Translate this page into:
Pomegranate nanoparticle mitigates cisplatin-induced testicular toxicity and improves cisplatin anti-cancer efficacy in Ehrlich carcinoma model
⁎Corresponding authors at: King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia (S. Harakeh). sharakeh@gmail.com (Steve Harakeh), shaker.mousa@acphs.edu (Shaker Mousa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cisplatin (CISP) ranks among the most used chemo-therapeutic agents with exceptional efficacy against testicular cancer among other diverse types of cancers. However, it has been associated with nephrotoxicity among other side effects. Pomegranate (PE) is an effective anti-inflammatory and antioxidant compound, protecting against several chemotherapy-linked toxicities. The use of PE are limited due to its low bioavailability and poor solubility. We investigated the potential of a novel nanoparticle (NP) encapsulating PE formulation to surmount its poor solubility, improve its bioavailability, and augment its protective efficacy against CISP-induced testicular toxicity in an Ehrlich solid carcinoma (ESC) mice model. All animals were randomly grouped into four treatment groups: 1) control, 2) tumor, 3) CISP, and 4) CISP + PE-NPs. The results obtained demonstrated that PE-NPs efficiently prevented testicular toxicity induced by CISP in ESC mice and enhanced its functions. PE-NPs effectively improved CISP-induced oxidative stress in testicular tissues by elevating the levels of antioxidants (GSH, SOD and CAT). Importantly, PE-NPs, also, substantially decreased testicular inflammation induced by CISP, via reducing the levels of IL-1β, TNF-α, and NF-kB. PE-NPs did not impede the CISP’s antitumor activity as shown by histological examination data and tumor weight. It is, therefore, conceivable that PE-NPs may serve as an adjuvant therapy to CISP in the treatment of cancer, to ameliorate the associated testicular toxicity and other unwanted effects without compromising the antitumor efficacy of CISP.
Keywords
Cisplatin
Pomegranate
Testicular toxicity
Ehrlich
Anti-inflammatory
Nano formulation
Dedication: This article is dedicated to the memory of Prof. Dr. Fatimah O. Nassief, former Vice President of female campus at KAU and Co-founder of the Prophetic Medicine Chair for her dedicated life in serving science and humanity.
1 Introduction
The global burden of cancer has been reported to be on a geometric rise and, given the increasing risk factors associated with globalization and growing economies, it is expected to reach 28.4 million cases in 2040 (Sung, Ferlay et al. 2021). There are several ongoing studies investigating the use of different drugs against different types of cancer. Cisplatin (CISP) is an effective chemotherapeutic agent widely used in the treatment of solid tumours owing to its ability to bind to DNA and induce DNA destruction (Siddik 2003) leading to apoptosis induction (Desoize and Madoulet 2002). CISP causes testicular toxicity (Sherif, Abdel-Aziz et al. 2014), nephrotoxicity (Nematbakhsh, Pezeshki et al. 2017) myelosuppression, vomiting and nausea among other notable undesirable adverse side effects (Tsang, Al-Fayea et al. 2009). Testicular toxicity is implicated as a major complication associated with the use of CISP owing to the concomitant rise in the levels of cytokines including TNF-α and IL-6 (Sherif, Abdel-Aziz et al. 2014, (Kohsaka et al., 2020)). Cisplatin-induced testicular damage has also been shown to occur via mitochondria mediated inflammation, apoptosis, and oxidative stress in rodents (H and B 2020). Although massive necrosis is reported as the key histopathological feature associated with CISP-induced testicular and nephrotoxicity (Dobyan, Levi et al. 1980, Fallahzadeh, Rezaei et al. 2017), there is a major controversy about the mechanism of action of CISP-induced toxicity. Prominent among the suggested mechanisms include apoptosis, free radicals generation, inflammation, and hypoxia (Uehara, Yamate et al. 2011). Complementary therapy when applied with conventional treatment may lead to better results with less desirable side effects and lower drug resistance. Consequently, in recent years several research studies have been centred on the development of candidate cisplatin adjuvants to mitigate the associated adverse effects. Although several herbal and synthetic antioxidants have been investigated for this purpose, there is a paucity of information on a definite supplement that can effectively prevent CISP-induced testicular toxicity (Wang, Lai et al. 2020). Pomegranate (Punica granatum L.) belonging to the Punicaceae family which is a common fruit derived from the deciduous tree of Punica L. genus (Lavoro, Falzone et al. 2021). Off note, the pomegranate has a number of therapeutic and pharmacological properties, which may be ascribed to the presence of numerous phytochemicals including phenolic acids, alkaloids phytocompounds, anthocyanins, anthocyanidins, flavonoids, flavanones, flavanols, ellagitannins, Gallo tannins, organic acids, fatty acids and lipids, and lignans have been isolated from pomegranate ((Akefe et al., 2020), Zhao and Yuan 2021). Pomegranate has been used to ameliorate cancer, memory impairment, and arteriosclerosis and lowering high cholesterol levels (Viuda-Martos et al., 2010; Almuhayawi et al., 2020). The employment of nanotechnology in the area of herbal medicine has contributed to an increase in the use of herbal medicine in the management of many cancer and chronic diseases (El-Shitany et al., 2019). The formulation of natural remedies nano-particles (NP) enhances its bio-availability and effectiveness (Watkins et al., 2015). This study aimed to: (1) prepare and characterize Pomegranate - nanoparticles (PE-NPs), (2) evaluate the beneficial role PE-NPs in CISP-induced testicular toxicity in Ehrlich solid carcinoma (ESC) mice model, (3) investigate the possible protective mechanism (4) examine the impact of PE-NPs on the anticancer effect of CISP using ESC mice model.
2 Materials and methods
2.1 Preparation and characterization of Pomegranate - nanoparticles (PE-NPs), PE-PLGA NPs
NP PE was synthesized by double emulsion methods of pomegranate encapsulated into PLGA and PVA natural polymers and Pluronic 127 non anionic surfactant (Alam et al., 2012; Feczkó et al., 2008). In brief, 60 mg PLGA, 20 mg Pluronic and 30 mg PE were dissolved in 0.1 ml DMSO under stirring for 30 min. The entire solution was then emulsified with 0.7 ml 2% PVA under prob sonication for an additional 90 sec. forming the first nano emulsion. Then, the emulsion was sonicated for 60 sec with 0.2 ml 1% chitosan forming the second emulsion. NP PE was washed twice with deionized water using centrifugation (14,800 × g, 4 °C, 60 min). The precipitate was dispersed in 1 ml of chitosan.
2.2 Characterization of PE-NPs
The size distribution and zeta potential of NP PE in aqueous dispersions was determined using Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) techniques using a Malvern zeta sizer (Malvern Instrumentation Co, Westborough, MA, USA). 10 µl of the prepared NP were resuspended in 1 ml of water. PE concentration was determined spectroscopically by establishing standards calibration curve.
2.3 Encapsulation efficiency (EE) and loading ratio (LR)
The EE of NP PE as determined by analyzing the PE encapsulated in the NP compared to the amount of PE fed initially. After lyophilization, weighed nano-encapsulated powder was dispersed in 3 ml of DMSO for 30 min. The amount of PE in the DMSO was determined spectroscopically by calibration curve. The EE was calculated according to Eq. (1):
The LR was determined by measuring the amount of encapsulated PE and the weight of whole NP according to Eq. (2):
2.4 Animals and treatment:
Eight weeks adult female Swiss albino mice (n = 20) weighing between 20 and 25 g were reared at the animal house unit, King Fahd Medical Research Centre, King Abdulaziz University (KAU), Jeddah, Saudi Arabia. The animals were treated in a very humane way and all handling of animals were done according to the set rules put forward by the ethical committee at KAU university. Before the experiment, animals will be acclimatized for 1 week with the new surrounding fed commercial diet and allows access to water ad libitum. CISP of Mylan Institutional LLC, Rockford, IL, USA, was used in this study.
2.5 Induction of Ehrlich solid carcinoma
ESC was induced in the mice using Ehrlich ascites carcinoma (EAC) cells purchased from the National Cancer Institute, Cairo, Egypt. The viability of ESC cells was measured using the trypan blue exclusion assay, and it was found to be 98 percent. On day zero 2.5 × 106 viable EAC cells (0.2 ml PBS/mouse) were injected intramuscularly in the left thigh of each mouse to induce a solid tumor (Aldubayan et al., 2019). The tumor grew in 100% of the mice, with a palpable solid tumor mass within 10 days of implantation. The mice with the tumor were divided into 3 equal groups (n = 5) besides a group of non-tumor bearing mice which served as the control group. The tumor groups were designed as follows: tumor group, tumor + CISP (3.5 mg/kg), and tumor + CISP + PE-NPs (3 mg/kg). The dose of CISP were according to a study published by (Elkhawaga, Gebril et al. 2019). CISP was intraperitoneally injected as a single dose while PE-NPs was orally administered daily for 2 weeks. Both the control and the tumor groups were orally injected daily with PBS.
2.6 Samples collection and storage
Two weeks after CISP, CISP + PE-NPs, and PBS injection, the mice were ether anesthetized and blood was withdrawn via cardiac puncture, centrifuged at 3,000 rpm to separate the serum. Serum samples were collected and stored at − 80 °C. The mice were then sacrificed, and the tumor samples and testes samples were excised and weighed and were formalin-fixed for histopathology. Parts of the testis’s samples were kept frozen at − 80 °C for assessment of the markers of oxidative stress, antioxidants, and inflammation.
2.7 Assessment of kidney function and testosterone
Serum concentrations of urea, uric acid, and testosterone, were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS9719084, MBS9719085, MBS2903804, MBS2600001, MBS7606443, MBS749827, and MBS1600166 respectively, according to the manufacturer's instructions.
2.8 Determination of testicular tissue oxidative stress/antioxidants markers
Testicular tissue concentrations of oxidative stress marker, malondialdehyde (MDA), and the antioxidants markers, reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS268427, MBS265966, MBS036924, and MBS726781, respectively according to the manufacturer's instructions.
2.9 Determination of testicular inflammatory markers
Testicular tissue concentrations of inflammatory markers, tumor necrosis factor alfa (TNF-α), interleukin-1beta (IL-1β), interleuken-10, C- reactive protein, and cystatin C and nuclear factor kappa beta (NF-kB) were determined using ELISA kits of MyBioSource (USA) catalogue No. MBS2507393, MBS825017, MBS763996,and MBS268833, respectively according to the manufacturer's instructions.
2.10 Histopathological investigation
The preparation of hematoxylin and eosin (H & E) stained sections of the collected tumor and testes samples was carried out as previously described by (Suvarna et al., 2018). The sections were investigated and photographed by a blind pathologist for the pathological findings.
2.11 Statistical presentation of data
Data was presented as mean ± SD (n = 5). Comparison among groups were carried out using ANOVA followed by Tukey HSD post-hoc test, using GraphPad Prism version 5. The level of significance was settled as p ≤ 0.05.
3 Results
3.1 Preparation and physicochemical characterization
Results of Z-average particle size and zeta potential were 210.9 nm and + 32.8 mV, respectively (Fig. 1).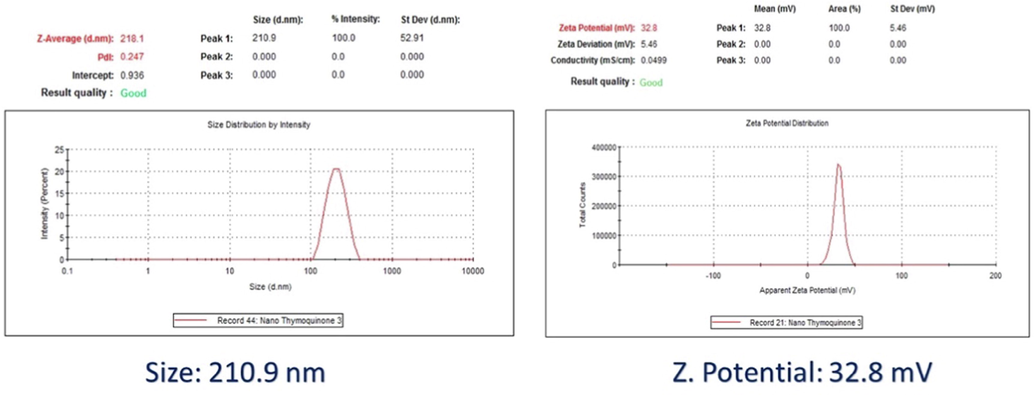
Nanoencapsulation of Pomegranate into PLGA nanoparticles.
3.2 Entrapment efficiency (EE) and drug loading capacity
The results showed that EE was 96% which reflects the success of the preparation method to prevent loss of the active drug. The drug loading ratio was 10.2% measured by HPLC-UV as this has been showed in one of our previous study (Almuhayawi et al., 2020).
3.3 Effect of PE-NPs on-serum kidney function markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumor with CISP significantly increased kidney function markers (Serum concentrations of urea, uric acid, creatinine, and cystatin C) compared to both the control and Ehrlich tumor groups. Conversely, the level of testosterone was significantly decreased in the CISP treated groups compared to the to both the control and Ehrlich tumor groups. The mice treated with PE-NPs together with CISP showed improvement in kidney function assessed in serum as significantly decreased serum urea, uric acid, creatinine, and cystatin C was observed compared with the CISP group while the level of serum testosterone was restored to normal (Table 1). These results showed that adding PE-NPs to CISP protects against CISP-induced testicular and nephrotoxicity. a = Significantly different from the value in the Control group (P < 0.05). b = Significantly different from the value in the Tumor group (P < 0.05). c = Significantly different from the value in the CISP group (P < 0.05).
Urea
(mg\dl)
U.A
(mg\dl)
Creatinine (mg\dl)
Testosterone (ng\dl)
CRP
(mg\dl)
IL-10
(pg\dl)
C. cysteine (mg\l)
Control
14.2 ± 0.48
6.2 ± 0.37
0.596 ± 0.01
279.6 ± 3.80
6.24 ± 0.36
6.68 ± 0.18
0.686 ± 0.03
Tumour
13.84 ± 0.88
4.72 ± 0.29a
0.686 ± 0.05
319.4 ± 13.22a
5.68 ± 0.29
5.96 ± 0.39
0.8 ± 0.03
CISP
45 ± 2.25ab
8.98 ± 0.10ab
1.818 ± 0.16ab
130.2 ± 4.71ab
29.8 ± 1.62ab
15.26 ± 1.15ab
2.61 ± 0.08ab
CISP + PE-NPs
14.78 ± 0.17c
5.36 ± 0.39c
0.518 ± 0.04c
266.8 ± 4.16bc
6.84 ± 0.09c
6.56 ± 0.58c
0.766 ± 0.05c
P value
<0.0001
<0.0001
<0.0001
<0.0001
<0.0001
<0.0001
<0.0001
3.4 Effect of PE-NPs on testicular tissue oxidative stress/antioxidant markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumor with CISP significantly reduced testicular tissue antioxidants markers (GSH, SOD, and CAT) compared to both the control and Ehrlich tumor groups. Treatment of mice bearing Ehrlich solid tumor with CISP significantly increased testicular tissue oxidative stress marker (MDA) compared to both the control and Ehrlich tumor groups. The mice treated with PE-NPs together with CISP showed a significantly decreased MDA concentration compared with the CISP group (Fig. 2). The mice treated with PE-NPs together with CISP showed significantly increased GSH, SOD, and CAT concentrations compared with the CISP group (Fig. 2 A, B, C, and D). These results indicated that adding PE-NPs to CISP enhances the antioxidant protection against CISP-induced oxidative stress in the testicular tissue.![Effect of Pomegranate - nanoparticles (PE-NPs), on testicular antioxidant enzyme activities [A] CAT [B] GSH, [C] SOD, oxidative stress marker [D] MDA, and inflammatory marker [E] TNF-α, [F] IL-10, and [G] NF-kB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). **p ≤ 0.01, ***p ≤ 0.001. and ****p ≤ 0.0001 are significantly different in the compared groups.](/content/185/2023/35/4/img/10.1016_j.jksus.2023.102631-fig2.png)
Effect of Pomegranate - nanoparticles (PE-NPs), on testicular antioxidant enzyme activities [A] CAT [B] GSH, [C] SOD, oxidative stress marker [D] MDA, and inflammatory marker [E] TNF-α, [F] IL-10, and [G] NF-kB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). **p ≤ 0.01, ***p ≤ 0.001. and ****p ≤ 0.0001 are significantly different in the compared groups.
3.5 Effect of PE-NPs on testicular tissue inflammatory markers measured during treatment with CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumor with CISP significantly increased testicular tissue inflammatory markers (TNF-α, IL-1β, and NF-kB) compared to both the control and Ehrlich tumor groups. The mice treated with PE-NPs together with CISP showed significantly decreased TNF-α, IL-1β, and NF-kB concentrations compared with the CISP group (Fig. 3). These results indicated that adding PE-NPs to CISP prevents CISP-induced inflammatory reaction in the testicular tissue.![Effect of Pomegranate - nanoparticles (PE-NPs), on Ehrlich tumor tissue antioxidant activities [A] CAT [B] GSH, [C] SOD, oxidative stress marker [D] MDA, and inflammatory marker [E] TNF-α, [F] IL-10, and [G] NF-kB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). **p ≤ 0.01, ***p ≤ 0.001. and ****p ≤ 0.0001 are significantly different in the compared groups.](/content/185/2023/35/4/img/10.1016_j.jksus.2023.102631-fig3.png)
Effect of Pomegranate - nanoparticles (PE-NPs), on Ehrlich tumor tissue antioxidant activities [A] CAT [B] GSH, [C] SOD, oxidative stress marker [D] MDA, and inflammatory marker [E] TNF-α, [F] IL-10, and [G] NF-kB measured during treatment with cisplatin (CISP) in Ehrlich solid carcinoma mice model. Results are expressed as mean ± SD (n = 5). **p ≤ 0.01, ***p ≤ 0.001. and ****p ≤ 0.0001 are significantly different in the compared groups.
3.6 Effect of adding PE-NPs to CISP on the antitumor activity of CISP in Ehrlich solid carcinoma mice model
Treatment of mice bearing Ehrlich solid tumor with CISP significantly decreased tumor weight compared to Ehrlich tumor group. The mice treated with PE-NPs together with CISP showed also a significantly decreased tumor weight compared to Ehrlich tumor group. There was no significant difference observed between the tumor weight observed in the mice treated with CISP alone and those treated with CISP combined with PE-NPs (Fig. 4).![Effect of Pomegranate - nanoparticles (PE-NPs), on [A] weight of Ehrlich tumor tissue and [B] correlation of IL-10 and levels of testosterone in serum. r2 = 0.02711. Results are expressed as mean ± SD (n = 5). asignificant difference against Ehrlich tumor group. ***P ≤ 0.001.](/content/185/2023/35/4/img/10.1016_j.jksus.2023.102631-fig4.png)
Effect of Pomegranate - nanoparticles (PE-NPs), on [A] weight of Ehrlich tumor tissue and [B] correlation of IL-10 and levels of testosterone in serum. r2 = 0.02711. Results are expressed as mean ± SD (n = 5). asignificant difference against Ehrlich tumor group. ***P ≤ 0.001.
3.7 Effect of CISP alone and combined with PE-NPs on testicular tissues histopathology in Ehrlich solid carcinoma mice model
Histological structure of control mice testicular tissues revealed normal regularly circular seminiferous tubules with full germ layer thickness and mature sperms. Interstitial cells in-between show normal appearance (Fig. 5A). In solid Ehrlich tumor mice group, marked degeneration of germ cells into the lumen in some seminiferous tubules and disintegration of interstitial cells. Meanwhile, disrupted germ cell layers in nearby infiltrated neoplastic cells was also prominent compared to control (Fig. 5B). After administration of cisplatin (CISP) to tumor mice revealed deformed and disorganization of seminiferous tubules, neoplastic cells looked degenerated cells and the interstitial Leydig cells (Fig. 5C). Co-administration of PE-NPs with CISP showed marked preservation of seminiferous tubules to the normal appearance and their germ layer thickness but few of seminiferous tubules contain desquamated cells (Fig. 5D).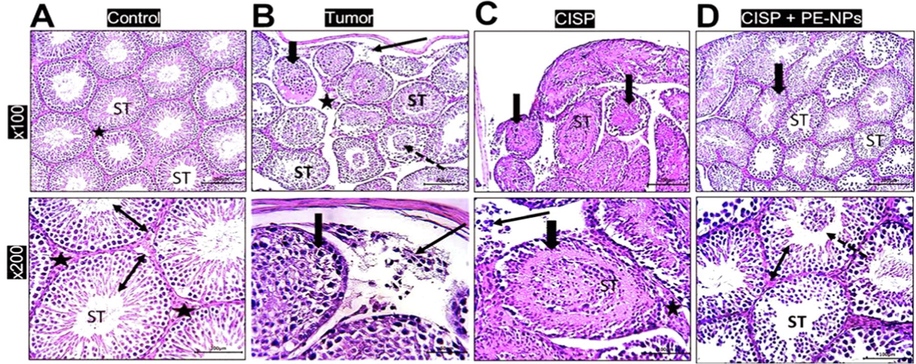
Photomicrograph sections in rat testis stained by H&E showing; A) Control group: Normal regularly circular seminiferous tubules (ST) and interstitial cells (star). Higher magnification showed seminiferous tubules with full germ layer thickness (double arrows head) and mature sperms. Interstitial cells in-between are of normal appearance (stars). B) tumor group: marked degenerative changes of some tubules (thick arrow & ST), desquamation of germ cells into the lumen (dotted arrow) and disintegration of interstitial cells (star). Higher magnification section showed disrupted germ cell layers (thick arrow) in nearby infiltrated neoplastic cells (thin arrow). C) CISP group: deformed and disorganization of seminiferous tubules (thick arrow & ST). Using higher magnification, neoplastic cells looked degenerated cells (thin arrow) and also the interstitial Leydig cells (star) and deformed seminiferous tubules (ST). D) CISP + PE-NPs group: Under low and higher magnifications, marked preservation of seminiferous tubules (ST) normal appearance and germ layer thickness (thick arrow & double head arrow) except for few seminiferous tubules contain desquamated cells (dotted arrow).
3.8 Effect of CISP alone and combined with PE-NPs on histopathological features in Ehrlich solid carcinoma tumor model.
Investigation of tumor group sections revealed highly vascular bed and massive proliferating neoplastic cells infiltrating the muscle; they have pleomorphic vesicular active nuclei with numerous mitotic figures (Fig. 6A). Whereas CISP group revealed marked decrease of tumor vascularity and neoplastic cells in their density with wide regions of tumor necrosis. Also, neoplastic cells showed small degenerated pyknotic nuclei (Fig. 6B). In CISP + PE-NPs group, more decrease in proliferating cell density compared to the previous group. Meanwhile, wide regions of tumor necrosis were increased, degeneration of neoplastic cells which become more evident with fragmented darkly stained nuclei (Fig. 6C).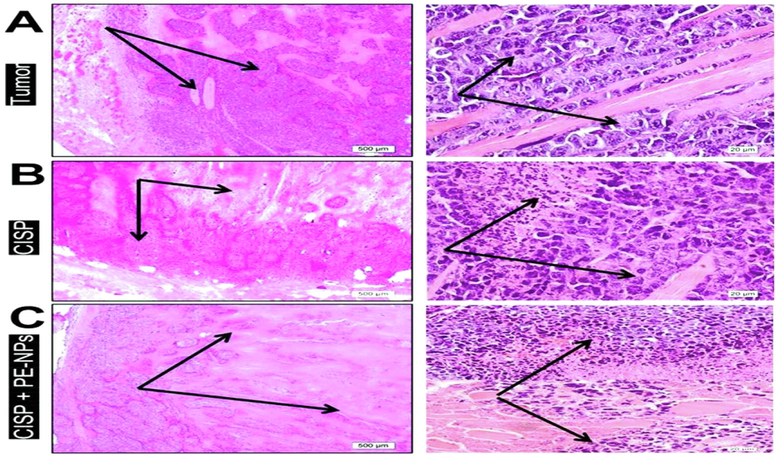
Low (x40) and high (x600) powers of induced Ehrlich’s solid tumor in mice stained by H&E to showing; A) tumor group: highly vascular bed (star) and massive proliferating neoplastic cells infiltrating the muscle; they have pleomorphic vesicular active nuclei with numerous mitotic figures (arrows). B) CISP group: decreased tumor vascularity and neoplastic cells markedly decreased in density with wide regions of tumor necrosis (dark pink stained area).under high magnification, neoplastic cells showed small degenerated pyknotic nuclei (arrows). C) CISP + PE-NPs group: more prominent decrease in proliferating cell density (arrows). In higher magnification, more wider regions of tumor necrosis, degeneration of neoplastic cells became evident with fragmented darkly stained nuclei.
4 Discussion
Pomegranate is an antioxidant derived from the tree of Punica L., but apparently has low aqueous solubility and bioavailability (Akefe, Yusuf et al. 2019, Lamidi, Mikail et al. 2021, Lavoro, Falzone et al. 2021, Harakeh, Almuhayawi et al. 2022). Bioavailability of a drug to the cells, whether in-vitro or in-vivo, is critical for its optimal efficacy (Dehghani, Hashemi et al. 2015). In the present work a PE-NPs was formulated and characterized to improve the solubility of PE. Consequently, a low dose of PE-NPs was used in this experiment (3 mg/kg/day orally). In this study, we aimed to simulate what happens in cancer patients. Most researchers usually examine the effect of cancer chemotherapy toxicity in healed PE animals (i.e., without cancer), while what happens is the emergence of associated side effects of chemotherapy on cancer patients being treated with these drugs. Therefore, this study investigated the possible protective effect of PE-NPs on testicular toxicity of CISP in mice bearing Ehrlich solid tumor. The results of this study showed that PE-NPs was effective in preventing CISP-induced testicular toxicity in ESC mice. This was confirmed by the decreased kidney function markers and improved testicular pathology observed. PE-NPs effectively ameliorated CISP-induced oxidative stress conditions in the testicular tissue via increasing the levels of antioxidants both non-enzymatic (GSH) and enzymatic (SOD and CAT). PE-NPs also significantly reduced CISP-induced testicular inflammation by reducing TNF-α, IL-1β, and NF-kB levels. This is the first study to show the protective effect of this novel formulation of PE-NPs against CISP-induced testicular toxicity in an in vivo cancer model. Furthermore, this study showed that PE-NPs didn’t hinder the antitumor action of CISP in this in vivo ESC model as shown by the tumor weight and the histological examination results. Ample evidence supports the key role of increased oxidative stress in the etiology of testicular toxicity triggered by CISP (Fallahzadeh, Rezaei et al. 2017, H and B 2020). Hence, the finding that treatment with PE-NPs significantly ameliorated CISP-induced oxidative as indicated by the decreased level of MDA and improved activity of the measured antioxidant enzymes is quite interesting. This is in line with previous reports supporting the antioxidant potential of PE in reducing oxidative stress, mitigating free radical damage and stimulating the endogenous antioxidants system (Benzer, Kandemir et al. 2011, Al-Olayan, El-Khadragy et al. 2014, Ameh, Mohammed et al. 2020). Also, Minisy et al., recently reported the protective effect of PE against tramadol-induced testicular toxicity (Minisy, Shawki et al. 2020), while Nasser et al., demonstrated that pomegranate juice extract decreases cisplatin toxicity on peripheral blood mononuclear cells (Nasser, Damaj et al. 2020).
Besides, the severity of CISP-induced testicular toxicity is associated with increased concentration of TNF-α and IL-6 (Fallahzadeh et al., 2017; Sherif et al., 2014; H et al., 2020) and a number of researchers have reported that TNF-α is the most important cytokine induced during CISP toxicity, and proved that inhibition of TNF-α protects against the deleterious action of CISP (Ramesh and Reeves 2002).
In line with these reports, the protective effect of PE-NPs, on CISP-induced testicular toxicity was further demonstrated in our study as indicated by the improved levels of kidney function markers and reduced testicular pathology observed following treatment in this study. These observed effects may be ascribed to the anti-lipoperoxidative and antioxidant potential of PE in hepatic and renal tissue of the treated animals. Our data suggest that PE and its nanoformulation might be useful adjunct nano-nutraceuticals in cisplatin chemotherapy to ameliorate the accompanying testicular pathology in long term cancer chemotherapy treatment. These protective effects were achieved without compromising the anti-cancer effects of cisplatin but rather enhanced it.
5 Conclusions
In conclusion, the results of this study provided evidence that nano formulation of PE could be an adjuvant therapy to CISP in treatment of cancer. The novel combination of PE-NPs and CISP prevents CISP-induced testicular toxicity. The underlying mechanism may be the antioxidant plus the anti-inflammatory potential of PE.
Institutional review board statement
The animal study protocol was approved by the Animal Care and Use Committee at King Fahd Medical Research Center, KAU (protocol code 02-CEGMR-Bioeth-2022 on 24.8.2021.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 473–140-1442. The authors, therefore, acknowledge with thanks The DSR for their technical and financial support.
CRediT authorship contribution statement
Mohammed Qari: Formal analysis, Project administration, Funding acquisition. Steve Harakeh: Conceptualization, Writing – original draft, Resources, Supervision. Isaac O. Akefe: Methodology. Saber H. Saber: Methodology, Validation, Investigation. Rajaa Al-Raddadi: Software. Zakaria Y. Abd Elmageed: Validation, Investigation. Turki Alamri: Software, Writing – review & editing. Nagla El-Shitany: Validation, Investigation. Soad S. Ali: Methodology, Validation, Investigation. Mohammed Almuhayawi: Formal analysis, Data curation. Shaker Mousa: Conceptualization, Visualization, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kaempferol and zinc gluconate mitigate neurobehavioral deficits and oxidative stress induced by noise exposure in Wistar rats. PLoS One. 2020;15(7):e0236251.
- [Google Scholar]
- C-glycosyl flavonoid orientin alleviates learning and memory impairment by radiofrequency electromagnetic radiation in mice via improving antioxidant defence mechanism. Asian Pac. J. Trop. Biomed.. 2019;9(12):518-523.
- [Google Scholar]
- Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int. J. Nanomed.. 2012;7:5705.
- [Google Scholar]
- Aldubayan MA, Elgharabawy RM, Ahmed AS, Tousson E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid Med Cell Longev. 2019 Jan 13;2019:5162687. doi: 10.1155/2019/5162687. PMID: 30755785; PMCID: PMC6348884.
- The potential role of pomegranate and its nano-formulations on cerebral neurons in aluminum chloride induced Alzheimer rat model. Saudi J Biol Sci. 2020;27(7):1710-1716.
- [Google Scholar]
- Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014;14(1):164.
- [Google Scholar]
- Detoxifying Action of Aqueous Extracts of Mucuna pruriens Seed and Mimosa pudica Root Against Venoms of Naja nigricollis and Bitis arietans. Recent Pat Biotechnol. 2020;14(2):134-144.
- [Google Scholar]
- Effect of Pomegranate Seed Extract on Free Radical Damage and Antioxidant Activity Under Cisplatin-Induced Oxidative Stress Conditions in Rabbit Testes. Asian J. Chem.. 2011;23(7):3231-3234.
- [Google Scholar]
- The comparison of anticancer activity of thymoquinone and nanothymoquinone on human breast adenocarcinoma. Iranian journal of pharmaceutical research: IJPR. 2015;14(2):539.
- [Google Scholar]
- Particular aspects of platinum compounds used at present in cancer treatment. Critical reviews in oncology/hematology. 2002;42(3):317-325.
- [Google Scholar]
- Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J. Pharmacol. Exp. Ther.. 1980;213(3):551-556.
- [Google Scholar]
- Evaluation of anti-tumor activity of metformin against Ehrlich ascites carcinoma in Swiss albino mice. Egyptian Journal of Basic and Applied Sciences. 2019;6(1):116-123.
- [Google Scholar]
- Nanoparticles Ellagic Acid Protects Against Cisplatin-induced Hepatotoxicity in Rats Without Inhibiting its Cytotoxic Activity. Int. J. Pharmacol.. 2019;15(4):465-477.
- [Google Scholar]
- Evaluation of the Effect of Pentoxifylline on Cisplatin-Induced Testicular Toxicity in Rats. Toxicol Res. 2017;33(3):255-263.
- [Google Scholar]
- Comparison of the preparation of PLGA–BSA nano-and microparticles by PVA, poloxamer and PVP. Colloids Surf A Physicochem Eng Asp. 2008;319(1–3):188-195.
- [Google Scholar]
- H, A. A. A. and G. E. B 2020. “Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: impact of resveratrol.” Endocr J 67(9), 969-980.
- Novel Pomegranate-Nanoparticles Ameliorate Cisplatin-Induced Nephrotoxicity and Improves Cisplatin Anti-Cancer Efficacy in Ehrlich Carcinoma Mice Model. Molecules. 2022;27(5)
- [Google Scholar]
- Efficacy of relaxin for cisplatin-induced testicular dysfunction and epididymal spermatotoxicity. Basic Clin Androl. 2020;30(1):3.
- [Google Scholar]
- Flavonoid fractions of diosmin and hesperidin mitigate lead acetate-induced biochemical, oxidative stress, and histopathological alterations in Wistar rats. Toxicol Res. 2021;37(4):473-484.
- [Google Scholar]
- Pomegranate: A promising avenue against the most common chronic diseases and their associated risk factors (Review) International Journal of Functional Nutrition. 2021;2(2):6.
- [Google Scholar]
- Pomegranate Seeds Extract Possesses a Protective Effect against Tramadol-Induced Testicular Toxicity in Experimental Rats. Biomed Res Int. 2020;2020:2732958.
- [Google Scholar]
- Pomegranate Juice Extract Decreases Cisplatin Toxicity on Peripheral Blood Mononuclear Cells. Medicines (Basel). 2020;7(10)
- [Google Scholar]
- Cisplatin-Induced Nephrotoxicity; Protective Supplements and Gender Differences. Asian Pac J Cancer Prev. 2017;18(2):295-314.
- [Google Scholar]
- TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest.. 2002;110(6):835-842.
- [Google Scholar]
- Cisplatin-induced testicular toxicity in rats: the protective effect of arjunolic acid. J Biochem Mol Toxicol. 2014;28(11):515-521.
- [Google Scholar]
- Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265-7279.
- [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
- [Google Scholar]
- Bancroft's theory and practice of histological techniques E-Book. Elsevier Health Sciences; 2018.
- Comparative nephrotoxicity of cisplatin and nedaplatin: mechanisms and histopathological characteristics. Journal of toxicologic pathology. 2011;24(2):87-94.
- [Google Scholar]
- Pomegranate and its Many Functional Components as Related to Human Health: A Review. Compr. Rev. Food Sci. Food Saf.. 2010;9(6):635-654.
- [Google Scholar]
- Counteracting Cisplatin-Induced Testicular Damages by Natural Polyphenol Constituent Honokiol. Antioxidants (Basel). 2020;9(8)
- [Google Scholar]
- Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomed.. 2015;10:6055.
- [Google Scholar]
- Anthocyanins from Pomegranate (Punica granatum L.) and Their Role in Antioxidant Capacities in Vitro. Chem Biodivers. 2021;18(10):e2100399.
- [Google Scholar]







