Translate this page into:
A pH-stable alkaline pectate lyase produced by the newly identified strain Bacillus altitudinis CAS-WZS-08
⁎Corresponding authors at: Tobacco Research Institute of Chinese Academy of Agricultural Sciences, No. 11, Keyuanjing 4th Road, Laoshan District, Qingdao 266101, China(H. Liu), Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, NO.189, Songling Road, Laoshan District, Shandong Province, 266101, China(H. Zhang). liuhaobao@caas.cn (Haobao Liu), zhanghb@qibebt.ac.cn (Haibo Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Thermal and pH stabilities are extremely important for the application of pectate lyase. In this study, we aimed to obtain a strain that is able to produce pectate lyase with good pH stability.
Methods
In this study, screening for pectate lyase was performed using plate assays. Fermentation parameters for pectate lyase production were optimized utilizing a single variable optimization. To get insight into the pectate lyase, its enzyme property, purification, identification, and application were performed.
Results
Bacillus altitudinis CAS-WZS-08, producing pectate lyase with good pH stability, was isolated. The optimal fermentation conditions of CAS-WZS-08 are 4 g/L pectin, 20 g/L yeast extract, 2% inoculum size, pH 7.0, and 33 °C, which the production of pectate lyase can reach up to 0.71 ± 0.001 U/mL. The optimal pH and temperature of the pectate lyase were 10.0 and 60 °C, respectively. Stored at 4 °C, the pectate lyase was able to keep its full enzyme activity for 24 h under a wide range of pH (4.0–10.0) condition. With pH 10.0, this pectate lyase was stable under 30-45 °C. In addition, it can be activated by Mn2+, Cu2+, Co2+, and Ca2+, while inhibited by Fe3+, Ba2+, and Mg2+. Later, the electrophoretic pure protein was acquired through ammonium sulfate precipitation, cation exchange column, and Sephadex G-75. Liquid chromatography tandem-mass spectrometry (LC/MS-MS) further confirmed that the purified protein was pectate lyase with a molecular weight of ∼ 40 kDa. At last, the result of pectate lyase in extracting apple juice demonstrated that it has an excellent juice extraction ability.
Conclusion
This study provides an excellent pH-stable pectate lyase with good thermal stability that is a potential candidate for industrial applications.
Keywords
Bacillus altitudinis
Isolation
pH-stable
Pectate Lyase
Thermal Stability
1 Introduction
Enzymes, catalyzing substances to products specifically and in an environmentally friendly manner, have an extensive range of applications (Uzuner and Cekmecelioglu, 2019). Pectate lyase plays a significant role in current industries, such as paper making, wastewater treatment, textile processing, wine clarification, animal feed industry, and coffee or tea fermentation (Hugouvieux-Cotte-Pattat, et al., 2014; Wu, et al., 2020). The catalytic attributes and stability of pectate lyase in various physio-chemical environments are significant for their commercialization (Xiang, et al., 2019). In particular, the temperature stability and pH stability are extremely vital for its application. For example, the degumming process is usually executed at a temperature from 40 °C to 70 °C and in alkaline pH conditions (8–11) (Bekli, et al., 2019; Wu et al., 2020).
The methods for obtaining pH stability of pectate lyase including immobilization, rational protein design or directed enzyme evolution, isolation newly strains, and so on. Through these methods, some studies on the pH stability have been reported with promising results. Ran et al., (2017) immobilized alkaline polygalacturonate lyase to the surface of bacterial polyhydroxyalkanoate nanogranules, enhancing the thermostability and pH stability under certain conditions. Rational protein design and directed enzyme evolution were reported in improving the stability of pectate lyase (Zhou and Wang, 2021). Screening new strains for obtaining special enzyme is another efficient method due to the abundant microbial resources. Zhou et al., (2017) reported the B. subtilis PB1with a broad pH stability from pH 5 to pH 11, maintaining 80% relative enzyme activity after 2 h. Sasaki et al., (2015) screened a strain of Georgenia muralis JAM 3H7-3 that could produce the pectate lyase with an optimal pH of 10.0 and was stable at pH 6.5–11.0. Therefore, screening pectate lyase with good stability is great significance for the practical application of enzymes.
In this study, an excellent pH-stable pectate lyase with good thermal stability, secreting from Bacillus altitudinis CAS-WZS-08, was reported. CAS-WZS-08 was preserved in China General Microbiological Culture Collection Center (CGMCC NO. 22763). The culture medium and conditions were optimized using single-factor analysis for obtaining higher production. Enzyme property and purification were performed in a subsequent study. Finally, the pectate lyase was displayed through sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and detected using LC-MS/MS. This study provides an excellent pH-stable pectate lyase with good thermal stability. It also provides candidate for researching the mechanism of pH stability in the future.
2 Materials and methods
2.1 Media and reagents
Isolation medium: pectin (galacturonic acid ≥ 74.0%, Shanghai Macklin Biochemical Co., Ltd.) 2 g/L, (NH4)2SO4 10 g/L, NaCl 2 g/L, KH2PO4 0.3 g/L, K2HPO4 1.0 g/L, MgSO4 0.3 g/L, FeSO4 0.1 g/L, agar 15 g/L, pH 7.0, sterilized at 115 °C for 30 min. Primary fermentation medium: pectin 2 g/L, (NH4)2SO4 10 g/L, NaCl 2 g/L, KH2PO4 0.3 g/L, K2HPO4 1.0 g/L, MgSO4 0.3 g/L, FeSO4 0.1 g/L, pH 7.0, sterilized at 115 °C for 30 min. Regents were purchased from Sinopharm Chemical Reagent Co., Ltd. Shanghai, China.
2.2 Isolation and identification of strain
2.2.1 Isolation the strains
Samples were gathered from the tobacco leaf conserved in the laboratory, and rotting leaves were acquired from the park of Qingdao Institute of Bioenergy and Process Biotechnology. The ability of strain to secrete pectate lyase was measured using congo red and NaCl for measuring the Hc value (Prajapati, et al., 2021). Each measurement was carried in triplicate.
2.2.2 Identification of strain
The strain was identified according to the 16S rRNA gene sequence and tested the biochemical characteristics using microbial biochemical identification tubes (Qingdao Hope Bio-Technology Co., Ltd., China), including the voges-proskauer (V-P), citrate, propionate, D-xylose, L-arabinose, D-mannose, gelatin liquefaction, 7% NaCl, nitrate reduction, amylolysis, resistance to antibiotics (Ampicillin 100 ug/mL; Chloramphenicol 34 ug/mL; Kanamycin 35 ug/mL; Spectinomycin 50 ug/mL), and gram staining. The temperature and pH range of strain growth were studied under diverse temperature and pH. The phylogenetic tree was constructed through MEGA 7.0 (Kumar, et al., 2016) using the neighbor-joining method and 1000 bootstrap analysis.
2.3 Determination the enzyme activity
Enzyme activity was measured using the 3, 5-dinitrosalicylic acid (DNS) method. To obtain the crude enzyme, 1.0 mL of freshly grown culture was centrifuged at 10, 000 rpm for 10 min. The substrate was prepared by mixing 0.1% (w/v) pectin in Glycine-NaOH buffer (50 mmol/L). The control experiment was prepared using a deactivated enzyme (100 °C, 5 min) instead of the normal pectate lyase. The supernatant (10 μl) was mixed with 500 μl substrate and incubated at 60 °C for 30 min. Adding the DNS solution (500 μl), then boiled the reaction mixture 5 min for detecting the absorbance at 540 nm. Enzyme activity (U/mL) is defined as the amount of enzyme required to catalyze the substrate to form 1 μmol of galacturonic acid per minute under the given conditions.
2.4 Effects of fermentation parameter
To obtain a higher enzyme activity, fermentation parameters, including pectin concentration, nitrogen types, nitrogen concentration, the initial pH of the media, inoculum size, and culture temperature, were determined step by step using single-factor analysis at one time (El-Ghomary, et al., 2021). The pectin concentration was designed for 2, 4, 6, 8, and 10 g/L. Nitrogen types, including urea, NH4Cl, (NH4)2SO4, yeast extract, tryptone, and beef powder, were measured at 10 g/L. In nitrogen concentration, seven concentrations were designed, including 4, 8, 12, 16, 20, and 24 g/L. Initial pH of the media was set to 5, 6, 7, 8, 9, and 10. Moreover, the inoculum size (v/v), including 1%, 2%, 3%, 4%, 5%, 6%, and 7%, were performed. Experiments were performed in triplicate (n = 3). Statistical analysis was carried out using analysis of variance in SAS studio. The p-values were compared to the significance level of 5% and used to determine the significance.
2.5 Characterization of enzyme property
Regarding the enzyme properties, the optimum temperature and stability in temperature, the optimum reaction pH and pH stability, the effects of metal ions on enzyme activity, and effects of buffer concentration were organized.
The optimum temperature was determined from 45 to 60 °C (tested in interval of 5 °C) in Glycine-NaOH (50 mmol/L, pH 9.0). To determine the temperature stability, the crude enzyme was preincubated at 30 °C, 35 °C, 40 °C, 45 °C, 50 °C, 55 °C, and 60 °C, determining the remaining enzyme activity. The optimum pH was determined at 60 °C for 30 min in different buffers (50 mmol/L) with pH from 3.0 to 10.5, including Na2HPO4-Citric acid, KH2PO4-NaOH, Tris-HCl, and Glycine-NaOH (Jadhav and Pathak, 2019). The pH stability was determined by measuring the relative enzyme activity (Jalil and Ibrahim, 2021) after the enzyme incubation at 4 °C at different pH buffer from 3.0 to 10.0. The enzyme activity without preincubation was defined as 100%. Metal ion influences were calculated through 1 mM K+, Ca2+, Mg2+, Zn2+, Mn2+, Cu2+, Ni2+, Fe2+, Fe3+, Ba2+, and Co2+ in the reaction mixture. The buffer concentrations were set as 0, 50, 100, and 200 mmol/L to determine the influence of buffer concentrations.
2.6 Purification of pectate lyase
To obtain the purified enzyme, several purification methods were evaluated in this study. First, the ammonium sulfate precipitation was deployed with supernatant. The saturation of ammonium sulfate, containing 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, and 100%, was applied. Subsequently, the ion exchanger column (HiTrap SP HP, 5 mL) was applied to obtain purified pectate lyase using low-pressure liquid chromatography (2001-A-I, Shanghai Jiapeng). Then, liner gradient elution was carried out at 0.8 mL/min, and collection was carried out using a separator. After that, the sample was purified through Sephadex G-75 with pH 7.2 (Tris-HCl, 50 mmol/L) at 0.3 mL/min. Each tube was collected after 10 min.
Protein content was measured by BCA (bovine serum albumin) standard curve. The weight and purity of pectate lyase were measured using SDS-PAGE (X. Zheng et al., 2020).
2.7 Identification of pectate lyase through LC-MS/MS
The signal bond was cut off from the SDS-PAGE gel and subjected to LC-MS/MS (Vogeser and Parhofer, 2007) analysis by Beijing BGI Technology Co., Ltd. The top 23 charge ions from each scan were selected for MS/MS (tandem-mass spectrometry) analysis. Subsequently, MASCOT 2.2 (Matrix Science, London, UK) was applied to search the MS/MS spectrum and identify the protein by searching the Uniport.
2.8 Application of pectate lyase in extraction of apple juice
Two grams of apple pulp were incubated with 5 mL of partially pure pectate lyase for 1 h at 50 °C. Apple juice was acquired by centrifugation at 5,000 rpm for 30 min. After cooling at room temperature, the volume of supernatant and the weight of residue acquired were assessed (Rahman, et al., 2020).
3 Results
3.1 Screening of strains for pectate lyase
Through the primary and secondary screening, five strains were obtained, including CAS-WZS-08, MEI-2–27, MEI-2–29, CAS-MEI-2–33, and CAS-07, showing in Fig. 1. The CAS-WZS-08 strain had a higher Hc value than others and was selected for further studies.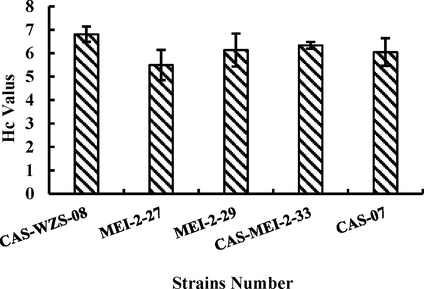
Hc value in pectin isolation medium.
3.2 Phylogenetic tree and characteristics of the strain
Phylogenetic tree of CAS-WZS-08 was shown in Fig. 2. It had 96% similarity to B. altitudinis 41KF2b T. 23 MN543854.1:1–1423. The sequence of the 16S rDNA was deposited in GenBank, with SUB11024406 CAS-WZS-08 OM462373. The physiological and biochemical characteristics were shown in Table 1. L-arabinose, D-mannose, gelatin liquefaction, nitrate reduction, and gram staining were positive, it also could grow under 7% NaCl. Other tests were negative, including V-P, citrate, propionate, D-xylose, and amylolysis. Antimicrobial susceptibility tests demonstrated that CAS-WZS-08 was susceptible to ampicillin, chloramphenicol, kanamycin, and spectinomycin. Note: ‘+’ means positive, ‘-’ means negative. ‘S’ means sensitive.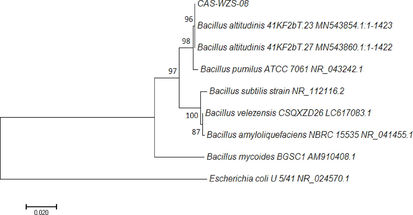
The phylogenetic tree of Bacillus altitudinis CAS-WZS-08.
Biochemical Test
Results
V-P
–
Citrate
–
Propionate
–
D-xylose
–
L-arabinose
+
D-mannose
+
Gelatin liquefaction
+
7% NaCl
+
Nitrate reduction
+
Amylolysis
–
Ampicillin, 100 ug/mL
S
Kanamycin 35 ug/mL
S
Chloramphenicol, 34 ug/mL
S
Spectinomycin 50 ug/mL
S
Gram staining
+
3.3 Growth characteristic of CAS-WZS-08
The strain grows in a range of pH 5.0 to pH 10.0. The highest biomass was 4.75 ± 0.12 at pH 7.0, while it was markedly inhibited at pH 10.0 (Fig. 3a). The influence of temperature shown that the CAS-WZS-08 grown better at 33 °C (Fig. 3b).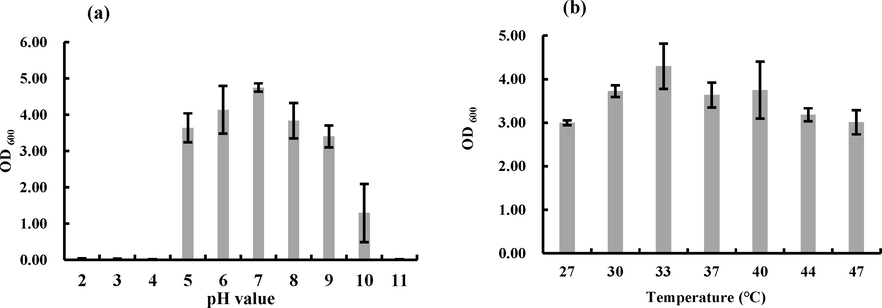
The growth of CAS-WZS-08. (a). Growth under different pH conditions; (b). Growth under different temperatures.
3.4 Optimization of fermentation conditions
The results of fermentation parameter were shown in Fig. 4, including the pectin concentration, nitrogen source, nitrogen concentration, medium pH, inoculum size, and culture temperature. Pectate lyase activity was highest (0.064 ± 0.002 U/mL) at 4 g/L pectin than other (Fig. 4a). Yeast extract, with 0.263 ± 0.013 U/mL enzyme activity, was better than the tested media (Fig. 4b). Organic nitrogen sources were better than inorganic nitrogen in the experiments. The enzyme activity was enhanced 4.1-fold compared to that under a 4 g/L pectin concentration. The influence of yeast extract concentration on pectin lyase activity shown that the enzyme activity increased gradually from 4 g/L to 20 g/L. The highest enzyme activity was 0.54 ± 0.017 U/mL at 20 g/L (Fig. 4c). The effects of medium pH shown the enzyme activity reached 0.69 ± 0.040 U/mL at pH 7.0 (Fig. 4d). Subsequently, the influence of inoculum size on enzyme activity was shown in Fig. 4e, and the highest enzyme activity was obtained with 0.79 ± 0.001 U/mL at 2% (v/v). Finally, the influences of culture temperature from 27 °C to 47 °C were shown in Fig. 4f. The enzyme was no significant difference in enzyme activity between 27 °C and 40 °C, otherwise, it was significantly declined with the temperature exceeded 44 °C.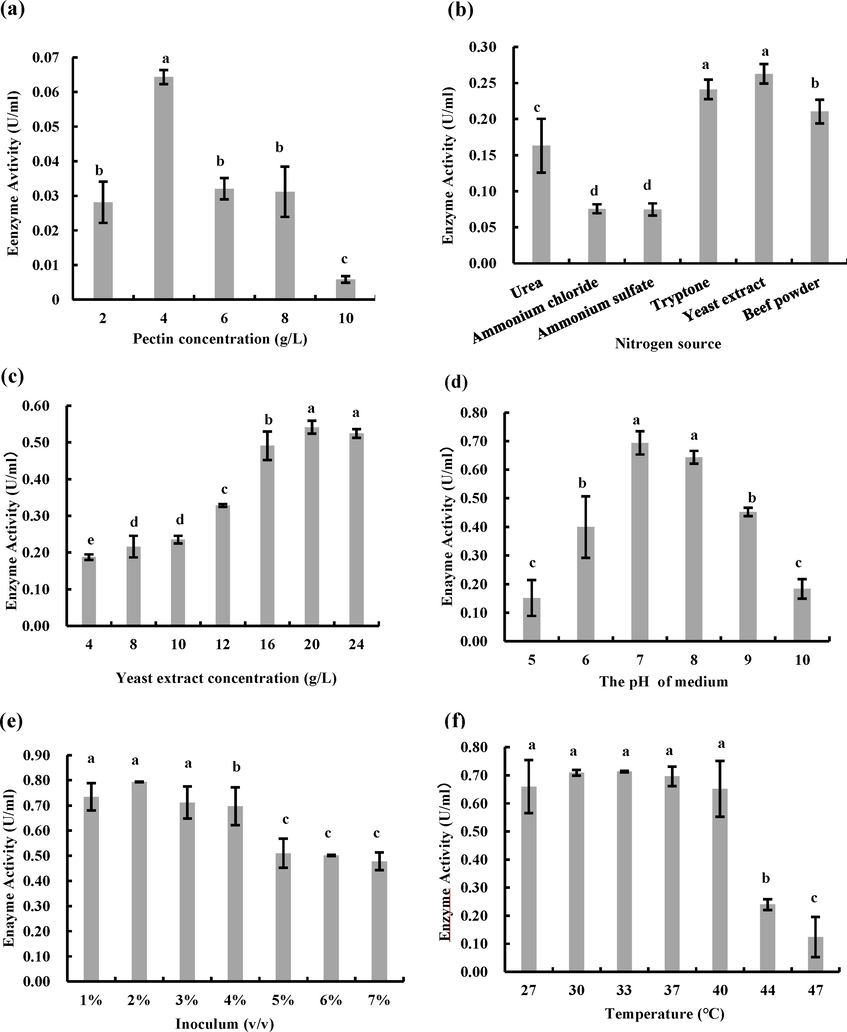
The optimum condition of CAS-WZS-08 for pectate lyase activity. (a). Pectin concentration. (b). Nitrogen source. (c). Yeast extract concentration. (d). Medium pH. (e). Inoculum size. (f). Cultivation temperature. Note: Identical letters indicate a lack of significant difference.
3.5 Enzyme properties
Enzyme properties were shown in Fig. 5. Under the experiment, the pectate lyase increased gradually with increasing temperature until 60 °C (Fig. 5a). The results of different pH buffer on enzyme activity demonstrated that it was an alkaline pectate lyase, with the highest enzyme activity occurred in pH 10.0 (Fig. 5b). The activity was also affected by the category of the buffer. The Tris-HCl was more suitable for pectate lyase activity than KH2PO4-NaOH and Glycine-NaOH. Enzyme activity was activated by Mn2+, Cu2+, Co2+, and Ca2+. Especially in the Mn2+ condition, the enzyme activity was 2.11-fold that of the control. It was inhibited by Fe3+, Ba2+, and Mg2+, especially Fe3+, in which the pectate lyase activity was only 18% of the control. K+, Zn2+, Ni2+, and Fe2+ have little effect on enzyme activity (Fig. 5c). The enzyme activity was also affected by buffer concentration, and the highest activity occurred at 50 mmol/L (Fig. 5d). Temperature stability demonstrated that it was stable at 30-50 °C, especially since the enzyme activity was not affected at 30 °C and 35 °C after 180 min (Fig. 5e).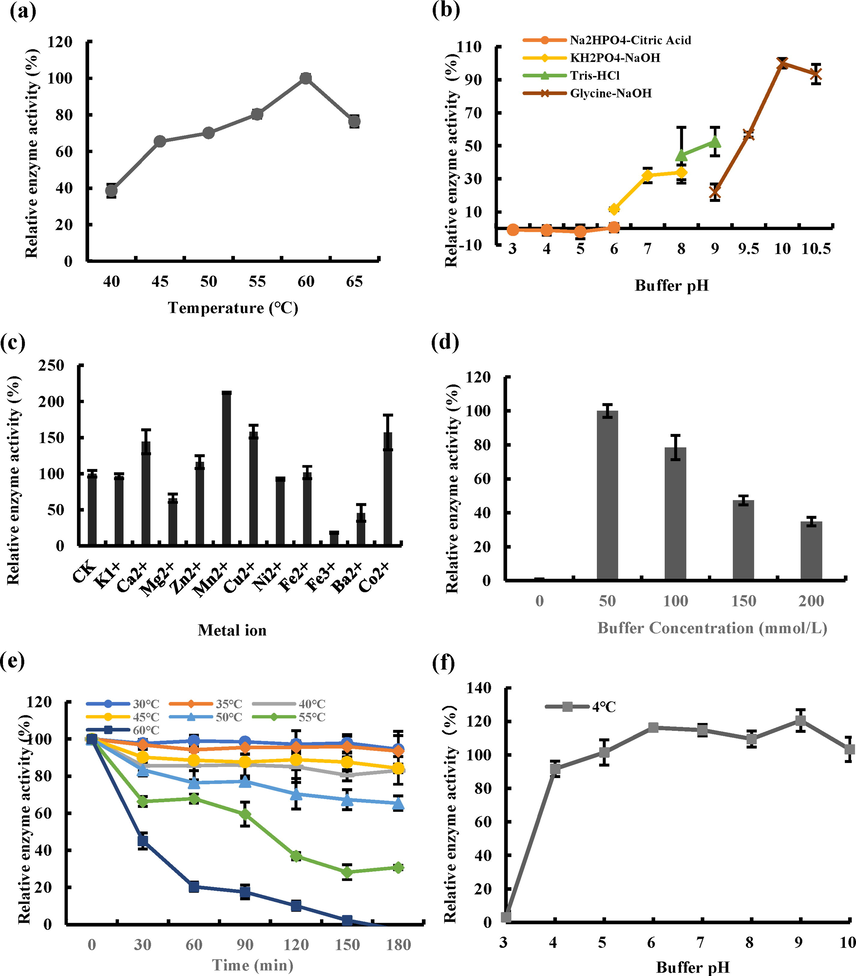
Pectate lyase properties of CAS-WZS-08. (a). Optimal temperature. (b). Optimal buffer pH. (c). Metal ions. (d). Buffer concentration. (e). Temperature stability. (f). pH stability.
Pectate lyase has a wide range of stability from pH 4.0–10.0 at 4 °C, maintaining 100% relative enzyme activity after 24 h incubation (Fig. 5f). The studies on the stability of pectate lyase were summarized in Table 2, including isolation and heterogeneous expression in bacteria. Note: “/” means not mentioned.
Organism
Optimal Tm
Temperature stability
Optimal pH
pH stability
Reference
Isolation
B. subtilis PB1
50 °C
50 °C ∼ 2h ∼ 90%
9.5
pH 5.0–11.0 ∼ 2 h ∼ 80%
(M. Zhou et al., 2017)
Georgenia muralis JAM 3H7-3
50 °C
/
10.0
pH 6.5–11.0 ∼ 40 °C ∼ 1h
(Sasaki et al., 2015)
B. tequilensis SV11
60 °C
50 °C ∼ 24 h ∼ 50%
9.0
pH 11.0 ∼ 24 h ∼ 75%
(Chiliveri and Linga, 2014)
B. pumilus BK2
70 °C
30 °C ∼ 75 h ∼ 50%
8.5
/
(Klug-Santner et al., 2006)
CAS-WZS-08
60 °C
50 °C ∼ 3h ∼ 65.39%
10.0
pH 4.0–10.0 ∼ 4 °C ∼ 24 h ∼ 100%
This study
Heterogeneous expression
E. coli (Paenibacillus sp. 0602 G241A)
67.5 °C
60 °C ∼ 61.86 min ∼ 50%
/
/
(Zhou and Wang, 2021)
E. coli (Paenibacillus polymyxa KF-1)
40 °C
40 °C ∼ 60 min > 50%
10.0
pH 5.00–11.0 ∼ 25 °C
(Y. Yuan et al., 2019)
E. coli (Antarctic
bacterium)30 °C
40 °C ∼ 10 min ∼ 0%
10.0
pH 9–10.5 ∼ 4 °C ∼ 70%
(Tang et al., 2019)
A. luchuensis var. saitoi
30 °C
60 °C ∼ 60 min ∼ 71%
8.0
pH 6–12 ∼ 4 °C ∼ 24 h ∼ 80%
(Kamijo et al., 2019)
E. coli (Paenibacillus polymyxa KF-1)
50 °C
< 50 °C
9.0
pH 5.0–11.0 ∼ 24 h ∼ 50%
(Yan et al., 2018)
E. coli (Bacillus clausii)
70 °C
75 °C ∼ 30 min ∼ 140%
10.0
pH 6.5–11.5 ∼ 30 °C ∼ 6h ∼ 80%
(C. Zhou, Xue, & Ma, 2017)
P. pastoris (Volvariella volvacea)
60 °C
40 °C ∼ 1h ∼ 60%
10.0
pH 4.0–11.0 ∼ 4 °C ∼ 24 h ∼ 80%
(Shi, Hu, Zheng, Long, & Ding, 2015)
E. coli (Paenibacillus sp. 0602)
65 °C
50 °C ∼ 9h ∼ 50%
9.8
pH 7.1–11.6 ∼ 45 °C ∼ 1h ∼ 80%
(X. Li et al., 2014)
E. coli (Streptomyces sp. S27)
60 °C
50 °C
10.0
pH 7.0–12.0 ∼ 37 °C ∼ 1h ∼ 55%
(P. Yuan et al., 2012)
B. subtilis WB600 (Bacillus subtilis)
50 °C
50 °C ∼ 2h ∼ 65%
9.0
pH 7.0–10.0 ∼ 80%
(Liu et al., 2012)
3.6 Purification and characterization of pectate lyase
To obtain purified pectate lyase, ammonium sulfate fractional precipitation, cation exchange column, and Sephadex G-75 were applied (Fig. 6). The pectate lyase activity was markedly decreased when the ammonium sulfate saturation reached 50% in the supernatant (Fig. 6a). When the saturation reached 70%, the pectate lyase activity diminished to 0 in the supernatant, while the relative activity in the corresponding precipitation reached 93.17% (Fig. 6a). Ultimately, the saturation of ammonium sulfate precipitation was determined to be 50%-70% (Fig. 6a). Subsequently, the crude enzyme was further purified by cation exchange column and Sephadex G-75 (Fig. 6b, 6c). The collection tubes were detected through enzyme activity and SDS-PAGE (Fig. 6d). In the purification process, the enzyme activity and protein content were measured as shown in Table 3. The 1.5 L fermentation liquid was obtained under optimal fermentation conditions, with 927.71 U total enzyme activity and 12936.72 μg protein content. After ammonium sulfate precipitation, 50 mL dialysate was obtained with 221.41 U and 245.30 μg protein. After cation exchange column, the total enzyme activity was 65.09 U with 61.33 μg protein content, and the purification factor was 14.80 with 29.39% yield. After Sephadex G-75, 18.54 U total activity was obtained in 7.51 μg protein with 34.41 purification factor. The purification steps were shown in Fig. 6d with ∼ 37 kDa. LC/MS-MS results showed that the molecular weight of the pectate lyase was 40 kDa with the accession NO. Q56806, which was consistent with the SDS-PAGE results. The matched proteins with a prot-score ≥ 32 were selected, and their detail information were listed in Table S1.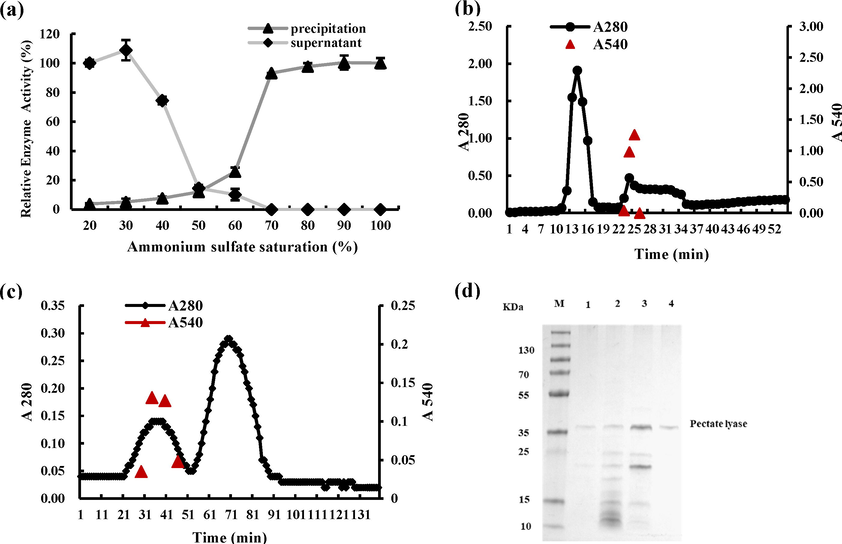
Purification of pectate lyase from CAS-WZS-08. (a). Ammonium sulfate fractional precipitation. (b). Cation exchange column. (c). Sephadex G −75. (d). SDS-PAGE. M: Marker, 1: Fermentation liquid. 2. Ammonium sulfate precipitation. 3: Cation exchange column. 4. Sephadex G −75.
Total activity (U)
Total protein (μg)
Specific activity (U/mg)
Purification fold
Yield (%)
Fermentation liquid
927.71
12936.72
71.71
1
Ammonium sulfate
221.41
245.30
902.61
12.59
23.87
Cation exchange column (pH 6.0)
65.09
61.33
1061.27
14.80
29.39
Sephadex G-75
18.54
7.52
2467.437
34.41
28.48
3.7 Application of pectate lyase in extraction of apple juice
Many studies have investigated the effect of pectinase on the yield of fruit juice, including diverse of treatment conditions, the enzyme concentration, and the kinds of enzyme. In this work, the apple juice yield (treated by pectate lyase) increased by 8.29% compared with untreated. The enzyme hydrolysis the pectin of cell wall, releasing the sap inside the cells of pulp, increasing the juice yield (Sharma et al., 2017). The results showed that pulp treated with pectate lyase exhibited better pressing characteristics (Table 4). The weight of residue reduced considerably after enzyme therapy of apple pulp as contrasted with controlling. Rahman et al. (2020) reported that apple juice yield increased by 11.54% through the pectinase, secreting from Bacillus subtilis subsp. Makebe et al., (2020) reported the treatment conditions of soursop pulp using pectinase, including enzyme concentration, temperature, and time, with the 75.20% yield. Mohanty et al., (2018) reported that palm was treated with pectinase and cellulase, and the juice yield reached 87.9 ± 0.66% under optimized conditions. To get better result in the latter studies, the treatment conditions of pectate lyase still need more detailed study.
Apple juice extraction
Untreated
Treated by pectate lyase
Volume of pulp (mL)
7.00
7.00
Volume of juice (mL)
5.79 ± 0.06
6.37 ± 0.03
Weight of residue (g)
0.61 ± 0.01
0.31 ± 0.01
Juice yield (%)
82.71%
91.00%
4 Discussion
4.1 The production of pectate lyase in strain
The production capacity of screened strain was an important parameter (Rebello et al., 2017). In this study, the fermentation condition for higher pectate lyase activity was determined, with activities ranging from 0.03 ± 0.01 U/mL to 0.79 ± 0.001 U/mL. Nevertheless, compared with previous studies, the production should be optimized for higher yield. A pectin-rich substrate, such as lemon peel, wheat bran, and orange bagasse, should be utilized instead of pure pectin (Muslim, et al., 2015, Ferreira, et al., 2010). Thite et al., (2020) reported the strain to generate the xylanase and pectinase with four different agro-waste biomasses. The pectinase activity was 220–280 units using the citrus peel. Li et al., (2020) reported that the highest yield of pectinase produced by A. niger NRRL 322 was 9.5 U/mL using soybean hull. Jadhav (2019) screened the B. licheniformis UNP-1 with the 55.2 U/mL pectinase activity. However, the studies on pectate lyase production using agricultural wastes or industrial wastes were limited. To obtain a relatively high fermentation yield, the method of response surface design was used. For example, plackett burman design and central composite design were carried out for optimization of pectate lyase production in B. subtilis PB1 with 19.50 ± 0.28 U/ml activity (Zhou et al., 2017). In the latter study, the higher pectate lyase activity from CAS-WZS-08 might be researched using statistical methods.
4.2 Property of the pectate lyase
Temperature and the pH determine the changes in enzyme activity collectively. The collision between enzyme and substrate was encouraged due to the temperature increased, increasing enzyme activity. However, when a certain limitation was reached, the tertiary structure of the protein was damaged, destroying the enzyme activity (Peterson, et al., 2007). Alkaline pectate lyase is generally applied in textile refining, wastewater treatment, and tea or coffee fermentation (Wu et al., 2020; Zheng et al., 2021). The stability of pectate lyase is extremely important for its application. Zhou et al., (2017) reported the pectate lyase maintained 90% relative activity at 50 °C after 2 h. Prajapati et al., (2021) reported the pectate lyase was stable at pH 4.0–10.0, with 80% relative enzyme activity after 3 h. Zhou et al., (2017) isolated B. subtilis PB1 strain at pH 5 ∼ 11 after 2 h. In this study, the pectate lyase has a wide range of stability from pH 4.0–10.0 at 4 °C. The pH stability not only has a great advantage for storage but also has a wide selection in purification. This result is also a basis for researching the mechanism of pH stability to further enhance the stability of industrial enzymes.
Among the different roles that metal ions can play in the catalytic event, the most common is their ability to orient the substrate correctly for the reaction, exchange electrons in redox reactions, and stabilize negative charges (Prejanò, et al., 2020). Bacterial pectate lyase works predominantly requires Ca2+ ions, which could acidify the C5 proton of galacturonic acid binding to the + 1 subsite of pectate lyase (Bekli et al., 2019; Zheng, et al., 2021). Ca2+ also was important for pectate lyase producing with CAS-WZS-08.
5 Conclusion
In this study, a pH-stable alkaline pectate lyase with good thermal stability was obtained. It has broad pH stability between pH 4.0 and pH 10.0. This alkaline pectate lyase had the highest enzyme activity at 60 °C, pH 10.0. In addition, the pectate lyase was activated by Mn2+, Cu2+, Co2+, and Ca2+, but was inhibited by Fe3+, Ba2+, and Mg2+. This study provided a new strain that generated a fairly pH-stable alkaline pectate lyase. At last, the results of pectate lyase application in extracting apple juice demonstrated that it has an excellent juice extraction ability. All the results indicated that the enzyme is not only a potential candidate for researching the mechanism of pH stability to further enhance the stability of industrial enzymes, but also for industrial applications.
Funding
This work was supported by Shandong Provincial Science Fund for Distinguished Young Scholars (No. ZR2020JQ11), the National Natural Science Foundation of China (NSF 32170084), Young Taishan Scholars (No. TSQN201909159), and Research and Innovation Fund of Shandong Energy Institute (No. SEI I202135;No. SEI I202113).
7 Ethics approval
This rerearch does not contain any studies with human participants or animals performed by any of the authors.
Authors contributions
Ge Zhang carried out the experiments in this study and drafted the manuscript. Fan Wang revised the manuscript and participate in some experiments. Shuaijun Deng and Guoqiang Chen assisted with the fermentation experiments. Haobao Liu and Haibo Zhang conducted the design of the experiment, helped draft, and finalize the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biochemical and molecular characterizations of a novel pH- and temperature-stable pectate lyase from Bacillus amyloliquefaciens S6 for industrial application. Mol. Biotechnol.. 2019;61:681-693.
- [CrossRef] [Google Scholar]
- A novel thermostable, alkaline pectate lyase from Bacillus tequilensis SV11 with potential in textile industry. Carbohydr. Polym.. 2014;111:264-272.
- [CrossRef] [Google Scholar]
- El-Ghomary, A. E., Shoukry, A. A., EL-Kotkat, M. B., 2021. Productivity of pectinase enzymes by Aspergillus sp. isolated from Egyptian soil. Al-Azhar J. Agricultural Research, 46. 134-142. https://doi.org/10.21608/ajar.2021.245617
- Production of pectate lyase by Penicillium viridicatum RFC3 in solid-state and submerged fermentation. Int. J. Microbiol.. 2010;2010
- [CrossRef] [Google Scholar]
- Bacterial pectate lyases, structural and functional diversity. Environ. Microbiol. Rep.. 2014;6:427-440.
- [CrossRef] [Google Scholar]
- Production and characterization of a thermo-pH stable pectinase from Bacillus licheniformis UNP-1: a novel strain isolated from Unapdev hot spring. Indian J. Geo Marine Sci.. 2019;48:670-677.
- [Google Scholar]
- Partial purification and characterisation of pectinase produced by Aspergillus niger LFP-1 grown on pomelo peels as a substrate. Trop Life Sci. Res.. 2021;32:1-22.
- [CrossRef] [Google Scholar]
- Identification and characterization of a thermostable pectate lyase from Aspergillus luchuensis var. saitoi. Food Chem.. 2019;276:503-510.
- [CrossRef] [Google Scholar]
- Purification and characterization of a new bioscouring pectate lyase from Bacillus pumilus BK2. J. Biotechnol.. 2006;121:390-401.
- [CrossRef] [Google Scholar]
- MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol.. 2016;33:1870-1874.
- [CrossRef] [Google Scholar]
- Aspergillus niger production of pectinase and alpha-galactosidase for enzymatic soy processing. Enzyme Microb. Technol.. 2020;134:109476
- [CrossRef] [Google Scholar]
- Cloning, expression and characterization of a pectate lyase from Paenibacillus sp. 0602 in recombinant Escherichia coli. BMC Biotechnol.. 2014;14(18)
- [CrossRef] [Google Scholar]
- Efficient expression of an alkaline pectate lyase gene from Bacillus subtilis and the characterization of the recombinant protein. Biotechnol. Lett.. 2012;34:109-115.
- [CrossRef] [Google Scholar]
- Optimization of pectinase-assisted extraction of Annona muricata L. juice and the effect of liquefaction on its pectin structure. J. Sci. Food Agric.. 2020;100(15):5487-5497.
- [CrossRef] [Google Scholar]
- Optimisation of enzymatic extraction and characterization of palm (Borassus flabellifer) juice. J. Food Meas. Charact.. 2018;12(4):2644-2656.
- [CrossRef] [Google Scholar]
- Detection of the optimal conditions for pectate lyase productivity and activity by Erwiniachrysanthemi. J. Med. Bioeng.. 2015;4:184-191.
- [CrossRef] [Google Scholar]
- The dependence of enzyme activity on temperature: determination and validation of parameters. Biochem. J.. 2007;402:331-337.
- [CrossRef] [Google Scholar]
- Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: optimization, characterization, and application for fruit juice clarification. Biocatal. Agri. Biotechnol.. 2021;35:102063
- [CrossRef] [Google Scholar]
- The effects of the metal ion substitution into the active site of Metalloenzymes: a theoretical insight on some selected cases. Catalysts. 2020;10:1038.
- [CrossRef] [Google Scholar]
- Purification and identification of novel alkaline pectinase PNs31 from Bacillus subtilis CBS31 and its immobilization for bioindustrial applications. Korean J. Chem. Eng.. 2020;37:1942-1950.
- [CrossRef] [Google Scholar]
- Immobilization of alkaline polygalacturonate lyase from Bacillus subtilis on the surface of bacterial polyhydroxyalkanoate nano-granules. Appl. Microbio. Biotechnol.. 2017;101:3247-3258.
- [CrossRef] [Google Scholar]
- Recent advancements in the production and application of microbial pectinases: an overview. Rev. Environ. Sci. BioTechnol.. 2017;16:381-394.
- [CrossRef] [Google Scholar]
- A pectate lyase from a deep subseafloor Georgenia muralis with unusual molecular characteristics. Extremophiles. 2015;19:119-125.
- [CrossRef] [Google Scholar]
- Sharma, H. P. Patel, H. Sugandha, 2017. Enzymatic added extraction and clarification of fruit juices. Crit Rev Food Sci Nutr. 57(6), 1215-1227. https://doi.org/10.1080/10408398.2014.977434
- Shi, A., Hu, H., Zheng, F., Long, L., Ding, S., 2015. Biochemical characteristics of an alkaline pectate lyase PelA from Volvariella volvacea: roles of the highly conserved N-glycosylation site in its secretion and activity. Appl Microbiol Biotechnol. 447-3458. https://doi.org/10.1007/s00253-014-6146-0
- A new cold-active and alkaline pectate lyase from Antarctic bacterium with high catalytic efficiency. Appl. Microbiol. Biotechnol.. 2019;103:5231-5241.
- [CrossRef] [Google Scholar]
- Optimization of concurrent production of xylanolytic and pectinolytic enzymes by Bacillus safensis M35 and Bacillus altitudinis J208 using agro-industrial biomass through response surface methodology. Sci. Rep.. 2020;10:3824.
- [CrossRef] [Google Scholar]
- Uzuner, S., Cekmecelioglu, D., 2019. Enzymes in the beverage industry. https://doi.org/10.1016/B978-0-12-813280-7.00003-7
- Liquid chromatography tandem-mass spectrometry (LC-MS/MS)–technique and applications in endocrinology. Exp. Clin. Endocrinol. Diabetes. 2007;115:559-570.
- [CrossRef] [Google Scholar]
- Origins and features of pectate lyases and their applications in industry. Appl. Microbiol. Biotechnol.. 2020;104:7247-7260.
- [CrossRef] [Google Scholar]
- Improving the specific activity and pH stability of xylanase XynHBN188A by directed evolution. Bioresour. Bioprocess.. 2019;6
- [CrossRef] [Google Scholar]
- Screening of a novel polysaccharide lyase family 10 pectate lyase from Paenibacillus polymyxa KF-1: cloning, expression and characterization. Molecules. 2018;23:1-14.
- [CrossRef] [Google Scholar]
- An alkaline-active and alkali-stable pectate lyase from Streptomyces sp. S27 with potential in textile industry. J. Ind. Microbiol. Biotechnol.. 2012;2012:909-915.
- [CrossRef] [Google Scholar]
- A novel PL9 pectate lyase from Paenibacillus polymyxa KF-1: cloning, expression, and its application in pectin degradation. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Pectinolytic lyases: a comprehensive review of sources, category, property, structure, and catalytic mechanism of pectate lyases and pectin lyases. Bioresour. Bioprocess.. 2021;8
- [CrossRef] [Google Scholar]
- High-level expression and biochemical properties of a thermo-alkaline pectate lyase from Bacillus sp. RN1 in pichia pastoris with potential in ramie degumming. Front. Bioeng. Biotechnol.. 2020;8:850.
- [CrossRef] [Google Scholar]
- Rational design and structure-based engineering of alkaline pectate lyase from Paenibacillus sp. 0602 to improve thermostability. BMC Biotechnol.. 2021;21:32.
- [CrossRef] [Google Scholar]
- Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming. Appl. Microbiol. Biotechnol.. 2017;101:3663-3676.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102649.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







