOA, MHA and MNR made a substantial contribution in the interpretation of data and revising the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSP2023R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mubarak Blood chemical changes and renal histological alterations induced by gentamicin in rats Saudi. J. Biol. Sci.. 2012;19:103-110.
- [Google Scholar]
- Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7:1-15.
- [Google Scholar]
- Gentamicin-induced nephrotoxicity: do we have a promising therapeutic approach to blunt it? Pharmacol. Res.. 2010;62:179-186.
- [Google Scholar]
- Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues Life. Sci.. 2008;82:450-459.
- [Google Scholar]
- PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle. 2009;8:2692-2696.
- [Google Scholar]
- Cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid Med. Cell. Longev.. 2016;2016:3565127.
- [Google Scholar]
- Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. Gen. Sub.. 2013;1830:3217-3266.
- [Google Scholar]
- Role of modulator of inflammation cyclooxygenase-2 in gammaherpesvirus mediated tumorigenesis. Front. Microbiol.. 2017;8:538.
- [Google Scholar]
- Iridoid glycoside Aucubin protects against nonylphenol-induced testicular damage in male rats via modulation of steroidogenic and apoptotic signaling. Sci. Rep.. 2022;12:1-4.
- [Google Scholar]
- High glucose downregulates the number of caveolae in monocytes through oxidative stress from NADPH oxidase: Implications for atherosclerosis. BBA-Mol. Basis. Dis.. 2007;1772:364-372.
- [Google Scholar]
- Orientin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. Pak. Vet. J.. 2021;4:574-578.
- [Google Scholar]
- Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep.. 1996;2:385-391.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase Indian. J. Biochem. Biophy.. 1984;21:130-132.
- [Google Scholar]
- Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed. Pharmacother.. 2018;105:981-991.
- [Google Scholar]
- Prevention of CCl4-induced nephrotoxicity with Sonchus asper in rat. Food Chem. Toxicol.. 2010;48:2469-2476.
- [Google Scholar]
- Single-dose daily gentamicin therapy in urinary tract infection. Antimicrob Agents. Chemother.. 1974;6:465-470.
- [Google Scholar]
- Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep.. 2018;8:1-9.
- [Google Scholar]
- Pharmacological properties of glabridin (a flavonoid extracted from licorice): A comprehensive review. J. Funct. Foods.. 2021;85:104638.
- [Google Scholar]
- Evaluation of KIM-1 and NGAL as early indicators for assessment of gentamycin-induced nephrotoxicity in vivo and in vitro Kidney. Blood Press. Res.. 2016;41:911-918.
- [Google Scholar]
- Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. CancerRes.. 2018;137:37-75.
- [Google Scholar]
- Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria dependent pathway. Kidney. Int.. 2010;77:861-869.
- [Google Scholar]
- Isorhamnetin: a flavonoid, attenuated doxorubicin-induced testicular injury via regulation of steroidogenic enzymes and apoptotic signaling gene expression in male rats. Toxicol. Res.. 2022;11:475-485.
- [Google Scholar]
- Enzyme applications in silk processing. In: Advances in Silk Science and Technology. Woodhead Publishing; 2015. p. :111-120.
- [Google Scholar]
- Glabridin alleviates inflammation and nociception in rodents by activating BKCa channels and reducing NO levels. Biol. Pharm. Bull.. 2020;43:884-897.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-induction by multiple nonphagocytic stimuli. Cell. Immunol.. 1981;59:301-318.
- [Google Scholar]
- Outcomes in patients with gram-negative sepsis treated with gentamicin. Ther. Adv. Drug. Saf.. 2012;3:109-113.
- [Google Scholar]
- Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem.. 2006;108:365-371.
- [Google Scholar]
- An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol. Sci.. 2011;119:245-256.
- [Google Scholar]
- Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J.. 2017;16:388-399.
- [Google Scholar]
- Cadmium-induced nephrotoxicity via oxidative stress in male Wistar rats and capsaicin protects its toxicity. Bull. Environ. Pharmacol. Sci.. 2016;5:5-11.
- [Google Scholar]
- Dysregulation of renal sodium transporters in gentamicin-treated rats. Kidney Int.. 2006;70:1026-1037.
- [Google Scholar]
- Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd. 3) Toxins. 2014;6:1742-1760.
- [Google Scholar]
- Proximal tubule damage in patients treated with gentamicin or amikacin. Pol. J. Pharmacol.. 2003;55:631-637.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin and kidney diseases. Child Kidney Dis.. 2015;19:79-88.
- [Google Scholar]
- Protective effects of Fraxinus xanthoxyloides (wall.) leaves against CCl4 induced hepatic toxicity in rat BMC. Comp. Alter. Med.. 2016;16:407.
- [Google Scholar]
- Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl4-challanged nephrotoxicity in rats. Pak J. Pharm. Sci.. 2018;3:1475-1484.
- [Google Scholar]
- SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189-4198.
- [Google Scholar]







