Translate this page into:
Dental calculus – An emerging bio resource for past SARS CoV2 detection, studying its evolution and relationship with oral microflora
⁎Corresponding author at: Department of Periodontology & Oral Implantology, Dr. H. S. J. lnstitute of Dental Sciences & Hospital, Punjab University, Chandigarh. vishakha_grover@rediffmail.com (Vishakha Grover)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The most grievous threat to human health has been witnessed worldwide with the recent outbreak of Corona virus disease 2019 (COVID-19). There is mounting evidence available regarding theconnect of COVID −19 and oral cavity, particularly periodontal disease. The current review provides an update on the diagnostic potential of dental calculus and how this bio resource may help in providing us huge amount of diagnostic regarding the causative virus. Contemporary standard method of diagnosis via nasopharyngeal swabs (NPS) is tedious, may enhance the risk of aerosol contamination by inducing sneezing and detects the presence of active infection only.However,dental calculus being a mineralized deposit serves as a reservoir for biomoleculesand provides detection of past SARS CoV2 infection. Further, the abundance of information that can be obtained from this remarkable mineralized deposit on teeth regarding the viral genome, its evolution and interactions with the oral microflora shall enhance the understanding of the viral disease process and its connection with the periodontal disease. Additional diagnostic information, which may be obtained from this simple bio reservoir can complement the contemporary diagnostic strategies adopted in the management of COVID-19pandemic and enhance our existing knowledge for developing improvised novel approaches to mitigate the effects of mutated variants of the infectious agent.
Keywords
COVID-19
SARS CoV2
Diagnosis
Evolution
Calculus
Saliva
Periodontal Diseases
Nasopharyngeal swabs.
1 Introduction
COVID-19 pandemic,caused by the 2019 novel coronavirus has appeared as an unprecedented health emergency situation borne by the mankind. SARS CoV 2 is highly transmissible and responsible for causing a disease known as COVID-19. However, these organisms are prime agents for infecting animals but can infect humans too (Naji, 2020). Coronaviruses harbour single‐stranded RNA and are enveloped viruses that infect humans—composed of four universal proteins, viz. nucleocapsid, spike, a membrane protein and glycoprotein, with more variable proteins as well (Rottier, 2013). Coronaviruses are capable of infecting humans with the primary modes of transmission through droplet inhalation or contact transmission. (Khurshid et al., 2020).
The oral cavity hosts several bacteria and viruses, including SARS CoV 2. Thus a non-invasive technology such as salivary diagnostics may be an aid for the detection of the virus. Saliva constantly bathes the oral lining and tissues and therefore bears a deterministic potential also on the microbial ecology of the oral cavity. Dental calculus develops when mature dental plaque, become mineralized with calcium phosphate, present in saliva and gingival crevicular fluid (Socransky and Haffajee, 2000). The rate of calculus formation, and its composition, may differ depending on the location of teeth in the oral cavity. Nonmineralized dental plaque entraps oral microbes, host and microbial proteins, viruses, and food particles etc, and preserves their DNA (Jin and Yip, 2002). The current review provides an update on the diagnostic potential of dental calculus and how this novel approach may help in the detection of past SARS CoV2 infection. It further, highlights the plethora of additional diagnostic information, which may be obtained from this simple bio reservoir and can complement the contemporary diagnostic strategies adopted in the management of Covid-19 pandemic.
2 Calculus as a detection tool for SARS-CoV- 2 - literature search and a proof of concept
Physicians have used dental calculus as a tool for the evolutionary studies of micrbiome of oral cavity (Brealey et al, 2020). An extensive literature search was carried out on diverse scientific databases viz Science Direct, Nature, Molecular biology, and evolution along with specialized COVID issues of PubMed (https://www.ncbi.nlm. nih.gov/sars-cov-2/) (accessed 13 March 2021), WHO (https://www.coronavirus.gov.https:// https://www.coronavirus.gov) (accessed 13 March 2021), along with hand search and cross-reference tracking. The terms used for the search were “Calculus,” “Sample Tool,” and free text forms “COVID-19”, “COVID,” “SARS,” and “CORONA.” There has been a clinical trial on dental calculus as a sample tool for SARS CoV 2 (Berton et al, 2021). The study included the patient pool who were infected with SARS CoV 2 virus two months back, as diagnosed by nasopharyngeal swab to ascertain the detection of the virus. The authors reported findings from five patients, including their antibody response elicited, and it was suggested that calculus probably could help reveal all cases of SARS CoV 2 infection by the mere presence of SARS CoV 2 RNA, regardless of the presence of symptoms. However, the analysis of the sensitivity/specificity of the diagnostic method could not be reported by the authors owing to the limited number of the study sample. However, the diagnostic potential of dental calculus in revealing the SARS CoV 2 infection exhibits a strong biologic rationale. The bioarchaeology findings of dental calculus revealed an immense potential for diagnostic research, as it harbours many informative biomolecules that remain preserved. Based on such evidence from contemporary literature, we suggest that dental calculus can be a novel promising potential analytical resource for the detection of SARS CoV 2, which needs to be further researched in the light of the existing knowledge.
3 The Hypotheses
3.1 Dental Calculus-A tool for diagnostic information
The earliest evidence of dental calculus as a potential diagnostic tool dates back to 1975 when for the first time, plant phytoliths were extracted from animal teeth in order to understand the ancient diet patterns of animals (Armitage, 1975). Many researchers studied the dental calculus in its demineralized form from ancient life forms and observed that there were preserved mineralized food particles within the calculus (Dobney and Brothwell, 1986). Came from the same group of researchers that the microorganisms are also maintained within the matrix of calculus based on the scanning electron microscope observations. The same group of researchers observed that the microorganisms are also maintained within the matrix of calculus (Dobney and Brothwell, 1988). These findings were, since then, recapitulated in many other investigations (Dobney, 1994; Santiago-Rodriguez et al., 2017; Pap et al., 1995). Linossier, Gajardo, and Olavarria (Pap et al., 1995) documented a variety of ancient oral bacteria preserved within the dental calculus using immunohistochemistry and microscopy. However, they couldn’t establish the intactness and viability of DNA. It was after the advent of improvised technologies such as gold-labeled antibody transmission electron microscopy; it was ascribed that microbial DNA survived in archaeological material (Preus et al., 2011), and now, with the development of high-throughput sequencing (HTS) technology, millions of DNA fragments from thousands of different microorganisms may be recovered from the mineralized deposit (Adler et al., 2013; Warinner et al., 2017) (See Fig. 1).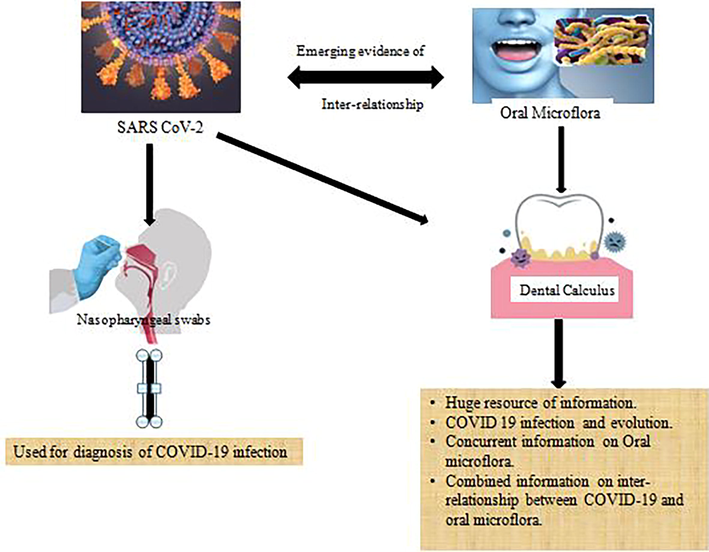
Graphic abstract/Flow chart of the conceptual flow of ‘Dental Calculus – An emerging bio resource for diagnostic information regarding SARS CoV2 and oral microflora.
3.2 Sustainability and dental calculus
A very small amount of the dental calculus sample may serve the purpose as it contains ahuge amount of biomolecules within the deposit. Therefore, dental calculus is very appealing as a bioresource. Also, due to its existence on multiple teeth in the oral cavity, an ample amount for future reference analysis can be available at any time (Mackie et al, 2017). Layer-wise, mineralization in multiple cycles during the formation and maturation instils strength and rigidity. The strength and rigidity of calculus owe to the layer-wise mineralization in multiple cycles during the formation and maturation of calculus, making it sturdy and highly resistant to environmental influences due to the presence of minimal organic substances (Jin and Yip, 2002; White, 1997). Retrieving viable DNA from archaeological sources is challenging due to degradative processes, including oxidation, DNA fragmentation by nuclease activity or degradation of DNA by microorganisms, or degradative processes including oxidation, etc. (Rotola et al, 2008). Identification of pathogens from the genomic content can provide information regarding the past infectious exposures of the individual. Furthermore, dental calculus has the ability to correlate with the biomarkers which could be indicative of various life processes or diseases, including both local and systemic factors, allowing for a far greater understanding of individual life histories(Mackie et al, 2017). Calculus has the ability to entrap influences from the environmental by inhalational ingress of micro debris as documented in previous research (Hardy et al, 2016; Henry and Piperno, 2008; Henry et al, 2012; Fox et al, 1996; Li et al,2010; Buckley et al, 2014; Radini et al,2016). In light of increased environmental concerns, dental calculus provides the following major benefits: 1) ample amounts in archaeological sites; 2) enriched pool of preserved biomolecules and 3) minimal susceptibility of contamination from extraneous agents. A huge and diverse data originating from proteomic, genomic, and microscopic factors can be processed simultaneously from a single specimen enabling a detailed insight into numerous aspects of an individual's health and diet, as well as their cultural customs (Mackie et al,2017). Unless entrenched in an acidic environment, the encapsulated bacteria are well preserved against post-mortem breakdown in highly mineralized calculus. Also, as nucleic acids are generally stabilized by divalent cations thus, genomic material is highly conserved in calculus due to the quantity of Ca2 + containing amorphous crystals in calculus (Preus et al, 2011). Several studies have highlighted the presence of the virus in the sub-gingival and supra-gingival plaque of patients with chronic periodontal disease (Rotola et al, 2008). Due to the emerging close relationship between COVID-19 and periodontal disease, the genomic material of the viral and periodontal microflora can be extracted as a whole from dental calculus; hence in most cases, it shall reveal the total genomic content thereby revealing the total genomic content of the mixed infection in most of the cases. Zhou et al found that secondary bacterial infections rather than viral infections were the prime reason for fatalities in 50% of patients with severe COVID-19 (Zhou et al, 2020). Thus, using dental calculus shall provide a holistic insight into the genetic content of the infectious agents.
4 Relationship between COVID-19 and periodontal diseases
Periodontitis is a multifactorial immune inflammatory disease, initiated by microbes and eventually.
leading to theloss of teeth by destruction of the tooth supporting tissues. Persistent accumulation of microbial dental plaque, causes acute or chronic inflammation of the periodontiumand results in progressive periodontal disease(Bartoldet al, 2000).Huge body of research has explored therelationship between periodontal disease and systemic conditions in last 50 years. Most recently, many mechanisms have been postulated in connection with oral and respiratory diseasessuch as direct aspiration of oral pathogens into the lungs, or indirectly substance released by oral /periodontal bacteria result in the modification of the respiratory epithelium thereby making it conducive to the adhesion of pathogens(Scannapieco and Mylotte, 1996).Consequently, these oral and systemic links between respiratory disease and periodontitis could exacerbate COVID 19 in people with poor oral health.Many plausible underlying mechanisms have been proposed such as systemic inflammation, cytokine connection, oral microbial load, gut dysbiosis and the endothelial function which may contribute to the connection of the two diseases (Pitones-Rubio et al, 2020).Further, an increase in acute necrotising periodontal lesions has been seen with rise in COVID-19 positive patients, as described by some of the authors. In fact, salient microbial co-infections along with the usual oral microflora e.g. prevotella intermedia has been described in much higher proportions by metagenomic studies in SARS CoV2 patients(Patel and Woolley, 2020).Many recent studies have reported an association between COVID-19 pathology and greater susceptibility of complications, hospital admissions, need for assisted ventilation and increase level of markers, mortality and fatal disease in periodontitis patients suffering from SARSCoV2 infection(Larvin et al,2020) (Marouf et al,2021).There is a sharp increase in the published literature exploring the relationship of COVID-19 and periodontal disease. A systematic extraction of literature and a narrative summary based upon 47 included articles has been provided suggesting a mutual relationship of periodontitis and COVID-19, however the literature regarding association of peri-implantitis was found to be scarce by the authors (Mancini et al., 2022). A recent opinion paper concluded based on some of the possible mechanisms of association between the two disease conditions based on the published literature in this domain (Silvestre and Márquez-Arrico, 2022.COVID-19). A retrospective cohort investigation fromSaudi Arabia has documented a statistically significant association between periodontitis and COVID-19 severity and reported that patients with periodontitis have a higher likelihood for COVID-19 complications (Alnomay et al,2022).Despite the lack of concrete prospective clinical data studies which is likely to emerge in due course of time, the huge amount of biological plausibility and emerging evidence suggest close association of a potential association of the two diseases. A case control study observed that there is an association between periodontitis severity and COVID-19(Anand et al., 2022).
5 Current sampling techniques for COVID 19
With various new and more accurate approaches, viral diagnosis has advanced enormously owing to the novel valid laboratory testing methods and different diagnostic modalities. The reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swabs (NPS) is the most reliable test for SARS CoV 2 diagnosis among the several diagnostic modalities. (Long et al, 2020; Pang et al, 2020).Saliva has been linked to human-to-human transmission by infected droplets and is used to detect SARS CoV 2. Sabino-Silva et al, researched and presented three routes of entry of CoV 2 virus into the oral cavity, i.e., respiratory tracts, blood entering the crevicular fluid, and infected salivary glands for COVID-19 to reach oral cavity (Sabino-Silva et al, 2020). Surprisingly, some investigations implied that SARS-CoV 2 is positive in salivary specimens while NPS remained negative in matched samples (Wyllie et al, 2020; Azzi et al, 2020). NPS had been considered as the standard method of sampling for establishing the molecular diagnosis of SARS CoV 2, however collection of the throat swabs NPS or throat swabs is a bit tedious, cumbersome, relatively invasive associated with few drawbacks such as significant patient discomfort, bleeding tonsil, posterior pharynx and may also induce sneezing, coughing which may exacerbate exposure to other team members and fellow beings in close vicinity. There is also emerging evidence that salivary samples may or may not be a potential source of disease transmission. On the contrary, the aerosol generated from dental procedures could be a primary and significant source of contamination by microorganisms and disease transmission (Meethil, 2021). The gingival crevicular fluid (GCF), seeping in the gingival crevice reflects the systemic status of the body, thus is considered diagnostic fluid for COVID-19 (Gupta et al, 2021). A recent study suggests the sensitivity of GCF and saliva, respectively, was 63.64% and 64.52%, compared to the contemporary gold standard NPS. GCF was displayed similar sensitivity as of saliva, but the collection of GCF is a tedious, technique-sensitive, time-consuming, costly, and labor-intensive procedure (Gupta et al, 2021). Given the limitations of current diagnostic tools, an additional convenient, valid and reliable diagnostic sample is urgently needed. WHO has prioritized and emphasized the diagnostic research for developing nucleic acid and protein tests to detect COVID-19 (Azzi et al, 2020).
6 Dental calculus –An oral mineralized bio reservoir
Lately, there has been a resurge of interest in analyzing ancient biomolecules in diverse arenas of science. Dental calculus is a mineralized accretion that deposits on human teeth due to the continuous presence of food debris, microbes, saliva, lack of hygiene, oral environment, and individual genetic susceptibility. A soft deposit, namely dental plaque, forms and resides on human teeth after cleaning. If it is allowed to mature undisturbed, it starts getting mineralized into dental calculus, though with a variable rate of accumulation person to person based on individual host related factors. The mineral content is mainly derived from calcium phosphate in saliva. The matrix of dental calculus preserves biomolecules including DNA,RNA, proteinsin calcified layers unaffected by the external influences and invasions after the host’s death. It thus preserves the original microbial signature as existed (Adler et al, 2013). Archaeological dental calculus has been proven to be a rich source of information for oral microbial pathogens, host genomic material, and nutritional components (Dobney and Brothwell, 1988). A great body of published evidence documenting the detection of a variety of viruses e.ghuman immunodeficiency, herpes, Epstein-Barr, cytomegalo, and Zikaviruses in saliva, GCF, subgingival plaque, and gingival tissues. (Pallos et al, 2020; Contreras et al, 2000; Musso et al, 2015; Maticic et al, 2000; Parra and Slots, 1996). Dental calculus has emerged as an excellent tool for the detection of microbial and host biomolecules and thereby aiding the health analysis of the population (FernandesMatuck et al, 2021). It has high physical hardness and longevity, retaining bio fingerprints in sequentially deposited events during an individual’s lifespan. Therefore it has been designated amongst the richest biomolecular sources in yet included list of resources for archaeological analysis (Warinner et al, 2015). It has a significant bearing on the host environment to the microbial ecology, especially in the pathology of periodontitis patients, where micro-ulcerations in the pocket epithelium contribute to the dissemination of pathogens and their products into the gingival vasculature and thereby the systemic circulation of the individual (Badran et al, 2020; FernandesMatuck et al, 2021).
7 Evolutionary biology as evidenced by dental calculus
Humans have experienced many pandemics in the past, which have led us to new queries, situations, and ways to mitigate the effects of pandemics worldwide. These situations require much historical, archaeological, clinical, public health, and biological research. The recent identification of dental calculus as a long-standing housing for preserved biomolecules from human-associated microbes is a landmark milestone in palaeomicrobiology (Adler et al, 2013; De La Fuente et al., 2012; Linossier et al, 1996; Warinner et al, 2014; Preus et al, 2011). This hypothesis focuses on information revealed by ancient biomolecules, such as DNA, RNA, proteins, etc., to model the evolution of infectious pathogens and decipher the course of pathogenesis. This shall pave the path for discovering new approaches to combat the contemporary evolved pathogens. Physicians have utilized genomic material to study hereditary and infectious diseases in the past (Witas and Zawicki, 2006).
An example is the study of victims of Spanish flu on the island of Spitsbergen, Norway, in 1998 (Duncan, 2003).In this attempt, they searched for a fragment of viral DNA/RNA to produce antibodies against a future viral pandemic. Another investigation (Witas and Zawicki, 2006) was conducted from a middle/late Neolithic human skull from the Hulbjerg passage grave, Langeland, Denmark, studied the transmission electron microscopy (TEM), which identified bacterial aDNA in archaeological human dental calculus. Shotgun-sequenced datafrom paired dental calculus and dentin samples from 48 globally distributed individuals were compared using a metagenomicapproach. DNA in dental calculus was found to be more abundant and less contaminated than dentin. The majority of DNA in dental calculus is microbial and originated from the oral microbiome; however, only a small proportion of DNA is derived from the host genome. (Mann et al, 2018).
8 Conclusion
As COVID-19 continues to burden public health systems and vaccination programs are still battling against emerging variants that might call for a change of management strategies, there remains a constant need for simple, accessible tools and tests to identify the infecting virus. Molecular diagnostics for SARS CoV 2 so far are dependent on the nasopharyngeal swab (NPS) or throat swabs, which have inherent limitations for testing on an extensive scale. Due to the emerging correlation between COVID-19 and periodontal disease, a novel reliable bio sampling source can be explored for its immense diagnostic potential, i.e., dental calculus. It not only shall provide insight into SARS CoV 2 and its evolution but also shall serve to study the virus interactions in co-inhabitation with the oral and periodontal microflora from a broader perspective. The authors emphasize the significance of dental calculus as an exceptional substrate and further research in this area that shall allow the direct investigation of the oral microbiome including SARS CoV 2. This novel diagnostic approach of using dental calculus shall enhance our existing knowledge and understanding regarding the microbial presence, evolution, their interactions with other microbes and host. It may pave path for developing improvised diagnostic and therapeutic methods/tools for early diagnosis, preventive strategies and better outcomes for patient management.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific research at the King Khalid University, Saudi Arabia for supporting this work through a Small Group Project under the grant number (RGP.1/351/43).
Ethics Statement
Not applicable (Review article)
Role of the funding source
The authors extend their appreciation to the Deanship of Scientific research at the King Khalid University, Saudi Arabia for supporting this work through a Small Group Project under the grant number (RGP.1/351/43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet.. 2013;45:450-455.
- [CrossRef] [Google Scholar]
- Association between periodontitis and COVID-19 severity in a tertiary hospital: a retrospective cohort study. Saudi Dent J. 2022
- [CrossRef] [Google Scholar]
- A case-control study on the association between periodontitis and coronavirus disease (COVID-19) J. Periodontol.. 2022;93:584-590.
- [CrossRef] [Google Scholar]
- The extraction and identification of opal phytoliths from the teeth of ungulates. J. Archaeol. Sci.. 1975;2:187-197.
- [CrossRef] [Google Scholar]
- Saliva is a reliable tool to detect SARS-CoV-2. J. Infect.. 2020;81:45-50.
- [CrossRef] [Google Scholar]
- Periodontal pockets: a potential reservoir for SARS-CoV-2? Med. Hypotheses. 2020;143:109907
- [CrossRef] [Google Scholar]
- Molecular and cell biology of the gingiva. J. Periodontol.. 2000;2000(24):28-55.
- [CrossRef] [Google Scholar]
- Dental calculus—a reservoir for detection of past SARS-CoV-2 infection. Clin. Oral Invest.. 2021;25:5113-5114.
- [CrossRef] [Google Scholar]
- Dental calculus as a tool to study the evolution of the mammalian oral microbiome. MolBiolEvol.. 2020;37:3003-3022.
- [CrossRef] [Google Scholar]
- Dental calculus reveals unique insights into food items, cooking and plant processing in prehistoric central Sudan. PLoS One. 2014;9:100808
- [CrossRef] [Google Scholar]
- Herpesviruses in periodontal pocket and gingival tissue specimens. Oral MicrobiolImmunol.. 2000;15:15-18.
- [CrossRef] [Google Scholar]
- Human bacterial DNA from dental calculus: a new source of genetic material. Am. J. BiolAnthropol. 2012;147:127.
- [Google Scholar]
- Dental calculus: its relevance to ancient diet and oral ecology. Teeth Anthropol.. 1986;291:55-81.
- [Google Scholar]
- A scanning electron microscope study of archaeological dental calculus. InScanning electron Microscopy Archaeol. 1988:372-385.
- [Google Scholar]
- Hunting the 1918 flu: one scientist's search for a killer virus. University of Toronto Press; 2003.
- Periodontal tissues are targets for Sars-Cov-2: a post-mortem study. J. Oral Microbiol.. 2021;13:1848135.
- [Google Scholar]
- Phytolith analysis on dental calculus, enamel surface, and burial soil: information about diet and paleoenvironment. Am. J. BiolAnthropol.. 1996;101:101-113.
- [Google Scholar]
- Dental calculus reveals potential respiratory irritants and ingestion of essential plant-based nutrients at Lower Palaeolithic Qesem Cave Israel. Quat. Int.. 2016;398:129-135.
- [Google Scholar]
- Using plant microfossils from dental calculus to recover human diet: a case study from Tell al-Raqā'i. Syria.J. Archaeol. Sci.. 2008;35:1943-1950.
- [CrossRef] [Google Scholar]
- Supragingival calculus: formation and control. Crit. Rev. Oral. Biol. Med.. 2002;13:426-441.
- [CrossRef] [Google Scholar]
- Human saliva: non-invasive fluid for detecting novel coronavirus (2019-nCoV) Int. J. Environ. Res. Public Health. 2020;17:2225.
- [Google Scholar]
- The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front. Med.. 2020;7:604980
- [CrossRef] [Google Scholar]
- Starch grains from dental calculus reveal ancient plant foodstuffs at Chenqimogou site, Gansu Province. Sci. China Earth Sci.. 2010;53:694-699.
- [Google Scholar]
- Paleomicrobiological study in dental calculus: Streptococcus mutans. Scanning Microsc.. 1996;10:1005-1014.
- [Google Scholar]
- Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? EMJ Radiol. 2020;126:108961
- [CrossRef] [Google Scholar]
- Mackie, M., Radini, A., Speller, C.F., 2017. The sustainability of dental calculus for archaeological research. In: Shallow Pasts, Endless Horiozons: Sustainability & Archaeology: Proceedings of the 48th Annual Chacmool Archaeology Conference, Feb 16, pp. 74-81.
- Impact of COVID-19 on Periodontitis and Peri-Implantitis: a narrative review. Front. Oral Health. 2022;3:822824
- [CrossRef] [Google Scholar]
- Differential preservation of endogenous human and microbial DNA in dental calculus and dentin. Sci. Rep.. 2018;8:9822.
- [CrossRef] [Google Scholar]
- Association between periodontitis and severity of COVID-19 infection: A case–control study. J ClinPeriodontol.. 2021;48:483-491.
- [CrossRef] [Google Scholar]
- Proviral HIV-1 DNA in gingival crevicular fluid of HIV-1-infected patients in various stages of HIV disease. J. Dent. Res.. 2000;79:1496-1501.
- [CrossRef] [Google Scholar]
- Sources of SARS-CoV-2 and Other microorganisms in Dental Aerosols. J. Dent. Res.. 2021;100:817-823.
- [CrossRef] [Google Scholar]
- The emerging of the 2019 novel coronavirus 2019-nCoV. Eur J Med HealthSci. 2020;2
- [CrossRef] [Google Scholar]
- Periodontal disease and detection of human herpesviruses in saliva and gingival crevicular fluid of chronic kidney disease patients. J. Periodontol.. 2020;91:1139-1147.
- [CrossRef] [Google Scholar]
- Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med.. 2020;9:623.
- [CrossRef] [Google Scholar]
- First scanning electron microscope analysis of dental calculus from European Neanderthals. Preliminary report. Bulletins etMémoires de la Sociétéd'Anthropologie de Paris. 1995;7:69-72.
- [Google Scholar]
- Detection of human viruses in periodontal pockets using polymerase chain reaction. Oral MicrobiolImmunol.. 1996;11(5):289-293.
- [CrossRef] [Google Scholar]
- Necrotizing periodontal disease: Oral manifestation of COVID-19. Oral Dis.. 2020;27:768-769.
- [CrossRef] [Google Scholar]
- Is periodontal disease a risk factor for severe COVID-19 illness? Med. Hypotheses. 2020;144:109969
- [CrossRef] [Google Scholar]
- Ancient bacterial DNA (aDNA) in dental calculus from archaeological human remains. J. Archaeol. Sci.. 2011;38:1827-1831.
- [CrossRef] [Google Scholar]
- Neanderthals, trees and dental calculus: new evidence from El Sidrón. Antiq.. 2016;90:290-301.
- [CrossRef] [Google Scholar]
- Human herpesvirus 7, Epstein-Barr virus and human cytomegalovirus in periodontal tissues of periodontally diseased and healthy subjects. J Clin Periodontal.. 2008;35:831-837.
- [CrossRef] [Google Scholar]
- The Coronaviridae. 115–137.Springer Science & Business Media; 2013.
- Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Invest.. 2020;24:1619-1621.
- [CrossRef] [Google Scholar]
- Insights of the dental calculi microbiome of pre-Columbian inhabitants from Puerto Rico. PeerJ. 2017;5:3277.
- [CrossRef] [Google Scholar]
- Relationships between periodontal disease and bacterial pneumonia. J. Periodontol.. 1996;67:1114-1122.
- [CrossRef] [Google Scholar]
- COVID-19 and Periodontitis: a dangerous association? Front. Pharmacol.. 2022;12:789681
- [CrossRef] [Google Scholar]
- Dental biofilms: difficult thera- peutic targets. Periodontol. 2000;2000(28):12-55.
- [CrossRef] [Google Scholar]
- Pathogens and host immunity in the ancient human oral cavity. Nat gen.. 2014;46:336-344.
- [CrossRef] [Google Scholar]
- A new era in palaeomicrobiology: prospects for ancient dental calculus as a long-term record of the human oral microbiome. Philosophical Trans. Roy. Soc. B: Biol. Sci.. 2015;370:20130376.
- [CrossRef] [Google Scholar]
- A robust framework for microbial archaeology. Annu. Rev. Genomics Hum. Genet.. 2017;18:321-356.
- [CrossRef] [Google Scholar]
- Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur. J. Oral Sci.. 1997;105:508-522.
- [CrossRef] [Google Scholar]
- Allele protecting against HIV (CCR5-delta32) identified in early medieval specimens from Central Poland. Preliminary results. Anthropol.. 2006;69:49-53.
- [Google Scholar]
- Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med.. 2020;383:1283-1286.
- [CrossRef] [Google Scholar]
- Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102646.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







