Translate this page into:
Anti-biofilm efficacy of marine actinomycete mediated zinc oxide nanoparticles increased the intracellular damages in biofilm forming K. pneumoniae
⁎Corresponding authors at: Department of Marine Science, Bharathidasan University, Tiruchirappalli- 620024, Tamil Nadu, India (Natesan Manoharan). Department of Biotechnology, K.S.Rangasamy College of Technology, Tiruchengode, Namakkal District, Tamil Nadu, India (B. Mythili Gnanamangai). mythilignanamangai@ksrct.ac.in (Balasubramanian Mythili Gnanamangai), biomano21@yahoo.com (Manoharan Natesan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, zinc oxide nanoparticles (ZnO NPs) were synthesized and characterized by marine endophytic actinomycete Streptomyces coeruleorubidus (S. coeruleorubidus). In result, the 380 nm spectrum of the synthesized peak was confirmed as ZnO NPs. Uniform sized nanoparticles that particle size was distributed in the range from 6 nm to 195 nm. The exact ZnO NPs morphology was clearly shown by transmission electron microscope analysis. In addition, the single crystalline or poly crystalline or amorphous nature of the ZnO NPs was clearly indicated by the result of SAED. The XRD diffraction and EDX values were more high and confirmed as ZnO NPs. Further, the antibiofilm properties of the biosynthesized ZnO NPs was exhibited 32 mm zone of inhibition against biofilm producing K. pneumoniae at 1000 µg/mL concentration. The 98% of biofilm eradication in K. pneumoniae was observed after treatment with ZnO NPs at 24 h. Furthermore, the survival fitness of the biofilm cells were degraded very high rate at 1000 µg/mL concentration of ZnO NPs. Altogether, the present result was proved that the biosynthesized ZnO NPs was very effective against biofilm forming K. pneumoniae at very low concentration, and it is an alternative drug candidate molecule in future drug discovery process.
Keywords
Zinc oxide nanoparticles
Actinomycetes
Biofilm
Minimum biofilm inhibition concentration
Survival rate
1 Introduction
Klebsiella pneumoniae (K. pneumoniae) is an important non-motile, lactose fermenting gram negative bacteria, which produce more virulence factors, that has the ability to develop more resistant behaviour to current antibiotics (Wenjian et al., 2022). They exhibit pathogenicity owing to their complex cell wall components and adhesion nature responsible for invading host immune mechanisms (John V. Ashrust and Adam Dawson). Some of the bacteria including K. pneumoniae have the innate capacity to produce biofilms. Biofilms are formed when the growing populations of microbial cells get embedded onto extracellular polymeric substances. They prevent physiological stresses such as temperature, light and environmental stress to inhibit the growth of bacterium to a larger extent. They are considered as one of the survival mechanisms to most bacterium. Mushin et al., (2018) elucidated the relationship between biofilm formation and associated severity of bacterial infections. Malaikozhundan et al., (2017a) elucidated the biopesticidal activity of bacterial coated nanoparticles for affecting the digestive activity of the pest. One of the most strongly believed hypothesis is that biofilms act as a barrier for antibiotics to enter into the cells, thereby increasing the virulence of the bacterium.
Recent years, synthesis of nanoparticle through chemical and physical methods is consuming the energy in high level and also need toxic substances to synthesis of nanomaterial. In addition, it is affected the environment and also they are not an eco-friendly process. Instead, the biosynthesized nanomaterial having more advantages than other chemical and physical methods due to the natural environment. Usually, plants, see weeds, algae, fungi, bacteria and marine crustaceans are frequently used to synthesis of nanoparticles in high level. Recent years, most of the researchers are working in the biosynthesized nanomaterial for biomedical application, because of the successive rate (Maruthupandy et al., 2018). Importantly, the synthesis method is easier and more convenient than other methods (Maruthupandy et al., 2020a). Based on the free toxic, less time, eco-friendly nature, large scale production, capping agents of phyto chemicals, hormones and other minerals of the biological mediated nanoparticle synthesis is the excellent choice for biomedical application. Recently, biofilm eradication properties of zinc oxide nanoparticles were reported by Malaikozhundan et al., 2020. The result of biofilm eradication property in human amniotic membrane ZnO NPs was indicated that the bacteria were killed by dose dependent manner. Especially, marine environment has unusal and unpredicted nature. Plenty of nutrients are uncalculated and it has enormous activities. It has the ability to produce unpredicted secondary metabolites, and it has supreme biological properties against various infections. The synthesis of nanoparticles using marine environment is most advantages than other environment. Because, the pH, temperature, salinity and other nutritional factors are improve the efficiency of nanoparticles in its biological properties. Hence, the present study was concentrated on anti-biofilm properties of ZnO NPs using S. coeruleorubidus for inhibition of biofilm forming bacteria.
2 Materials and methods
2.1 Growing of marine endophytic actinomycetes
Isolated and characterized strain of marine S. coeruleorubidus, Accession number: KY457708) was available as a slant culture. A single colony was selected and it was dissolved in distilled water. Quadrant plating method was followed in which 0.1 mL of culture was spread in a quadrant fashion on starch casein nitrate agar plate (Rajivgandhi et al., 2016). Single colony was isolated to start the fresh culture of endophytic marine actinomycetes in starch casein nitrate broth. It was allowed to grow sufficiently at 37 °C shaker incubator.
2.2 Biosynthesis of ZnO NPs
After extraction of actinomycete extract, the 50 mL of 0.1 M zinc sulphate was taken in 0.4 M sodium hydroxide was added. The metal oxide nanoparticles was allowed to form at 40 °C when the mixture was incubated for 15 min as explained in Govindan et al., (2022). The nanoparticles thus formed gradually pellet down forming white sediment. The reaction mixture was allowed to cool down and the supernatant was carefully decanted. The pellet was further suspended down by centrifugation at 3000 rpm for 10 min. It was then washed twice in deionized water to remove further impurities. The final precipitate was taken in a watch glass and was allowed to dry in hot air over set at 40 °C for 8 h. Powdery white zinc oxide nanoparticles formed were stored until it was utilized in sterile containers for characterization and bioactivity evaluation.
2.3 Characterization of ZnO NPs
2.3.1 UV–Visible spectrum
The ZnO NPs was resuspended in deionized water and Ultra-violet spectrum of the dispersed nanoparticles was read from 300 to 500 nm using Shimadzu UV–Visible spectrophotometer with path length of 1 cm.
2.3.2 Particle size analyser (PSA)
Particle size analyzer determines the size distribution of suspended nanoparticles and helps to derive the mean particle size based on intensity of particles reflecting light. If the particles are not of uniform dimension, it would result in a broad graph and if the particles are of similar dimensions, it would result in sharp peak. Based on the distribution, particle size distribution and purity can be ascertained.
2.3.3 Transmission electron microscopy (TEM)
Transmission electron microscopes have a greater magnification capability due to their electron beaming and capturing principles. Thus at such high magnifications, shape, structure, crystallinity and uniformity can be evaluated.
2.3.4 Selected area electron diffraction (SAD)
SAED pattern is an important tool in nanomaterial sciences to confirm the texture and crystallinity of synthesized nanoparticles. It forms a characterization tool to understand the diffraction pattern of selected area and thus can be tweaked to unearth valuable information from the given nanoparticles.
2.3.5 X-Ray diffraction (XRD)
XRD is a non-destructive testing and characterization tool used widely in physical sciences and nanomaterials to understand the crystallinity and chemical composition.
2.3.6 Energy dispersive X-ray spectroscopy (EDXA/EDAX)
EDAX is a confirmatory tool used for understanding the elemental composition of subject under study. EDA Spectrum can be used for the confirmation of synthesized nanoparticles based on its elemental composition; hence it is a powerful tool in the study.
2.4 Biomedical application
2.4.1 Anti-biofilm activity of K. Pneumoniae
The anti-bacterial ability of the ZnO NPs was performed against selected K. pneumoniae was initially confirmed by agar well diffusion experiment. In this method, the concentration of 100, 200, 250 µg/mL of ZnO NPs was applied into the wells of bacterial culture swabbed on nutrient agar surface of the wells respectively. After, the plate was put into the incubator at 37 ℃ for 24 h time interval. After required time, the plate was monitored whether the zone was formed by ZnO NPs or not (Palanisamy et al., 2014).
2.4.2 Minimum biofilm inhibition concentration using ZnO NPs
Aliquot 100 mL of fresh nutrient broth into the 96-well plate and added 48 h bacterial culture into the wells. Next, diluted concentration of 100–1000 µg/mL of ZnO NPs was treated into the all wells except first control wells. Then it was allowed to treatment process at 37 ℃ for 1 day time duration. After 1 day time, the turbidity based result was initially observed by naked eye in all the wells and noted the wells with turbidity range properly. Then, consecutively monitored the UV-range of O.D value by spectrophotometer at 600 nm. Based on the O.D values, the lowest concentration of ZnO NPs with highest inhibition of biofilm forming K. pneumoniae was monitored and confirmed the minimum biofilm inhibition concentration (Rajivgandhi et al., 2019a, 2019b).
2.4.3 Biofilm survival assay
The XTT assay was used to detect the live/dead variation between the control and treated wells after treatment with ZnO NPs using Uv-spectrometer at 640 nm. Briefly, the culture plus ZnO NPs of 100–1000 µg/mL was taken together into 96-well plate. The plate was permitted to run the treatment process till 48 h time duration at room atmospheric nature. Then, the XTT solution was inoculated into the treated wells of the plate after 48 h. Allowed 1 h for done the reaction, and added 50 µg/mL concentration of menadione acetate solution into the reaction mixture and then incubated at room atmosphere for 1 h. After 1 h, the precipitate of the treated result was seen in naked eye of ZnO NPs treated wells (Maruthupandy et al., 2018).
3 Results and discussion
3.1 Growing of marine Streptomyces coeruleorubidus
For synthesis of ZnO NPs, the well grown S. coeruleorubidus (Fig. 1) was used as a substrate. Also, fermentation of Streptomyces coeruleorubidus in starch casein broth was used to synthesis of ZnO NPs (Fig. 1b). Fig. 1c and Fig. 1d represent the solvent phase and aqueous phase respectively. Then, the aqueous phase of the Streptomyces coeruleorubidus extract was used to synthesize the Zno NPs.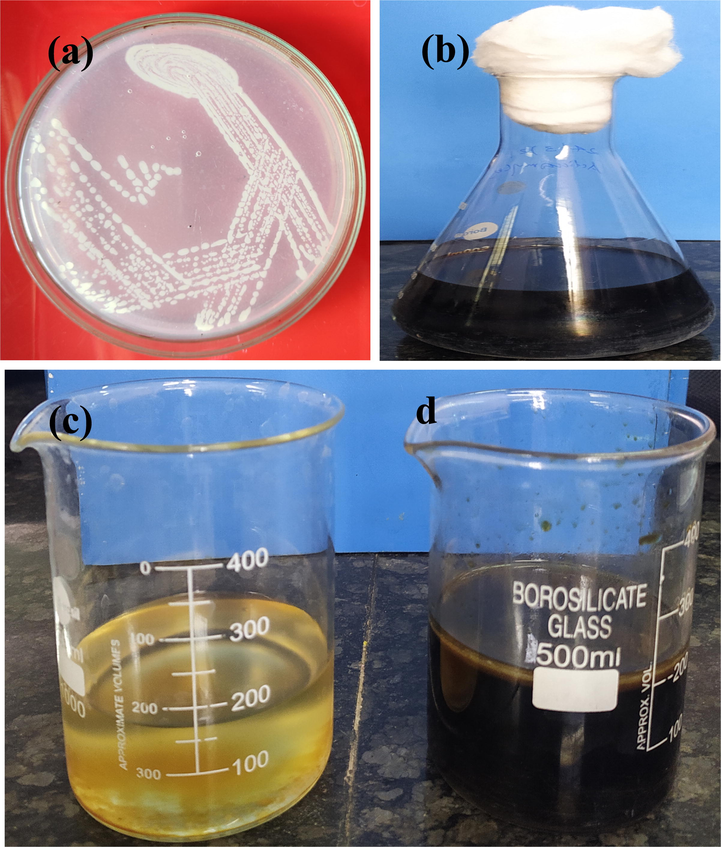
Culture of Streptomyces coeruleorubidus strain (a), fermentation of actinomycetes in starch casein agar (b), solvent phase (c) and aqueous phase (d) of Streptomyces coeruleorubidus strain for synthesis of ZnO NPs (c).
3.2 Characterization of ZnO nanoparticles
3.2.1 UV – Visible spectroscopy (UV–Vis)
UV–Visible spectrum of dispersed ZnO NPs in distilled water was evaluated for the confirmation of nanoparticles synthesis using distilled water as blank. The spectrum showed peak absorbance values at 380 nm, confirming the presence of ZnO NPs (Fig. 2a).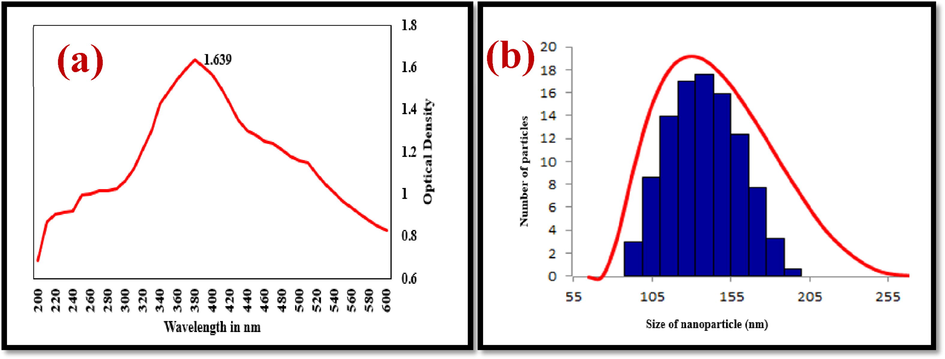
UV–Vis spectroscopy (a) and PSA (b) analysis of the Streptomyces coeruleorubidus mediated ZnO NPs.
3.2.2 Particle size analysis
The synthesized ZnO NPs are of various sizes as observed in Fig. 2b. The particle size distribution ranged from 6 nm to 195 nm. It was observed that about 75% of nanoparticles are within the size range of 125 nm – 165 nm and about 50% of nanoparticles are of the range 135–155 nm. This is considered fairly uniform distribution considering microbial synthesis. Ambika & Sundrarajan, 2015 synthesized ZnO NPs and found its size distribution in the range of 10–120 nm and the results corroborate with the current study.
3.2.3 Transmission electron microscopy
The magnified image of ZnO nanoparticles at various magnifications such as 0.5 µm and 100 nm are shown in Fig. 3a. From the images, it became evident that the nanoparticles exist as a polycrystalline structure. It can be inferred from the TEM image that the synthesized ZnO nanoparticles does not exist as amorphous powder. Some of the ZnO nanoparticles are of cuboid shaped, and few existed as triangular planes. The measurement of nanoparticles size by TEM estimated particles of about 80 nm, which was within the range observed in PSA study. TEM image of ZnO nanoparticles by Streptomyces plicatus was evaluated by Kalaba et al., 2021. The particles appeared polyhedral with mean size of 21 nm. Since the formation of nanoparticles is time, reagent and organism dependent, the size, shape and morphology of two different syntheses from two different studies cannot be compared to derive a meaningful opinion. This study however was found to result in fairly uniform sized and shaped ZnO nanoparticles as observed from TEM image at two different levels of magnification.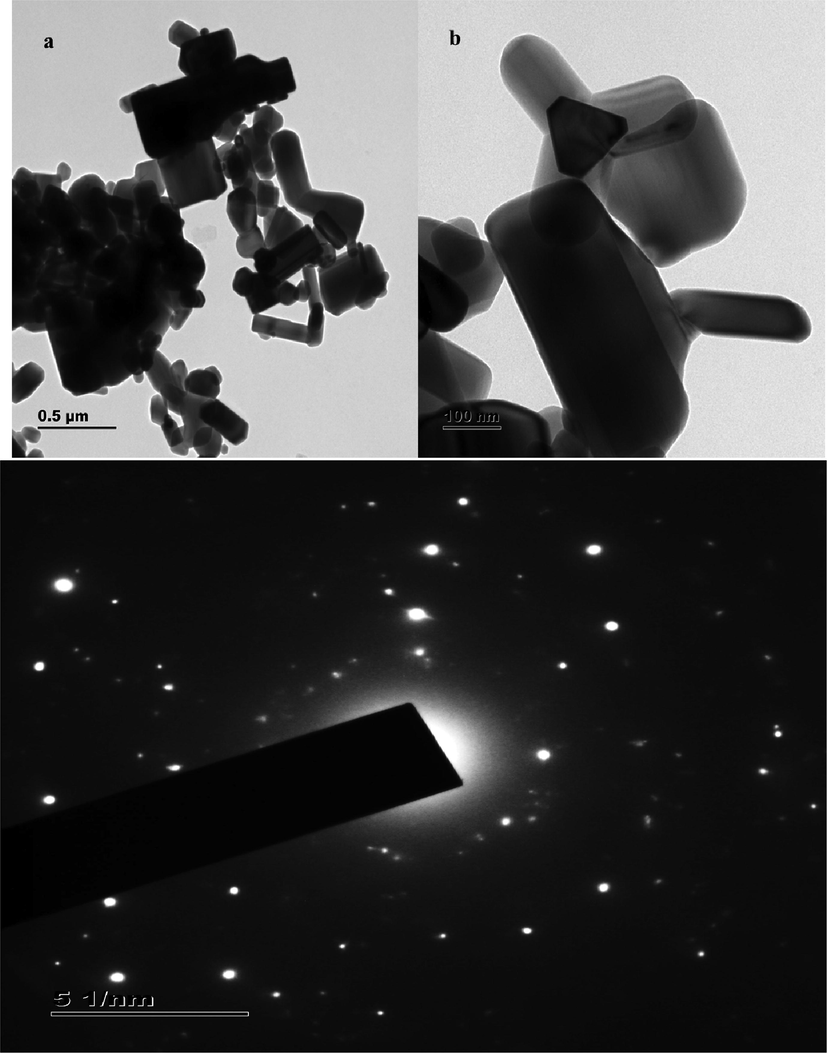
TEM (a) and SAED profile (b) analysis of the Streptomyces coeruleorubidus mediated ZnO NPs at two different magnifications.
3.2.4 Selected area electron diffraction
SAED pattern depicts diffraction spots of TEM image, when a desired area is selected for viewing and quantifying crystalline parameters. SAED confirms if the particle under study is single crystalline or poly crystalline or amorphous. Fig. 3b shows the SAED pattern image of synthesized ZnO nanoparticles. Concentric ring pattern along with bright spots were observed in the image. This further strengthens the crystalline nature of synthesized particles.
From the image the inter-planar distance was calculated using the formula given below
Inter-planar spacing (d) between two lattices for the synthesized ZnO nanoparticles was found to be 0.34 nm and 0.46 nm.
Compared to the previous study (Govindan et al., 2022) where the inter-planar spacing values were found to be 0.32 and 0.15 Å for one strain and 0.29 and 0.34 Å for another strain, these values are significantly different. Since the particle size synthesized earlier was much bigger than the current study, such substantiation could not be endorsed. Crystallinity was confirmed with inter planar spacing of 0.26 nm, which could be considered similar to the above stated observation.
3.2.5 X-Ray diffraction spectroscopy
XRD pattern graph as shown in Fig. 4 indicates that the synthesized ZnO nanoparticles are highly crystalline in nature as evident from the presence of large peaks. Also the presences of lesser number of peaks with minimal background noise indicate consistency in the structure of ZnO nanoparticles. The result was validated that the XRD profile is highly crystalline and devoid of impurities, Also it was interpreted with previous report of Rajivgandhi et al. (2018).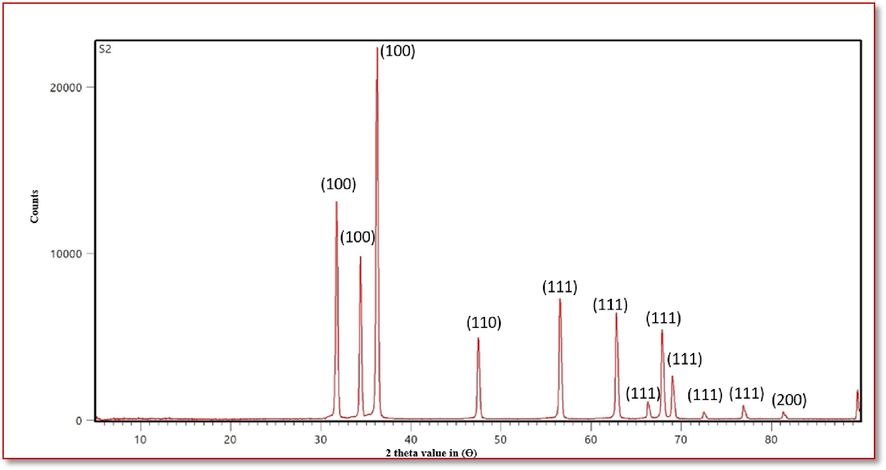
XRD pattern of analysis of the Streptomyces coeruleorubidus mediated ZNO NPs.
3.2.6 Electron dispersive X-ray analysis (EDAX)
EDAX profile of synthesized ZnO nanoparticles is given in Fig. 5. It signifies the presence of prominent peaks corresponding to zinc, copper and oxygen molecules. In case of Nagarajan and Arumugam Kuppusamy (2013), where ZnO nanoparticles were synthesized using seaweed, they observed the presence of zinc and oxygen molecules with fewer unidentified peaks. We could see the presence of copper molecules as impurities. This could be due to the source of organism and could not be found elsewhere.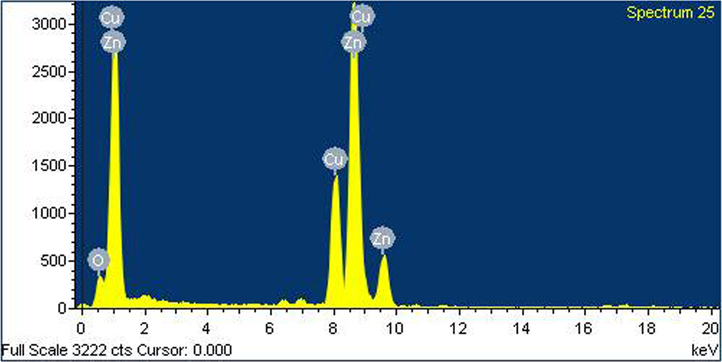
EDAX profile of analysis of the Streptomyces coeruleorubidus mediated ZNO NPs.
3.3 Biomedical confirmation
3.3.1 Agar well diffusion method of ZnO NPs
In agar well diffusion experiment, the result was exhibited with 12 mm zone of inhibition at 250 µg/mL concentration. Whereas, the 500 µg/mL concentration and 1,000 µg/mL concentration of the ZnO NPs was shown 20 mm and 32 mm zone of inhibition. It was conveyed the result that the ZnO NPs has efficient anti-bacterial activity against biofilm producing K. pneumoniae (Fig. 6a, b). In this result was indicated that the ZnO NPs was effectively inhibited the bacteria at increasing concentration with lowest concentration. When compared with previous result, the ZnO NPs has excellent anti-bacterial activity against K. pneumoniae.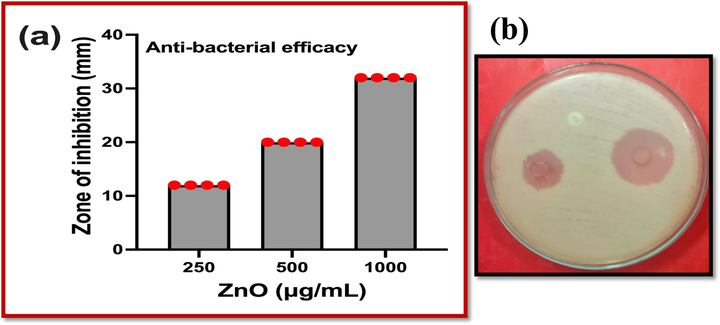
Anti-biofilm activity of Streptomyces coeruleorubidus mediated ZNO NPs against biofilm forming K. pneumonia at various concentration (a, b).
3.3.2 Minimum biofilm inhibition concentration
One could see the resulted 96-well plate, the more turbidity was shown around 900 and 1000 µg/mL concentration. The bacterial activity was not present in these wells except 100 µg/mL and 300 µg/mL treated wells. They may conveyed that the ZnO NPs was arrested the bacterial growth, but not their virulence factors (Maruthupandy et al., 2018). As same as, the turbidity level was also decreased initially and highest turbidity level was shown at 1,000 µg/mL concentration. The result of various concentrations of ZnO NPs against K. pneumoniae was available in Fig. 7a. The O.D values of 1,000 µg/mL concentration were exhibited with 98% cell death with virulence factor affect. It compared with previous results, it was very effective anti-biofilm agent. Among the O.D value results, the minimum biofilm inhibition was confirmed at 1,000 µg/ mL concentration. It was fixed for further morphological damages and other invitro experiments.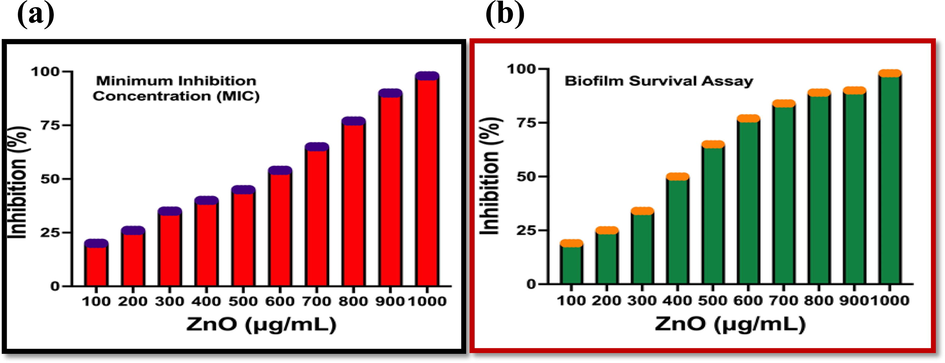
Liquid medium based biofilm inhibition assay (a) and Survival inhibition assay of Streptomyces coeruleorubidus mediated ZNO NPs against K. pneumoniae.
3.3.3 Survival fittest range of ZnO NPs
As per the confirmation of minimum biofilm inhibition concentration result, the biofilm survival assay result was also confirmed that the ZnO NPs has the efficient anti-biofilm activity against K. pneumoniae. The biofilm survival results were shown very effective against tested K. pneumoniae and the bacteria K. pneumoniae was compromised. In addition, the virulence factor eradication was also confirmed in survival assay. In result, there was no any survival of the bacterial colonies were observed. The death cells were higher compared with untreated cells (Fig. 7b). The O.D value of the result was more favoured to ZnO NPs and exhibited 98% death cells at 1,000 µg/mL concentration. Initially, 19% inhibition was observed against 10 µg/mL concentration of ZnO NPs.
4 Conclusion
Based on the observed result, the ZnO NPs was successfully synthesized and confirmed by spectroscopic results and morphological observation. Further the anti-biofilm effect and biofilm survival inhibition results of ZnO NPs were more efficient against biofilm forming K. pneumonia at 1000 µg/mL concentration. Thus ZnO NPs can reduce the virulence and can affect the pathogenicity in most dreadful microorganisms namely K. pneumoniae. Studies on in-depth investigation, evaluation of its toxicity and biocompatibility experiments can be further explored to confirm this ZnO NPs as more viable anti-biofilm agent in future.
Acknowledgement
G.Rajivagndhi and Franck Quero acknowledge the financial support from ANID-FONDECYT (Chile) under the Postdoctoral Fellowship No. 3220019. The authors extend their appreciation to the Researchers Supporting Project number (RSP2023R70), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol.. 2015;26:1294-1299.
- [Google Scholar]
- Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microbial Pathogenesis. 2018;121:224-231.
- [Google Scholar]
- Biologically synthesized copper and zinc oxide nanoparticles for important biomolecules detection and antimicrobial applications. Mater. Today Commun.. 2020;22:100766
- [Google Scholar]
- High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticle Biopro. Biosyst. Eng.. 2020;43:1533-1547.
- [Google Scholar]
- Bacterial biofilm and associated infections. J. Chinese Med. Associat.. 2018;81:7-11.
- [Google Scholar]
- Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J. Nanobiotech.. 2013;11:39.
- [Google Scholar]
- Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotech.. 2014;12:1-7.
- [Google Scholar]
- Molecular characterization and antibacterial effect of endophytic actinomycetes Nocardiopsis sp. GRG1 (KT235640) from brown algae against MDR strains of uropathogens. Bioact. Mater.. 2016;1:140-150.
- [Google Scholar]
- Antibiofilm activity of zinc oxide nanosheets (ZnO NSs) using Nocardiopsis sp. GRG1 (KT235640) against MDR strains of gram negative Proteus mirabilis and Escherichia coli. Process Biochem.. 2018;67:8-18.
- [Google Scholar]
- Biosynthesized silver nanoparticles for inhibition of antibacterial resistance and biofilm formation of methicillin-resistant coagulase negative Staphylococci. Bioorg. Chem.. 2019;89:103008
- [Google Scholar]
- Graphene/nickel oxide nanocomposites against isolated ESBL producing bacteria and A549 cancer cells. Mat. Sci. Eng. C. 2019;102:829-843.
- [Google Scholar]
- Distribution of type VI secretion system (T6SS) in clinical Klebsiella pneumoniae strains from a Chinese hospital and its potential relationship with virulence and drug resistance. Microb. Pathog.. 2022;162:105085
- [Google Scholar]
- Green Synthesized ZnO Nanoparticles Mediated by Streptomyces plicatus: Characterizations, Antimicrobial and Nematicidal Activities and Cytogenetic Effects. Plants. 2021;10:1760.
- [Google Scholar]
- Biosynthesized zinc oxide nanoparticles (ZnO NPs) using actinomycetes enhance the anti-bacterial efficacy against K. Pneumoniae, Journal of King Saud University –. Science. 2022;34:101731
- [Google Scholar]







