Translate this page into:
Rhamnetin abrogates polystyrene microplastics prompted hepatic damage by regulating Nrf-2/Keap-1 pathway
⁎Corresponding author. alihamzatabassum7@gmail.com (Ali Hamza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
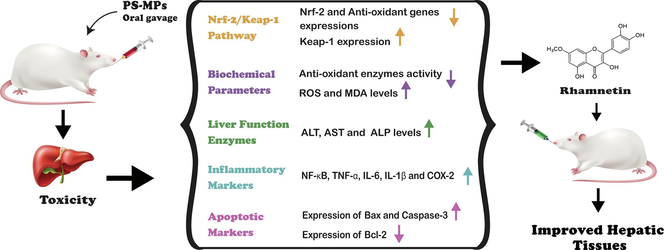
Polystyrene microplastics (PS-MPs) are environmental toxicants that exert adverse effects on organisms. Rhamnetin (RHM) is a natural flavone that shows multiple therapeutic potentials. Therefore, the present study was planned to determine the mitigative effect of RHM against PS-MPs induced liver damage. 48 rats were divided into 4 groups. Control group, PS-MPs (0.01 mg/kg) administered group, PS-MPs (0.01 mg/kg)+ RHM (50 mg/kg) co-administered group and RHM alone (50 mg/kg) administered group. PS-MPs reduced the expressions of Nrf-2 and anti-oxidant gene expressions, while increasing Keap-1 expression. PS-MPs also decreased the activities of catalase (CAT), glutathione reductase (GSR), heme oxygenase-1 (HO-1), glutathione-S-transferase (GST), superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione (GSH), besides elevating the levels of MDA and ROS. Additionally, PS-MPs augmented the levels of alanine transaminase (ALT), alkaline phosphatase (ALP) and aspartate aminotransferase (AST). Furthermore, there was an upsurge in the levels of inflammatory indices in PS-MPs treated group. PS-MPs intoxication also increased Bax and Caspase-3 expressions, while lowering the Bcl-2 expression. Nevertheless, RHM mitigated all the damages due to its hepatoprotective, anti-inflammatory, anti-oxidant and anti-apoptotic potentials.
Keywords
Hepatotoxicity
Rhamnetin
Polystyrene microplastics
Apoptosis
Oxidative stress
1 Introduction
Environmental pollution emerging from plastic is a prevailing issue due to its extensive manufacturing and application in industry and agricultural sector (Hwang et al., 2020). Plastic manufacture has accelerated exponentially reaching to 368 million metric tons in 2019, moreover within 20 years it is expected to be two folds (Al Mamun et al., 2023). Plastics production has surpassed the production of all other synthetic products worldwide. This shows that billions of kilograms of plastics are produced per day, of which only 9 % are recycled, 12 % are incinerated, whereas remaining 79 % are added in the natural environment (Geyer et al., 2017; Almadhi et al., 2023). The remaining plastics (79 %) undergo weathering and breaking down into minute particles that are known as microplastics (MPs) (<5 mm in diameter). Previous literature have described the presence of MPs in facial skin, hair, placenta, saliva, testis and liver. Additionally, MPs pollution contributes to global issues such as ocean acidification, global warming, and ozone depletion (Abbasi and Turner, 2021).
Polystyrene (PS) is one of the most renowned and frequently used type of plastics, which is valued for its persistent physical characteristics and inexpensive price (Cincinelli et al., 2017; Wang et al., 2022). Polystyrene microplastics (PS-MPs) is frequently used to manufacture disposable cups, paper clips, toys cosmetics, pharmaceuticals and trays. PS-MPs exposure to human occur directly through the drinking water, inhalation, skin and ingestion. PS-MPs can enter easily into the organisms owing to low density, smaller size and relatively large surface area; thus, they assemble in organic food chains (Campbell et al., 2017).
Mounting evidences have revealed that PS-MPs intoxication leads to gastrointestinal toxicity, nephrotoxicity, neurotoxicity, testicular toxicity and hepatotoxicity (Ge et al., 2023; Hamza et al., 2023a). PS-MPs treatment provokes ROS generation, which in turn enhances the oxidative and immunological processes. Excessive ROS production leads to DNA damage, impaired protein expression at biochemical as well as cellular level (Yu et al., 2018).
Flavonoids, is a group of polyphenolic compounds that exhibit remarkable pharmacological potentials (Alvi et al., 2022; Surien et al., 2023). Rhamnetin (C16H12O7) is a potent dietary bioflavonoid that is naturally extracted from diverse plant species i.e., halophytes (Halimione portulacoides, Salicornia europaea and Arthrocnemum macrostachyum) vegetables (Thymus nummularius, Ziziphus mistol) and fruits (clove, apple, sour cherries). RHM exhibits excellent therapeutic properties including neuro-protective, anti-tumor, anti-cancer and anti-bacterial (Mondal et al., 2013). Keeping these attributes under consideration, the present study was planned to determine the protective role of RHM against PS-MPs induced hepatic damage in rats.
2 Material and methods
2.1 Chemicals
PS-MPs (CAT No. 9003-53-6) and RHM (CAT 90-19-7) were purchased from Sigma-Aldrich, (Germany). The size of PS-MPs was 100 nm with monodispersed shape and 1±2 mV zeta potential. All the other chemicals used were of ultra-pure molecular biology grade. Formalin, sodium bicarbonate, ethanol, NADH, hydrogen peroxide, EDTA, ferric chloride and TRIzol reagent were purchased from Sigma-Aldrich, Germany.
2.2 Animals
The present research was conducted on 48 rats weighing 200–220 g. Rats were retained in the animal room at temperature (22–25 °C), 55–70 % humidity and 12 h day/night cycle at University of Agriculture, Faisalabad (UAF). All the rats were given free access to diet and water. The rats were treated by following the protocol of the European Union of Animal Care and Experimentation (CEE Council 86/609) that was further approved by the ethical committee of UAF.
2.3 Experimental design
48 rats were divided in 4 groups. Control group, PS-MPs administrated (0.01 mgkg−1), PS-MPs (0.01 mg/kg)+ RHM (50 mg/kg) co-administered and only RHM treated (50 mgkg−1). The doses were selected in compliance with the previous study (Hamza et al., 2023b). Furthermore, the oral toxicity analysis of RHM was performed by using 50, 100, 120, 150 and 200 mgkg−1 doses. Health parameters such as diet, changes in weight, fluid intake, and psychomotor changes were measured. RHM did not show any toxicity at all the tested doses. So lowest dose (50 mgkg−1) was selected. The experiment was performed for 30 days. Rats were anesthetized, beheaded, blood was collected in sterile tubes and centrifuged for 15 min (3000 rpm). Plasma was preserved at −20 °C for lateral observation. The hepatic samples were stored at −80 °C in a zipper bag for biochemical analysis.
2.4 Antioxidant enzymes activity
The technique demonstrated by Aebi (1984) was used to measure CAT activity. SOD activity was examined by following the protocol of Kakkar et al. (1984). GSH level was measured by using the methodology of Sedlak and Lindsay (1968). While, GPx activity was assessed by following the technique of Rotruck et al. (1973). GSR level was determined via the methodology of Carlberg and Mannervik (1985). The activity of GST was estimated by the method of Habig et al. (1974), while the activity of HO-1 was quantified by using the protocol of Magee et al. (1999). Moreover, MDA content was assessed by the protocol of Placer et al. (1966), while ROS content was analyzed by Hayashi et al. (2007) method.
2.5 Real-time polymerase chain reaction (qRT-PCR)
Anti-oxidant genes (CAT, SOD, GPx, GSR, GST and HO-1), Nrf-2/Keap-1 and apoptotic markers expressions were appraised by qRT-PCR. RNA was isolated with the help of TRIzol reagent that was converted to cDNA by employing Fast Quant RT kit. Alterations in the expressions were determined by 2-ΔΔCT, taking β-actin as inner regulator. Table 1 shows the primer sequences of the target genes as reported earlier (Hamza et al., 2023a; Ijaz et al., 2022).
Gene
Primers 5′ −> 3′
Accession number
Nrf-2
F: ACCTTGAACACAGATTTCGGTG
NM_031789.1
R: TGTGTTCAGTGAAATGCCGGA
Keap-1
F: ACCGAACCTTCAGTTACACACT
NM_057152.1
R: ACCACTTTGTGGGCCATGAA
CAT
F: TGCAGATGTGAAGCGCTTCAA
NM_012520.2
R: TGGGAGTTGTACTGGTCCAGAA
SOD
F: AGGAGAAACTGACAGCTGTGTCT
NM_017051.2
R: AAGATAGTAAGCGTGCTCCCAC
GPx
F: TGCTCATTGAGAATGTCGCGTC
NM_030826.4
R: ACCATTCACCTCGCACTTCTCA
GSR
F: ACCAAGTCCCACATCGAAGTC
NM_053906.2
R: ATCACTGGTTATCCCCAGGCT
GST
F: TCGACATGTATGCAGAAGGAGT
NM_031509.2
R: CTAGGTAAACATCAGCCCTGCT
HO-1
F: AGGCTTTAAGCTGGTGATGGC
NM_012580.2
R: ACGCTTTACGTAGTGCTGTGT
Bax
F: GCACTAAAGTGCCCGAGCTG
NM_017059.2
R: CCAGATGGTGAGTGAGGCAG
Bcl-2
F: ACTGAGTACCTGAACCGGCA
NM_016993.1
R: CCCAGGTATGCACCCAGAGT
Caspase-3
F: GTACAGAGCTGGACTGCGGT
NM_012922.2
R: TCAGCATGGCGCAAAGTGAC
β-actin
F: AGGAGATTACTGCCCTGGCT
NM_031144
R: CATTTGCGGTGCACGATGGA
2.6 Assessment of liver serum enzymes
The level of ALP, AST and ALT were determined with the help of standardized ELISA kits purchased from Wiesbaden (Wiesbaden, Germany).
2.7 Inflammatory indices assessment
The levels of inflammatory markers were evaluated by using ELISA kits. The quantifications were accomplished employing ELISA plate reader in compliance with instructor’s recommendations (BioTek, Winooski, USA).
2.8 Statistical analysis
Data were shown as Mean ± SEM. The whole data were interpreted with the help of One-way ANOVA & Tukey's test through Minitab software. The P<0.05 was taken as level of significance.
3 Results
3.1 Protective role of RHM on the expressions of Nrf-2/Keap-1
PS-MPs treatment induced a significant (P<0.05) reduction in the expressions of Nrf-2 and antioxidant genes, on the other hand elevated Keap-1 expression in PS-MPs administered rats in contrast to the control rats. Nevertheless, treatment of RHM increased the Nrf-2 and anti-oxidant genes expression and down-regulated the expression of Keap-1. Additionally, RHM alone administered rats presented these expressions near to the control rats (Figs. 1, 2 presenting the findings).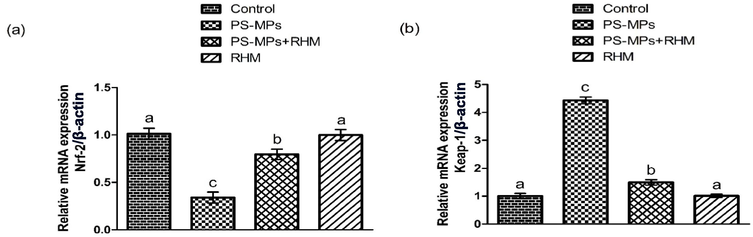
Impact of RHM and PS-MPs on a) Nrf-2 and b) Keap-1 expressions. The data were interpreted with the help of One-way ANOVA and Tukey's test. Different superscripts on the graphs are presenting significant difference.
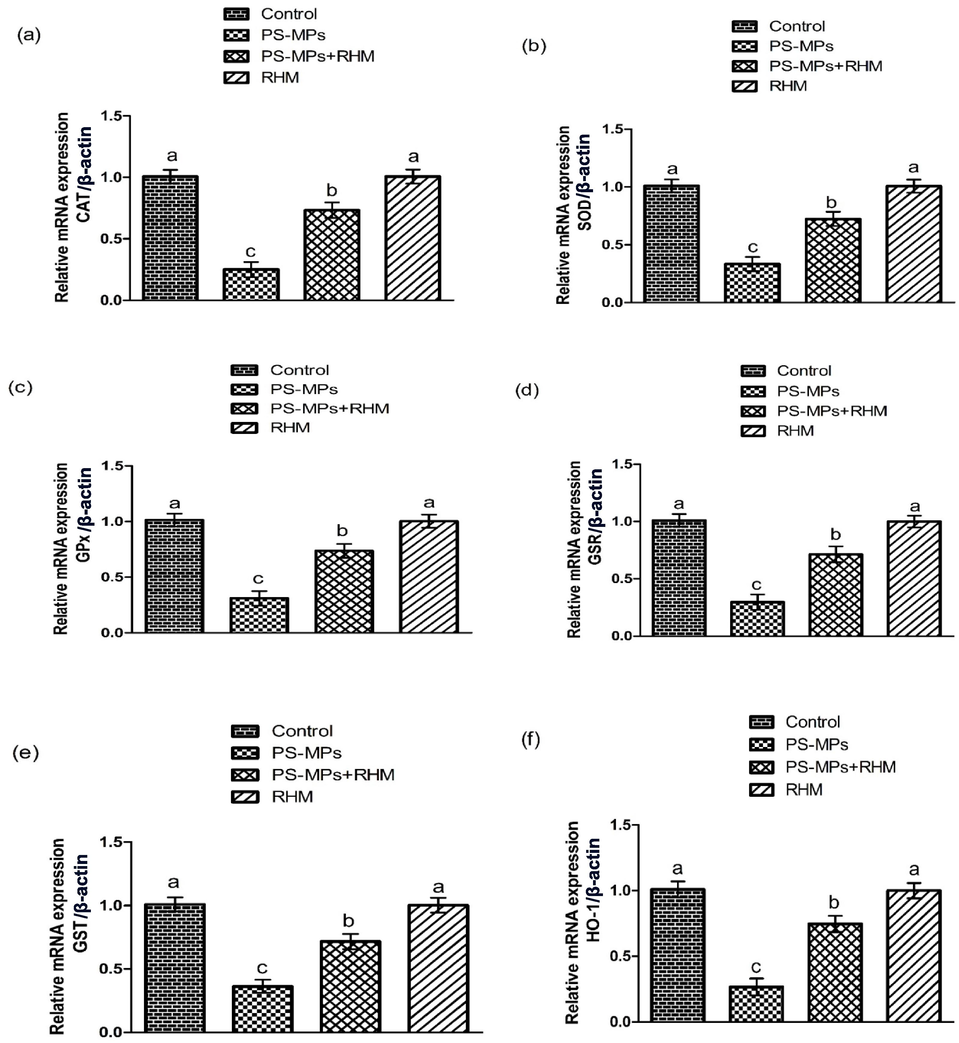
Impact of RHM and PS-MPs on a) CAT, b) SOD, c) GPx, d) GSR, e) GST and f) HO-1 expressions. The data were interpreted with the help of One-way ANOVA and Tukey's test. Different superscripts on the graphs are presenting significant difference.
3.2 Protective role of RHM on biochemical markers
PS-MPs treatment markedly (P<0.05) reduced the activities of GPx, GSR, HO-1, GST, CAT, GSH and SOD, while a remarkable elevation in ROS and MDA levels was observed in PS-MPs administered group as matched with the control group. Moreover, RHM + PS-MPs treatment noticeably increased the activities of anti-oxidants, while MDA and ROS contents were lowered relative to PS-MPs administered group. RHM only administered group exhibited these markers close to the control group (Table 2 presenting the findings). Values with different superscripts are significantly (P<0.05) different. The data were interpreted with the help of One-way ANOVA and Tukey's test.
Parameters
Groups
Control
PS-MPs
PS-MPs + RHM
RHM
CAT (U/mg protein)
9.11±0.21a
4.33±0.16c
8.11±0.14b
9.09±0.21a
SOD (U/mg protein)
8.08±0.16a
2.81±0.08c
6.53±0.12b
8.12±0.17a
GSR (nM NADPH oxidized/min/mg tissue)
6.86±0.13a
1.83±0.15c
5.23±0.12b
6.84±0.13a
GPx (U/mg protein)
25.63±1.08a
7.13±0.35b
21.55±0.77a
25.42±1.16a
GSH (µM/g tissue)
17.44±1.06a
4.52±0.28c
12.10±0.91b
17.94±1.15a
GST (nM/min/mg protein)
33.62±0.98a
12.73±0.59c
28.17±0.91b
34.48±1.37a
HO-1 (pmoles bilirubin/mg protein/h)
287.09±3.88a
58.22±1.56c
162.12±3.65b
285.01±3.74a
MDA (nmol/mg protein)
0.54±0.08a
6.33±0.13c
1.63±0.15b
0.52±0.08a
ROS (Umg−1 tissue)
1.63±0.09a
7.81±0.33c
2.34±0.12b
1.61±0.09a
3.3 Protective role of RHM on liver serum enzymes
PS-MPs significantly (P<0.05) augmented the levels of hepatic serum enzymes (ALP, AST & ALT) in PS-MPs treated rats as compared to the control rats. However, PS-MPs + RHM supplementation substantially lowered these levels in contrast to the rats treated with PS-MPs. RHM alone treated group showed the levels of these markers close to the control group (Table 3 presenting the findings). Values with different superscripts are significantly (P<0.05) different. The data were interpreted with the help of One-way ANOVA and Tukey's test.
Parameters
Groups
Control
PS-MPs
PS-MPs + RHM
RHM
ALT (U/I)
46.09±1.81a
87.93±1.74c
65.02±2.04b
45.29±1.74a
AST (U/I)
90.06±3.02a
188.05±4.03c
125.37±4.43b
89.30±3.21a
ALP (U/I)
125.87±4.84a
343.81±6.55c
187.31±3.75b
124.48±4.68a
3.4 Protective role of RHM on the expressions of apoptotic markers
PS-MPs intoxication markedly (P<0.05) increased Bax and Caspase-3 expressions. Although, Bcl-2 expression was lowered in rats treated with PS-MPs in contrast to the control rats. Nevertheless, RHM+PS-MPs supplementation considerably restored these expressions in contrast to the PS-MPs rats. RHM alone treated rats these expression were same as in the control rats (Fig. 3 presenting the findings).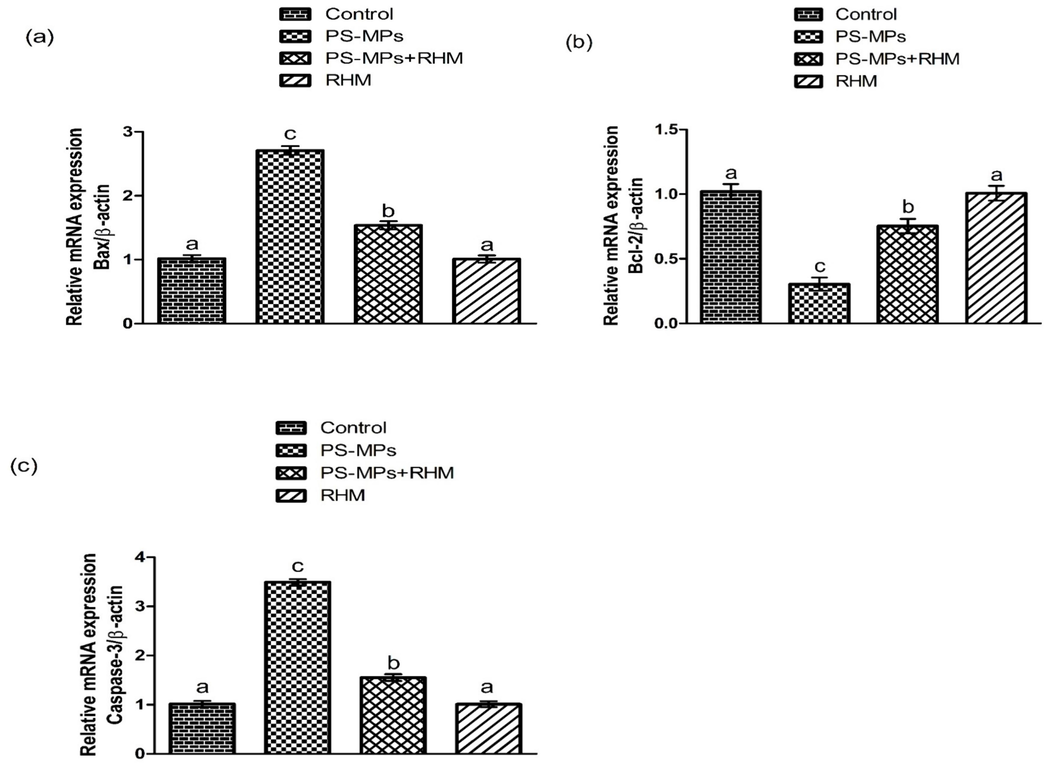
Impact of RHM and PS-MPs on a) Bax, b) Bcl-2 and c) Caspase-3 expressions. The data were interpreted with the help of One-way ANOVA and Tukey's test. Different superscripts on the graphs are presenting significant difference.
3.5 Protective role of RHM on the levels of inflammatory markers
PS-MPs significantly (P<0.05) elevated the levels of inflammatory indices such as NFκB, TNFα-, IL6, IL1β, and COX-2 as matched with the control group. Nevertheless, RHM + PS-MPs treatment considerably lowered inflammatory indices in contrast to the PS-MPs group. Only RHM administered rats presented these markers near to the control rats (Table 4 presenting the findings). Values with different superscripts are significantly (P<0.05) different. The data were interpreted with the help of One-way ANOVA and Tukey's test.
Parameters
Groups
Control
PS-MPs
PS-MPs + RHM
RHM
NF-kB (ng/g tissue)
17.22±0.73a
79.04±1.27c
27.48±1.31b
17.04±0.67a
TNF-α (ng/g tissue)
7.62±0.46a
57.50±1.29c
16.88±0.74b
7.57±0.44a
1L-1β (ng/g tissue)
23.15±0.93a
91.60±1.44c
36.07±0.91b
22.82±0.93a
IL-6 (ng/g tissue)
8.51±0.64a
65.55±1.45c
18.07±0.94b
8.44±0.66a
COX-2 (ng/g tissue)
12.64±1.07a
83.69±1.42c
26.64±1.67b
12.43±0.94a
4 Discussion
The pollution of plastic is one of the most prevalent issues, due to its massive production and detrimental effects on organisms (Zhang et al., 2020). PS-MPs are ubiquitous environmental contaminants that pose adverse effect on human health such as cancer, infertility, respiratory disease, brain damage, heart disease, gastrointestinal blockage, impairment in placental tissues and blood as well as remarkable dysfunctions in intestine, excretory systems and liver (Zolotova et al., 2022). PS-MPs have been reported in beer, salt and honey. According to the study of Hwang et al. (2019) humans are exposed to PS-MPs via skin, ingestion, drinking water and inhalation. PS-MPs exposure leads to OS and inflammatory responses (Kim et al., 2021). Moreover, PS-MPs administration disturb the apoptotic markers, anti-oxidants activities as well as liver serum marker enzymes in rats. RHM is a flavone that is abundantly reported in halophytes plants (Halimione portulacoides, Arthrocnemum macrostachyum), vegetables (Moringa oleifera), Haplopappus multifolius and fruits i.e. grapes, oranges, mangoes (Ahmad et al., 2023). RHM exhibits various protective activities i.e., anti-tumor, anti-arthritic, anti-tumor and anti-oxidant (Jia et al., 2016). Therefore, the present study was planned to assess the mitigative role of RHM against PS-MPs induced hepatotoxicity.
PS-MPs treatment reduced anti-oxidant genes and Nrf-2 expressions, while increasing the expression of Keap-1. Vomund et al. (2017), stated that Nrf-2 performs crucial function in the regulation of OS. While Keap-1 acts as the inhibitor of Nrf-2 and controls its stability. Keap-1 detaches from Nrf-2 during ROS production via some physical modifications, then travels into the nucleus, where it attaches to small MAF proteins (Pintard et al. 2004). Nrf-2 along with MAF proteins attach to the antioxidant responsive elements and induces anti-oxidants genes expressions. Therefore, Nrf-2 has a critical part in monitoring the stimulation of anti-oxidant genes (Hawkes et al. 2014). Nevertheless, during extreme OS Nrf-2 expression is decreased, whereas increasing the Keap-1. Consequently, reduced Nrf-2 lowers the genes expressions of anti-oxidants, as Nrf-2 has a crucial role in regulating the genes expressions anti-oxidants (Yang et al., 2022). Nevertheless, RHM administration elevated anti-oxidant genes and Nrf-2 expressions, while lowering Keap-1 expression. Therefore, it is assumed that RHM has the ability to regulate Nrf-2 and Keap-1 expressions.
PS-MPs administration reduced the anti-oxidants activities such as GPx, GSH, GST, SOD, GSR, HO-1, CAT enzymes, besides increased ROS and MDA level. These anti-oxidants are the first wall of protection that shield DNA, lipids and proteins from oxidative damage (Ighodaro et al., 2018). CAT is referred as primary enzyme, which has important role in the catabolism of hydrogen peroxide (H2O2) (Selamoglu Talas et al., 2008). SOD splits superoxide radicals into oxygen (O2) and H2O2. According to the study of Ulhaq and Tse (2023), GSR maintains reduced GSH levels in cells, via reducing ROS levels. HO-1 is an essential enzyme, which helps in the breakdown of heme and performs a crucial function in homeostasis. MDA is a marker of LP and directly indicates the level of LP and ROS (Samad et al., 2019). The body's anti-oxidant defense mechanism is overwhelmed when excessive level of ROS are created that results in OS. Besides natural anti-oxidants, these anti-oxidants from different plants may be used to treat OS (Nahid et al., 2017). Nevertheless, RHM+PS-MPs supplementation attenuated the PS-MPs damages by escalating anti-oxidants activity and lowering ROS and MDA contents owing to its ROS salvaging property. Moreover, according to previous study flavonoids have the anti-oxidant potential due to the presence of phenolic rings and OH groups in their structural formula (Rodríguez De Luna et al., 2020).
The intoxication of PS-MPs substantially escalated the levels of hepatic function enzymes. Hepatic function enzymes are suitable indicators to evaluate the hepatic function. When the plasma membrane of hepatocytes is disrupted, these enzymes are released from the cytosol and enter into the blood, resulting in hepatic dysfunction. Our outcomes are also evinced by the study of Cheng et al. (2022) who reported that PS-MPs intoxication increased the concentration of liver function enzymes, indicating the adverse condition of liver tissues that consequently leads to liver dysfunction (Kandemir et al., 2020). However, RHM administration dramatically reduced the levels of these enzymes due to hepato-protective and ROS scavenging properties.
PS-MPs-intoxication increased the expressions of Bax and Caspase-3, while decreasing the expression of Bcl-2. Caspase and Bcl-2 family play a major role in apoptosis (Hou et al., 2021). Bcl-2 protects the cells from apoptotic cell death. Contrarily, pro-apoptotic marker (Bax) triggers the cell death (Ehsan et al., 2023). A decrease in in Bcl-2 and escalation in Bax prompts cytochrome-C eviction in cytosol that instigates Caspase-3 (Yen et al., 2012). Caspase-3 break down the proteins and alters their structural makeup, leading to cell death. However, RHM+PS-MPs administration led to substantial reduction in Caspase-3 and Bax expression, besides a notable escalation in Bcl-2 expression was observed. This protective role might be ascribed to the anti-apoptotic property of RHM. Our results are further endorsed by the study conducted by Hamza et al. (2023b), who reported that RHM has to potential to inhibit apoptosis in the testicular tissues of rats.
The intoxication of PS-MPs increased the levels of inflammatory markers including NFκB, TNFα-, IL6, IL1β, and COX-2. The instigation of NF-κB performs a pivotal function in the expressions of other inflammatory indices (TNFα-, IL6, IL1β, and COX-2) that shows severe inflammation. The stimulation of NF-κB boosts inflammatory cytokines (TNFα-, IL6, IL1β, and COX-2) production that results in inflammation. COX-2 is an additional biomarker that also contributes significantly to inflammation (Kim et al., 2019). However, RHM supplementation significantly decreased the levels of inflammatory markers due to its anti-inflammatory nature.
5 Conclusion
Our research revealed that RHM shows potential mitigative role against PS-MPs-instigated hepatic damage. The administration of RHM regulated the anti-oxidants activities, MDA and ROS levels and hepatic function enzymes disturbed by PS-MPs intoxication. Moreover, it also restored apoptotic markers expression as well as inflammatory markers level, owing to its hepato-protective, anti-inflammatory, anti-oxidant and anti-apoptotic activities. Under the light of these findings, it can be deduced that RHM could be used to treat PS-MPs induced liver toxicity.
6 Limitations and future directions
The study was performed by using rats as an animal model and clinical trials are recommend in future to check the relevancy of these findings in human. The testing of rhamnetin with other natural flavonoids or compounds is also recommended in future to check its efficacy. The histopathological examination was not performed in this study.
CRediT authorship contribution statement
Saba Yaqoob: Conceptualization, Investigation, Methodology, Writing – original draft. Ali Hamza: Conceptualization, Investigation, Methodology, Writing – original draft. Moazama Batool: Data curation, Formal analysis, Software, Validation. Aisha Khatoon: Validation, Visualization, Writing – review & editing. Shaik Althaf Hussain: Funding acquisition, Resources, Validation, Writing – review & editing. Mian Nadeem Riaz: Software, Validation, Visualization, Writing – review & editing.
Acknowledgement
The authors would like to acknowledge the funding support by the Researchers Supporting Project number (RSP2024R371), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Human exposure to microplastics: A study in Iran. J. Hazard. Mater.. 2021;403:123799
- [Google Scholar]
- Ameliorative effects of rhamnetin against polystyrene microplastics-induced nephrotoxicity in rats. Pak Vet J. 2023;43(3):623-627.
- [CrossRef] [Google Scholar]
- Microplastics in human food chains: Food becoming a threat to health safety. Sci. Total Environ.. 2023;858:159834
- [Google Scholar]
- Moving from linear to circular economy in saudi arabia: life-cycle assessment on plastic waste management. Sustainability. 2023;15:10450.
- [Google Scholar]
- Nephroprotective Effects of Delphinidin against Bisphenol A Induced Kidney Damage in Rats. Pak. Vet. J.. 2022;43(1):189-193.
- [CrossRef] [Google Scholar]
- Microplastics in the gastrointestinal tracts of fish and the water from an urban prairie creek. Facets. 2017;2:395-409.
- [Google Scholar]
- Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ.. 2022;806:150328
- [Google Scholar]
- Microplastic in the surface waters of the Ross Sea (Antarctica): occurrence, distribution and characterization by FTIR. Chemosphere. 2017;175:391-400.
- [Google Scholar]
- Attenuative effects of ginkgetin against polystyrene microplastics-induced renal toxicity in rats. Pak Vet J. 2023;43(4):819-823.
- [CrossRef] [Google Scholar]
- The hepatotoxicity assessment of micro/nanoplastics: A preliminary study to apply the adverse outcome pathways. Sci. Total Environ.. 2023;902:165659
- [Google Scholar]
- Production, use, and fate of all plastics ever made. Sci. Adv.. 2017;3:1700782.
- [CrossRef] [Google Scholar]
- Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249:7130-7139.
- [Google Scholar]
- Hepatoprotective effects of astragalin against polystyrene microplastics induced hepatic damage in male albino rats by modulating Nrf-2/ Keap-1 pathway. J. Funct. Foods. 2023;108:105771
- [CrossRef] [Google Scholar]
- Rhamnetin alleviates polystyrene microplastics-induced testicular damage by restoring biochemical, steroidogenic, hormonal, apoptotic, inflammatory, spermatogenic and histological profile in male albino rats. Hum. Exp. Toxicol.. 2023;42:0960327123117378
- [Google Scholar]
- Regulation of the human thioredoxin gene promoter and its key substrates: a study of functional and putative regulatory elements. Biophys. Acta Gen. Subj.. 2014;1840:303-314.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet.. 2007;631:55-61.
- [Google Scholar]
- Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater.. 2021;405:124028
- [Google Scholar]
- An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ.. 2019;684:657-669.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54:287-293.
- [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Saudi Pharm. J.. 2022;30(5):519-526.
- [Google Scholar]
- Kakkar, P., Das, B., Viswanathan, P.N., 1984. A modified spectrophotometric assay of superoxide dismutase. 21, 130–132.
- Anti-inflammatory actions of folate-functionalized bioactive ion-releasing nanoparticles imply drug-free nanotherapy of inflamed tissues. Biomaterials. 2019;207:23-38.
- [Google Scholar]
- Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater.. 2021;413:125423
- [Google Scholar]
- In Vitroandin VivoImmunomodulatory Effects of RDP1258, a Novel Synthetic Peptide. J. Am. Soc. Nephrol.. 1999;10:1997-2005.
- [Google Scholar]
- Anti-inflammatory effect of O-methylated flavonol 2-(3,4-dihydroxy-phenyl)-3,5-dihydroxy-7-methoxy-chromen-4-one obtained from Cassia sophera Linn in rats. J. Ethnopharmacol.. 2013;147:525-529.
- [Google Scholar]
- Antioxidant and antimicrobial potentials of Artemisia Indica collected from the Nepal region. J. Pharm. Sci. Res.. 2017;9:1822-1826.
- [Google Scholar]
- Cullin-based ubiquitin ligases: Cul3-BTB complexes join the family. EMBO J.. 2004;23:1681-1687.
- [Google Scholar]
- Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem.. 1966;16:359-364.
- [Google Scholar]
- Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J.. 2020;679–2069
- [CrossRef] [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Protective effect of gallic acid against arsenic-induced anxiety-/depression- like behaviors and memory impairment in male rats. Metab. Brain Dis.. 2019;34:1091-1102.
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- The investigation of the antioxidative properties of the novel synthetic organoselenium compounds in some rat tissues. Exp. Biol. Med.. 2008;233:575-579.
- [Google Scholar]
- Potential chemopreventive role of pterostilbene in its modulation of the apoptosis pathway. Int. J. Mol. Sci.. 2023;24:9707.
- [Google Scholar]
- Perfluorohexanesulfonic acid (PFHxS) induces oxidative stress and causes developmental toxicities in zebrafish embryos. J. Hazard. Mater.. 2023;457:131722
- [Google Scholar]
- Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci.. 2017;18:2772.
- [Google Scholar]
- Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem. Toxicol.. 2022;162:112904
- [Google Scholar]
- Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol.. 2022;283:114739
- [Google Scholar]
- Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch. Toxicol.. 2012;86:923-933.
- [Google Scholar]
- Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol.. 2018;200:28-36.
- [Google Scholar]
- Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean. Environ. Pollut.. 2020;259:113948
- [Google Scholar]
- Harmful effects of the microplastic pollution on animal health: a literature review. PeerJ.. 2022;10:13503.
- [Google Scholar]







