Translate this page into:
Leftover bread as a potential feed additive: Impact on growth, fatty acid content, and antioxidant properties in Tenebrio molitor larvae
⁎Corresponding author. mkhalifa@ksu.edu.sa (Mohamed S. Al-Khalifa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bread waste in Saudi Arabia is a significant environmental issue that has caused significant losses. Insects, specifically Tenebrio molitor larvae, offer potential bioconversion solutions for food waste. Larvae were fed diets comprising different proportions of leftover bread (LB) powder mixed with wheat bran (WB), and feeding trials were conducted over 50 days. Results indicated that including LB powder did not significantly affect larval survival, affirming its safety. The larvae fed the diets LB100, LB75WB25, LB50WB50, LB25WB75, and WB reached the final weights of 27.64, 95.27, 105.012, 98.74, and 67.64 mg. Similarly, the highest mean pupal weight, at 0.133 mg, was observed when larvae were reared on LB75WB25, while the lowest mean pupal weight, at 0.107 mg, was recorded on the WB diet. Protein content varied significantly among diets, with mixed diets exhibiting protein content ranging from 16.98 % to 55.26 %. The primary fatty acid found in mealworm oil was oleic acid (C18:9), which made up 54.11 % of the total fatty acid content for larvae raised on 75LB:25WB, 52.01 % for LB, and 46.942 % for WB, with LB incorporation positively impacting growth, pupal weight, protein content, and antioxidant properties. Utilizing LB powder highlights its potential for sustainable insect farming, addressing environmental concerns, and enhancing resource sustainability.
Keywords
Protein content
Growth performance
Larval survival
Food waste management
Tenebrio molitor
1 Introduction
Bread waste in the Kingdom of Saudi Arabia poses an environmental concern that warrants attention and intervention. The accumulation of bread remnants results from inefficient disposal practices, where they are often discarded in regular trash bins instead of being recycled or utilized efficiently. This issue can adversely affect the environment, contributing to the growing volume of waste in landfills. To address this challenge, raising awareness about the importance of recycling unconsumed bread, incorporating it into organic compost, or converting it into bioenergy (Baig et al., 2019).
Flour-Bread Loss and Waste (FLW) in Saudi Arabia, according to the international standard for FLW, is estimated at 30 %. This comprises a loss ratio of 5 % and a waste ratio of 25 %. These figures indicate that a significant portion of flour and bread products in the country is either lost during the production process or wasted at the consumption stage (Baseline, 2019). Addressing and reducing these losses and waste is essential for economic and environmental sustainability, emphasizing the need for improved consumption and production practices in the food supply chain.
Insects are vital in bioconverting waste into valued resources, including secondary industrial products, animal feed, and human food. This process, known as insect-based bioconversion, is sustainable and efficient for managing food waste (Fowles and Nansen 2020). Insects are noteworthy for their rich content of high-quality proteins, dietary fibres, various micronutrients, and polyunsaturated fatty acids (Rumpold and Schlüter 2013). Beyond their nutritional advantages, insects offer benefits such as a low environmental impact and reduced land dependency compared to traditional livestock production (Dossey et al., 2016; Van Huis 2013).
Meanwhile, the larvae of Tenebrio molitor L. (Coleoptera: Tenebrionidae) are one of the most used insects in bioconversion. They have an efficient feed conversion rate (Oonincx et al., 2015) and established technology (Ortiz et al., 2016). Mealworms have been commercially cultivated in many countries (Ha et al., 2022). Depending on the provided diet, they can quickly adapt their larval nutritional composition, weight, and development time. It stands out as a promising choice for insect rearing due to its well-balanced amino acid profile and high protein content (Rumpold and Schlüter 2013), ability to thrive on organic by-products (Oonincx et al., 2015), low water footprint (Miglietta et al., 2015), reduced land usage (Ortiz et al., 2016), potential health benefits (de Carvalho et al., 2019), and low greenhouse gas emissions (Oonincx and De Boer, 2012).
Shifting from primarily grain-based diets to incorporating vegetable waste as supplements or partial replacements offers a cost-effective alternative, conserving resources earmarked for human consumption and mitigating environmental footprint (López-Gámez et al., 2023).
This study aims to investigate the potential of incorporating LB as a feed additive for T. molitor larvae and its impact on larval growth, fatty acid composition, and antioxidant properties. when mixed with wheat bran (WB), LB powder offers a viable strategy for utilizing bread waste while providing a nutrient-rich diet for insect larvae. Understanding the effects of LB supplementation on larval development and nutritional profile is essential for optimizing insect rearing practices and promoting sustainable protein production.
2 Materials and methods
2.1 Insects
The T. molitor colonies were cultivated in plastic culture trays of 60 × 40 × 14 cm. By removing the top cover of the boxes, air circulation and ventilation within the box are not hindered. The stock colonies were accommodated within insect breeding facility at the Laboratory of Entomology, Department of Zoology at King Saud University, Saudi Arabia, under controlled conditions (23 ± 2 ͦ C, RH 40 ± 5 %, and 12 h light–dark cycle). The larvae of T. molitor were nourished with WB as a meal. Water was included in the diet to offset the loss of moisture from waste. The larvae were isolated from the WB through the process of sifting.
2.2 Feeding trial
The control larvae were provided with WB sourced from a local store in Diriyah, Riyadh, with around 2 mm particle size. The wheat bran for the various dietary treatments incorporated residual bread at four distinct weight ratios (100, 75, 50, and 25 % w/w). The LB was sliced and sundried for two days. The LB was pulverized into a fine powder using an electric grinder (LC, China) and stored at −80 ͦ C until it was needed again. The feeding trials utilized plastic insect breeding trays measuring 7 × 7 × 7 cm as the experimental units. Every tray contained 30 g of feed, which could be WB or bran enriched with leftover bread and 50 larvae. Five tray replicates were created for each dietary treatment. The larvae were placed in an incubator (Sanyo, Japan) at a temperature of 25 ͦ C and a relative humidity of 50 ± 5 %, where they were allowed to feed without interference. The larvae were weighed at intervals of 20, 30, 40, and 50 days using an analytical balance (Equinox EAB125i, England). To determine the average weight of each larva, divide the total weight of all larvae by the number of larvae in each replicate. On the fiftieth day, the larvae were collected for further analysis. Following the harvest, the larvae underwent 24 h of fasting, were then divided into groups, and their weight was measured.
Regarding pupal weight, 10 larvae from each diet were selected and kept for each replicate until they reached the pupal stage. Subsequently, their weights were assessed.
2.3 Mealworm extraction
Upon completion, the harvested larvae were euthanized by freezing them at −80 °C, followed by a 48-hour exposure to an oven set at 50 °C. Subsequently, the dried larvae were powdered and preserved in glass vials at −80 °C. One gram of oven-dried mealworm larvae was combined with 10 mL of solvents with varying polarity (hexane and methanol). The mixture underwent homogenization (IKA, Germany) and ultrasonication (100 %, 10 min) (Wise Clean, Korea), followed by extraction through agitation (250 RPM, 1 h) at 25 ͦ C using a shaker (GFL Orbital Shaker 3005, Germany). The samples were then filtered using a single-layer filter paper and a 0.25 syringe filter (Eppendorf, Germany). The resulting supernatants were evaporated using a rotary evaporator (Heidolph, Germany) to assess extraction efficiency. The extracts were utilized to determine total antioxidant capacity depending on their polarity.
2.4 Assessment of Radical-Scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was assessed following the method outlined by Abutaha et al. (Abutaha et al. 2020).
DPPH• (0.004 g) (Sigma, Germany) solution was dissolved in methanol (100 mL) to create a DPPH• reagent. Following this, 190 µL was mixed with 10 µL of the supernatant. Following a 30-minute incubation at room temperature (25 ͦ C) in the dark, the absorbance was measured at 515 nm using a spectrophotometer (ChroMate, England). The IC50 value, representing the concentration at which the DPPH• radical was inhibited by 50 %, served as an indicator of the scavenging activity. The DPPH• radical scavenging percentage was calculated using the formula:
Scavenging Percentage = (1 − Abscontrol/Abssample) × 100.
Here, Abs control represents the absorbance of the control, and Abs sample is the absorbance of the sample.
2.5 Protein content
One gram of mealworm larvae was sampled from each diet group, frozen at − 80 °C, homogenized for 5 min using a homogenizer (IKA, Germany), and later sonicated for 5 min. The resulting extract underwent centrifugation at 4 °C and 8000g for 10 min to proceed. Protein content was assessed using the Bradford method (Bradford, 1976), wherein 5 µL of the obtained supernatant (protein extract) was combined with 195 µl of Bradford's reagent (Sigma-Aldrich, Germany) and left to incubate at room temperature for 10 min. Absorbance readings were taken at 595 nm using a spectrophotometer. A PBS-based blank was prepared, and a standard curve was constructed using a known concentration of bovine serum albumin (Sigma-Aldrich, Germany).
2.6 Fatty acid analysis
The sample was injected via an autosampler injection system of the GC–MS-QP2010 ultra with the GC-2010 Plus system from Shimadzu. The chemical structures of the FAMEs were identified using the database-integrated software. For the separation of target compounds, the capillary column from the Agilent J&W DB-5 ms GC A column of 30 m, 0.25 mm, and 0.25 µm was used with helium as the carrier gas at a flow rate of 1 mL/min, an inlet temperature of 250 °C with a split mode ratio of 50, and an oven temperature ranging from 50 to 250 °C with a total analysis time of 77 min. The MS detector was set as follows: acquisition scan type, mass ranging from 40 to 500 g/mol, scan speed of 1.56, 9 min solvent delay, and 230 °C MS source temperature.
2.7 Statistical analysis
All statistical analyses were performed with the SPSS software package. The collected data were subjected to a one-way analysis of variance (ANOVA). The Tukey’s (HSD) multiple tests determined significant differences between means at P≤0.05.
3 Results
3.1 Survival and growth of t. Molitor
When evaluating a potential feed for insect larvae, the first aspect to consider is its effect on survival. This study examined the impact of different proportions of LB powder mixed with WB on larval survival. WB was chosen as the control feed due to its widespread use, which supports growth, high larval survival and weight gain. Larval survival was monitored every ten days over fifty days until pupation. Table 1 provides detailed survival rates for each assessment interval. Notably, no significant differences (P≤0.05) were observed among the control samples throughout the 50-day period, regardless of the LB powder supplementation variations. Thus, it can be concluded that LB powder is safe for T. molitor larvae. *There are no statistically significant differences (P < 0.05). aLeftover bread, bwheat bran.
Diets
Survival (%)*
20 days
40 days
50 days
60 days
LBa 100
98.8 ± 0.8
97.6 ± 0.75
93.2 ± 0.80
88.8 ± 1.36
LB 75 and WBb 25
98.8 ± 0.49
99.6 ± 0.40
96.8 ± 1.02
94.8 ± 1.02
LB 50 and WB 50
98.4 ± 0.0.98
96.8 ± 1.62
92.8 ± 2.94
89.6 ± 2.23
LB 25 and WB 75
96.4 ± 2.23
93.6 ± 2.71
93.2 ± 2.94
90.8 ± 2.42
WB 100
98.8 ± 0.49
98 ± 0.63
96.8 ± 0.80
91.6 ± 0.98
dr
4
4
4
4
F
0.77
2.21
1.38
1.84
Another critical aspect is to evaluate how LB powder on the development of T. molitor larvae. Fig. 1 illustrates the weights of the larvae for each food treatment. According to the findings, larvae fed with varying ratios of LB powder in the tested diets exhibited significantly larger sizes than the control (P≤0.05). The weight of larvae fed the five different diets is reported in Fig. 1.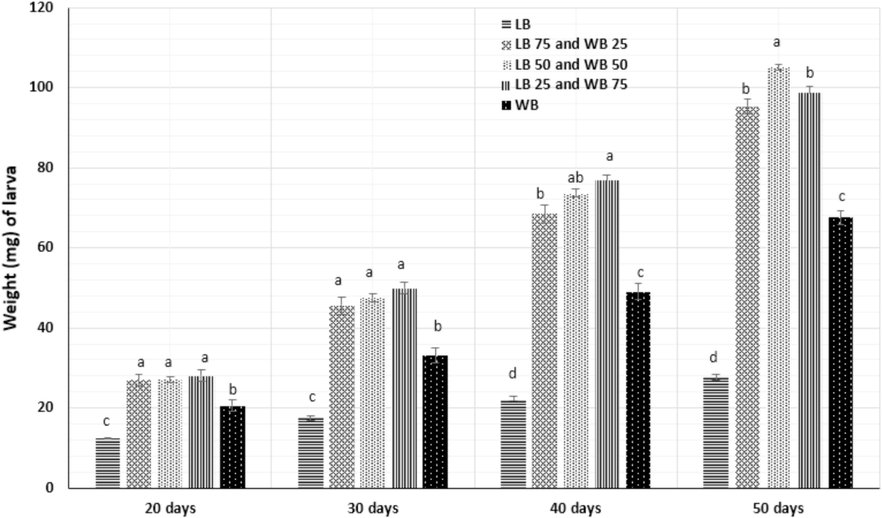
Individual weight (mg) of Tenebrio molitor larvae fed for sixty days with wheat bran (control) (LB 0 WB 100) and bran fortified with different rates of Leftover bread waste (LB 100 WB 0), LB 50 WB 50 and LB 25 WB 75 (n = 5). Statistically significant differences (P<0.05) are denoted with different letters. WB=wheat bran, LB=Leftover bread.
Minor variations in growth performance were noted with the introduction of a single diet, with discernible differences observed exclusively among larvae fed LB. As a result of the slower growth observed in the LB group, the trial concluded after 60 days, coinciding with the emergence of pupae in all other diet groups. The larvae fed the diets LB100, LB75WB25, LB50WB50, LB25WB75, and WB reached the final weights of 27.64, 95.27, 105.012, 98.74, and 67.64 mg.
The mean pupal weights of larvae reared on different diets are detailed in Fig. 2. The highest mean pupal weight, at 0.133 mg, was observed when larvae were reared on LB75WB25, while the lowest mean pupal weight, at 0.107 mg, was recorded on the WB diet. The rearing diet significantly influenced pupal weights, indicating a notable impact of diet composition on the developmental outcomes of the larvae.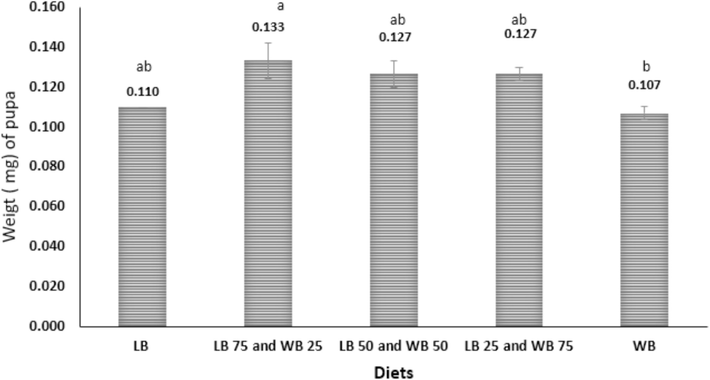
Pupal final weight of T. molitor fed on different ratios of WB (wheat bran) and LB (leftover bread) diets under laboratory conditions. Statistically significant differences (F=4.69P≤0.05) are denoted with different letters.
3.2 Protein content
In this study, larvae subjected to varying diets were analyzed for their crude protein content. Notably, the diet induced considerable variability (P≤0.05) in the larval protein concentrations.
Larvae fed different mixes exhibited protein content ranges ranging from 16.98 % to 55.26 %. A closer examination reveals more noticeable variations in individual diets, with crude protein increasing by 55 % between WB and LB. Larvae fed mixed diets exhibited elevated protein levels compared to those fed exclusively LB. The results indicate increased protein content with a decreased percentage of LB, as shown in Fig. 3. Even though the difference may not be statistically significant, adding 25 % of LB to 75 % of WB still resulted in slightly higher protein values than the control.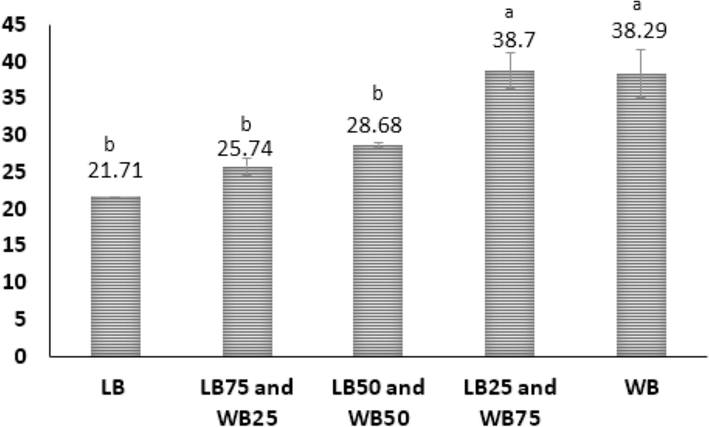
Protein Content of T. molitor fed on different ratios of WB (wheat bran) and LB (leftover bread) diets under laboratory conditions. Statistically significant differences (F=16P≤0.05) are denoted with different superscript letters.
3.3 Antioxidant properties
The analysis of the DPPH• free radical scavenging assay for methanol and hexane extracts was conducted and is provided in Table 2. The IC50% of the ranged from 22.55. to 38.98 µg/mL for the feed tested. The substrate with lower IC50% values had stronger antioxidant properties. Therefore, LB 75 WB 25 had the best antioxidant properties among the feeds tested. It reached 22.55 ± 1.43 µg/mL, which was comparatively higher than the other four tested feeds but had no substantial antioxidant property. *Statistically significant differences (P<0.05) are denoted with different letters.
Diets
Concentration
50
25
12.5
IC50*
LB 100 and WB 0
72.58 ± 1.72
53.93 ± 1.29
39.04 ± 0.23
22.11 ± 1.45b
LB 75 and WB 25
57.49 ± 2.37
55.34 ± 1.11
33.08 ± 2.88
19.71 ± 1.43b
LB 50 and WB 50
73.48 ± 0.76
50.78 ± 1.34
38.60 ± 0.15
24.25 ± 0.86c
LB 25 and WB 75
53.87 ± 0.95
48.95 ± 0.09
32.91 ± 1.41
31.21 ± 1.52 a
LB 0 and WB 100
71.43 ± 3.35
51.41 ± 2.79
46.54 ± 0.55
21.46 ± 4.56b
df
4
4
4
4
F
6.20
2.60
41.01
10.86
3.4 Fatty acid composition
The GC–MS was utilized to analyze the mealworm fatty acid composition of. Peak interpretation was based on profile matching in the library database. The primary fatty acid found was oleic acid (C18:9), which made up 54.11 % of the total fatty acid content for larvae raised on 75LB:25WB, 52.01 % for LB, and 46.942 % for WB. Following this, linoleic acid (C18:9, 12) was observed, with 24.888 %, 19.47 %, and 17.98 % for larvae raised on LB, 75LB:25WB, and WB, respectively. Palmitic acid (C16:0) ranked third, with 16.70 %, 16.039 %, and 12.96 % for larvae raised on LB, WB, and 75LB:25WB, respectively. Other fatty acids, including stearic acid (C18:0), myristic acid (C14:0), and palmitoleic acid (C16:7 and C16:9), exhibited varied proportions, with the highest amounts of stearic acid found in 75LB:25WB and the highest amounts of myristic acid and palmitoleic acid found in 75LB:25WB and WB, respectively (Table 3). aLeftover bread, bwheat bran.
Peak
Carbon chain
Common name
Area %
LB
75LBa: 25WB
WBb
1
(C10:0)
Capric
0.01
0.01
−
2
(C12:0)
Lauric
0.15
0.22
0.289
3
(C13:0)
Tridecylic
0.02
0.02
0.055
4
(C14:0)
Myristic acid
3.82
4.06
3.941
5
(C15-11)
Pentadecenoic
0.02
0.01
−
6
(C17:7)
Margaric acid
0.33
0.35
0.330
7
(C15:0)
Pentadecanoic acid
0.05
0.05
0.091
8
(C16:0)
Palmitic acid
16.72
12.97
16.105
9
(C16:7)
Palmitoleic acid
1.02
1.22
1.533
10
(C16:9)
Palmitoleic acid
2.61
2.37
2.463
11
(C16:7,10)
Hexadecadienoic acid
0.07
0.11
0.297
12
(C18:0)
Stearic acid
4.00
4.60
2.244
13
(C18:9)
Oleic acid
52.01
54.11
46.942
14
(C18:10,13)
Linoleic acid
0.14
0.08
0.023
15
(C18: 9,12)
Linoleic acid
17.98
19.47
24.888
16
(C20:9-Antiso)
Eicosenoic acid
0.37
−
−
17
(C18: 9,12,15)
Linolenic acid
0.10
0.22
−
18
(C20:11)
Eicosenoic acid
0.23
0.04
0.079
19
(C20: 11, 14)
Eicosadienoic Acid
0.04
0.08
−
20
(C20: 8,11,14)
Dihomo-γ-linolenic acid
0.02
−
−
Saturated fatty acid
24.79
21.95
22.725
Monounsaturated fatty acid
56.22
58.1
51.347
Polyunsaturated fatty acid
75.03
78.06
76.555
4 Discussion
The diet composition significantly impacts the growth and performance of insects, playing a pivotal role in their large-scale production. Our study found that the larval weight of T. molitor is greatly affected by the diet they consume, consistent with previous research (Amin and Usman, 2022; Zhang et al., 2019). Similar studies were conducted by rearing mealworms on distiller grain, soybean meal, corn stover, and WB diets. Their findings showed significant variations in larval weight among the different diets. The highest weight gain was observed in larvae fed on WB, while those fed on corn stover had the lowest weight gain. Our results align with this trend, as we observed the highest weight gain in larvae fed on a diet containing 50 % 4 and 50 % WB, while the lowest weight gain was recorded in larvae fed exclusively on 100 % LB.
Pupal weight showed significant dependence on larvae, especially those fed on LB and WB-containing diets in this experiment compared to WB alone. Our findings contrast with previous reports indicating that T. molitor pupae raised solely on WB exhibited significantly greater pupal weight compared to those on mixed diets or combined with various other feed compositions (Amin and Usman, 2022; Kim et al., 2019; Kim et al., 2017).
The diet's quality significantly influences the protein content of mealworm larvae. Integrating LB into mixed diets led to elevated carbohydrate levels, consequently promoting lipid accumulation in larval bodies. In contrast, the use of bread alone might have resulted in protein deficiency, restricting energy reserves. Notably, increasing bread amounts adversely influenced the diets' protein levels.
Larvae protein content followed this trend, decreasing in LB75WB25, LB50WB50, and B100 compared with WB100. However, the mixed diet with 75 % WB and 25 % LB exhibited a higher protein content than WB100, though not significantly. Similar results have been reported by Mancini et al., using spent grains instead of WB (Mancini et al., 2022).
Extractions were performed using hexane and methanol solvents to assess the lipophilic and hydrophilic antioxidant activity in T. molitor larvae. The total antioxidant capacities of the extracts were determined through DPPH assays (Table 2). The results of the antioxidant test indicated only hydrophilic antioxidants in mealworm larvae extracts. There was no significant difference in scavenging activity between LB 100 (21.9 %) and WB 100 (22.2 %). However, the scavenging activity increased with the reduced percentage of LB mixed with the WB. There was less scavenging activity in the methanol extract than in previous studies. This might have been because of variations in how the extract was made and how the larvae were dried, both of which affect scavenging activity variations (Keil et al., 2022).
In addition to serving as a sustainable protein source, insects also contribute a significant amount of fat to the diet, and this fat is typically rich in polyunsaturated fatty acids (PUFAs) (Rumpold and Schlüter, 2015). The lipid fractions of these biomasses exhibited notable levels of palmitic, linoleic, and oleic acids, consistent with prior research results (Dreassi et al., 2017). The predominant saturated fatty acids (SFAs) observed were stearic acid, myristic acid, and palmitic acid aligning with previous reports (Oonincx et al., 2015; Rumpold et al., 2014). The LB-based diet had a modest effect on the profile of fatty acid, elevating only the levels of oleic acids and monounsaturated fatty acids (MUFA) compared to the control diet. In contrast, the 75LB/25WB diet showed increased concentrations of oleic acids and elevated levels of total fatty acids, MUFA, and polyunsaturated fatty acids (PUFA) compared to the control treatment.
Conversely, the ratio of SFA to UFAconsistently remained below 0.5. These values align with the range documented in prior studies concerning T. molitor reared on wheat, bread, and oat flour (Lawal et al., 2021; Dreassi et al., 2017). They suggest that the fat content in all T. molitor biomasses maintains a balance of SA and USA suitable for human consumption.
Nonetheless, our findings suggest that the FA composition of T. molitor is responsive to variations in diet composition. This aligns with prior research conducted with diverse diets, such as those incorporating wheat flours, flax seeds, chia seeds, vegetables, or oats (Lawal et al., 2021; Dreassi et al., 2017; Ravzanaadii et al., 2012). The study recommends further investigation into elucidating the amino acid profiles of the dietary components. Additionally, it suggests exploring various methods to assess antioxidant activity comprehensively.
5 Conclusion
The study found that incorporating LB powder into the diets of T. molitor larvae is safe, and can improve growth, nutritional composition, antioxidant properties, and fatty acid profiles. Adding LB powder led to larger larval sizes and higher protein levels. The study also revealed varying fatty acid composition in mealworm oil, with different treatments influencing fatty acid profiles.
CRediT authorship contribution statement
Fahd A. Al-Mekhlafi: Writing – original draft, Methodology, Data curation, Conceptualization. Nael Abutaha: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Mohamed A. Wadaan: Writing – review & editing, Supervision, Funding acquisition. Mohamed S. Al-Khalifa: Writing – review & editing, Supervision, Funding acquisition.
Acknowledgements
The authors express their sincere appreciation to the Researchers Supporting Project number (RSP2024R112), King Saud University, Riyadh, Saudi Arabia.
Funding: This project was funded by Researchers Supporting Project number (RSP2024R112), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5. Open Chem.. 2020;18(1):472-481.
- [Google Scholar]
- Effects of different diets on life parameters and nutritional profile of mealworms (Tenebrio molitor l) under laboratory conditions. J. xi’an Shiyou Univ., Nat. Sci. Ed.. 2022;18(12):1212-1231.
- [Google Scholar]
- Food waste posing a serious threat to sustainability in the Kingdom of Saudi Arabia–A systematic review. Saudi J. Biol. Sci.. 2019;26(7):1743-1752.
- [Google Scholar]
- Food Loss & Waste Index in Kingdom of Saudi Arabia. Saudi Grains Organization: Riyadh, Saudi Arabia; 2019.
- Potential prebiotic activity of Tenebrio molitor insect flour using an optimized in vitro gut microbiota model. Food Funct.. 2019;10(7):3909-3922.
- [Google Scholar]
- Insects as sustainable food ingredients: production, processing and food applications. Academic Press; 2016.
- Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae) Lipids. 2017;52(3):285-294.
- [Google Scholar]
- Insect-based bioconversion: value from food waste. Food Waste Management: Solving the Wicked Problem 2020:321-346.
- [Google Scholar]
- Changes in Functionality of Tenebrio molitor Larvae Fermented by Cordyceps militaris Mycelia. Foods. 2022;11(16):2477.
- [Google Scholar]
- Systematic studies on the antioxidant capacity and volatile compound profile of yellow mealworm larvae (T. molitor L.) under different drying regimes. Insects. 2022;13(2):166.
- [Google Scholar]
- Nutritional analysis of alternative feed ingredients and their effects on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae) Entomol. Res.. 2017;47(3):194-202.
- [Google Scholar]
- Growth performance of the edible mealworm species, Tenebrio molitor (Coleoptera: Tenebrionidae) on diets composed of brewer's yeast. Int. J. Ind. Entomol.. 2019;39(2)
- [Google Scholar]
- Enrichment in specific fatty acids profile of Tenebrio molitor and Hermetia illucens larvae through feeding. Future Foods. 2021;3:100016
- [Google Scholar]
- López-Gámez, G., del Pino-García, R., Justicia-Rueda, A., Delgado-Vicedo, C., Quiles-Morales, J.L., 2023. Improvement of Tenebrio molitor Larvae Farming and Fatty Acid Composition by Supplementation with Vegetable Waste, Biology and Life Sciences Forum. MDPI, p. 24.
- Growth performances, chemical composition, and microbiological loads of mealworm reared with brewery spent grains and bread leftovers. Ital. J. Anim. Sci.. 2022;21(1):1419-1429.
- [Google Scholar]
- Environmental impact of the production of mealworms as a protein source for humans–a life cycle assessment. PLoS One. 2012;7(12):e51145.
- [Google Scholar]
- Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS One. 2015;10(12):e0144601.
- [Google Scholar]
- Insect mass production technologies. Insects as Sustainable Food Ingredients. Elsevier 2016:153-201.
- [Google Scholar]
- Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol.. 2012;25(1):93-98.
- [Google Scholar]
- Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res.. 2013;57(5):802-823.
- [Google Scholar]
- Comparison of volumetric and surface decontamination techniques for innovative processing of mealworm larvae (Tenebrio molitor) Innov. Food Sci. Emerg. Technol.. 2014;26:232-241.
- [Google Scholar]
- Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front.. 2015;5(2):20-24.
- [Google Scholar]
- Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol.. 2013;58:563-583.
- [Google Scholar]
- Van Huis, A., Van Itterbeeck, J., Klunder, H., Mertens, E., Halloran, A., Muir, G., Vantomme, P., 2013. Edible insects: future prospects for food and feed security. Food and agriculture organization of the United Nations.
- Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur. Food Res. Technol.. 2019;245:2631-2640.
- [Google Scholar]







