Translate this page into:
Detection of seed-borne fungal pathogens associated with wheat (Triticum aestivum L.) seeds collected from farmer fields and grain market
⁎Corresponding author. ziaghazali3@gmail.com (Hafiz Muhammad Zia Ullah Ghazali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The plant pathogens significantly affect quality and marketability of several cereal crops. Of these, seed-borne phytopathogens are responsible for quality and quantity losses in crops on commercial scale. The aim of this study was to isolate and detect the seed-borne mycoflora from seed lots of various bread wheat (Triticum aestivum L.) genotypes widely grown in Bahawalpur division of south Punjab, Pakistan.
Methods
Eleven commercial wheat cultivars/genotypes were investigated through standard blotter paper method. The samples were randomly collected from farmer fields and grain markets. The collected samples were brought to the laboratory and analyzed with different methods to detect the mycoflora.
Results
Twenty-one fungal species were recovered from seeds lots of diversified gene pool. The most frequently isolated fungi were Alternaria alternata, Fusarium monoliformae, Aspergillus flavus, Helminthosporium spp., Curvularia spp., Bipolaris sorokiniana, Phoma spp. and Penicillium spp. The one devasting fungal specie Alternaria alternata (88.8%) was the dominating species in all wheat genotypes included in the study.
Conclusion
These results divulge the presence of fungal pathogens in all wheat cultivars and could exert adverse effect on their allometric traits. So, there is a potential need to control these seed borne pathogens to minimize the crop yield losses.
Keywords
Ager plate method
Alternaria alternata
Blotter paper method
Mycoflora
1 Introduction
Wheat (Triticum aestivum L.) is a cereal crop and used as a staple food in several parts of the world. It is an essential cereal crop for the people of Pakistan and grown during winter from under irrigated conditions with water necessities range from 20 to 21 acre feet and is categorized first as vital food crop followed by rice and maize (Hussain et al., 2012). It yields the highest grain production as compared to any other crop in the world (Gulbitti-Onarici et al., 2009; Lamoureux et al., 2005). Foliar infections caused by fungal pathogens often have a negative impact on the yields of wheat. Necrotic regions appear on the leaf because of an infestation, and it may be challenging to identify the primary causal pathogen based only on the apparent symptoms, particularly in the case of two strains.
Wheat crop is affected by number of seed-borne pathogens, which can reduce its global production to significant extent. In Pakistan, 50 seed-borne phytopathogens are known to infest wheat. These phytopathogens, i.e., Alternaria alternata, Fusarium graminearum, Curvularia lunata, Bipolaris sorokinaina, Drechsleria, Helminthosporium sativum, Aspergillus and Penicillium are commonly account for mycoflora of wheat seeds. The wheat seeds affected by black point diseases involve Alternaria alternata, Curvularia lunata, Fusarium graminearum and Cladosporium. Bipolaris sorokinaina causes spot blotch in cereal grains and grasses, as it is the seed and soil-borne pathogen of wheat (Abdul Rehman, 2011; Hussain et al., 2013; Mehboob et al., 2015; Raza et al., 2014).
The awareness of the associated mycoflora with the specific frequency on commercially grown varieties runs with the source to access the threat related with unwanted organisms (Dinesh et al., 2015). For the controlling of plant diseases, the main step is to use disease-free and certified seeds. The germination test (Ozaslan et al., 2017) of seeds is substantial step in detecting the seed borne pathogen linked with wheat seeds and offers valuable information about mycoflora and their effective control (Anna, 2016; Majumder et al., 2013; Pathak and Zaidi, 2013).
The aim of study was to detect the mycoflora linked with wheat grains of eleven different wheat varieties available in the market by following International Seed Testing Association (ISTA) techniques. The results would help to provide the empirical information on the most infested genotypes and possible measures to control the pathogens associated with each genotype.
2 Materials and methods
The current study was conducted in the laboratory of Plant Pathology, Regional Agricultural Research Institute, Bahawalpur, Pakistan. Isolation, multiplication, and identification of fungi was carried out by using standard laboratory protocols as followed by ISTA.
2.1 Collection of wheat samples from grain market
The seed samples of eleven wheat genotype (i.e., ‘Gold-2016’, ‘Shafaq-2006’, ‘Millat-2011’, ‘Lasaani-2008’, ‘Punjab-2011’, ‘Sahar-2006’, ‘TD1’, ‘Bhakar-2002’, ‘Fareed-2006’, ‘Galaxy-2013’ and ‘Aas-2011’) were collected from six different wheat cultivated areas of Bahawalpur division of south Punjab in Pakistan. Similarly, three random samples were collected from local grain market according to standard sampling technique. The standard samples of 400 gm seed size were stored in craft paper bags and labeled.
2.2 Techniques used for isolation and detection of seed-borne mycoflora
For the isolation and detection of seed-borne mycoflora, the blotter paper (Habib et al., 2011; Mancini et al., 2016; Toma and Abdulla, 2013) and Agar plate method (Demeke et al., 2005; Ko et al., 2005) was followed. The collected seeds were surface sterilized with Clorox 1 % solution for 1–3 min, subsequently washed thrice with double distilled water (Butt et al., 2011; Chen et al., 2012; Habib et al., 2011).
2.3 Blotter paper method
The blotter paper method is the most popular and frequently used for the detection of several fungi, which can produce mycelial growth and fruiting structures during incubation. This method is most frequently used for the detection of seed-borne diseases. All collected seeds were plated in plastic Petri dishes, each having three layers of water soaked filter papers. The nine seeds per plate were incubated for seven days at 20–22 °C, for the period of 12 h under near the ultraviolet (NUV) light or in darkness. All slides were examined under the stereo-binocular microscope to detect the presence of mycoflora inside the seed (Abdullah and Atroshi, 2016; Butt et al., 2011).
2.4 Agar plate method
The ager plate method is used to identify and detect the microorganisms associated with seed bases on nutrient ager. The potato dextrose agar (PDA) was used in this method for the isolation of mycoflora. To obtain a better and reliable growth for various wheat mycoflora, PDA platting technique (Ahmad and Pathak, 2016; Tanveer et al., 2013) was followed. The modified PDA having (20 g potato extract, 20 g agar and 20 g dextrose) was prepared. All the medium was autoclaved and poured in 20 ml glass Petri dishes of 9-cm diameter. The nine seeds in each dish were plated with the help of sterile forceps. All Petri dishes were kept for 6 days at 20 °C under 12 h alternative cycles of near ultraviolet light and darkness. All the slides were examined under the stereoscopic microscope to observe the mycoflora inside the seed. Radial growth percentage of mycoflora prevalent inside the seed lots was also examined. The percentage and relative frequency of fungus was calculated by formula as described by Association of official seed analysts (Tanveer et al., 2013)
2.5 Identification of Soil-borne fungal microbes
All the corresponding samples were shifted into sterile Petri dishes for further analysis. All the cultured fungi were placed on slides for identification. All the slides covered with cover slip were stained with lactophenol-cotton blue to identify and measure the fungal structures. The identification and measurement of fungal structure with its colony morphology, spore formation and spore characteristics were examined under microscope and compared with already published literatures, identification keys and reference books (Guler et al., 2016; McClenny, 2007; Pornsuriya et al., 2008; Rønhede et al., 2005; Sanjotha et al., 2011; Tambekar and Wate, 2007).
3 Results
All fungi species associated with wheat samples were isolated by blotter paper method. The radial growth of all the isolated fungus were accomplished by PDA and its observance was by Ager plate method. The frequently isolated species included Alternaria alternata, Fusarium spp., Curvularia lunata, Penicillium, Aspergillus, Helminthosporium, Drechslera, Stemphylium and Phoma (Fig. 1).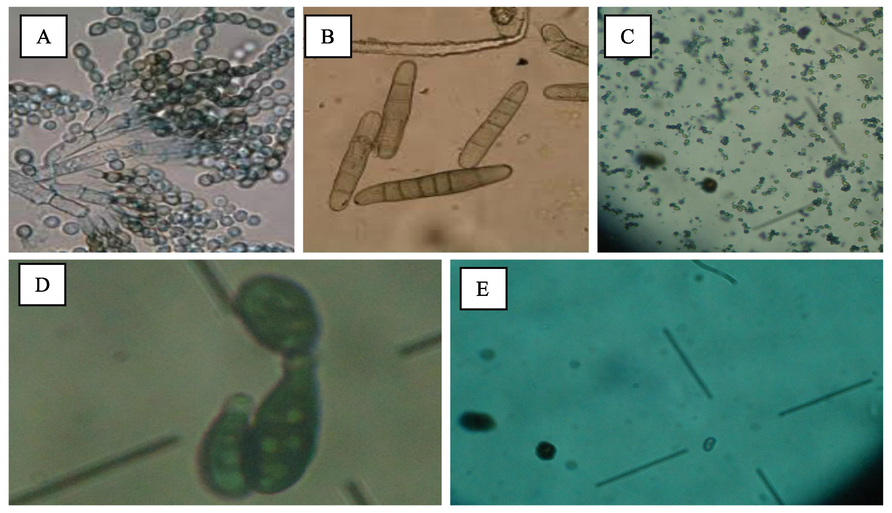
Mycoflora isolated from different wheat varieties, Penicillium (A), Drechslera (B), Aspergillus (C), Alternaria alternata (D) and Phoma (E).
From wheat cultivar ‘Gold-2016’, twenty-one fungal species were isolated including Alternaria alternata, A. tenuis, A. sesmicola, Fusarium monoliformae, Stemphylium, Phyllosticta, Curvularia, Hetrosporium, Penicillium and Cercospodium. The most frequently isolated species were A. alternata, A. sesmicola, Phyllosticta and Fusarium monoliformae (Table 1). The A. alternata, A. tenuis, Curvularia, Aspergillus, Penicillium, Fusarium monoliformae, Fusarium semitectum, Fusarium graminearum, Stemphylium and Bipolaris sorokiniana were isolated from genotype ‘TD1’. Most frequently isolated pathogens were A. alternata, A. tenuis, Bipolaris sorokiniana, Fusarium semitectum, F. monoliformae and Aspergillus (Table 1). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus, The S1-S6 denote samples collected from field, whereas S7-S9 are the samples collected from grain market.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
Gold 2016
1
Alterneria tenuis
+
+
−
+
+
+
+
−
+
77.7
2
Alterneria sesmicola
+
+
+
+
−
+
+
+
+
88.8
3
Alterneria alternate
−
+
+
+
+
+
+
+
+
88.8
4
Hetrosporium
+
+
−
−
+
−
−
−
+
44.4
5
Cercospodium
+
+
+
+
−
+
−
−
55.5
6
Curvularia
−
+
−
+
−
+
+
+
+
66.6
7
Phyllosticta
+
−
+
+
+
−
+
+
+
77.7
8
Stemphylium
−
+
−
+
+
−
−
+
+
55.5
9
Fusarium monoliformae
+
+
+
−
+
+
+
−
−
66.6
10
Pencillium
−
+
+
−
−
+
+
−
−
44.4
TD1
1
Fusarium semitectum
+
−
+
−
+
−
+
+
+
66.6
2
Fusarium monoliformae
+
+
−
+
+
+
−
+
+
77.7
3
Fusarium graminearum
+
−
+
+
−
−
+
−
−
44.4
4
Alternaria alternate
+
+
+
+
+
+
−
+
+
88.8
5
Drechslera
−
+
+
−
−
−
+
+
−
44.4
6
Alternaria tenuis
+
+
−
+
+
+
+
+
+
88.8
7
Bipolaris sorokiniana
+
+
+
−
−
+
+
+
+
77.7
8
Curvularia
+
−
−
+
+
−
+
+
+
66.6
9
Stemphylium
+
+
−
−
−
+
−
−
−
33.3
10
Pencillium
−
−
+
−
−
+
−
+
−
33.3
11
Aspergillus
+
−
+
+
+
+
+
−
+
77.7
The isolated fungal species from the cultivar ‘Millat-2011’ were A. alternata, A. tenuis, Phoma, Bipolaris sorokiniana, Stemphylium, Phyllosticta, Gonatoboryum, F. monoliformae, Curvularia and Penicillium. The most frequently isolated fungi included A. alternata, A. tenuis, Phyllosticta and F. monoliformae (Table 2). In ‘Shafaq-2011’ cultivar, the isolated pathogens include A. alternata, A. tenuis, Bipolaris, F. monoliformae, F. graminearum, F. oxysporum, Helminthosporium, Curvularia and Stemphylium. The most frequently isolated pathogens were A. alternata, A. tenuis and F. oxysporum (Table 2). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus, The S1-S6 denote samples collected from field, whereas S7-S9 are the samples collected from grain market.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
Millat-2011
1
Alterneria alternate
+
+
+
+
+
+
+
−
+
88.8
2
Alterneria tenuis
+
+
+
−
+
+
−
+
+
77.7
3
Bipolaris
+
+
−
+
−
+
+
+
−
66.6
4
Stemphylium
−
+
+
−
+
−
+
+
+
66.6
5
Phoma
+
+
−
+
−
+
−
−
+
55.5
6
Phyllosticta
+
−
+
+
+
−
+
+
+
77.7
7
Gonatoboryum
+
+
−
+
−
+
+
−
−
55.5
8
Fusarium monoliformae
+
−
+
−
+
−
+
+
+
66.6
9
Curvularia
+
−
+
+
−
−
−
+
−
44.4
10
Pencillium
+
+
−
−
−
+
+
−
−
44.4
Shaafaq-2011
1
Alterneria alternate
+
+
+
+
+
+
+
+
−
88.8
2
Alterneria tenuis
+
+
−
+
+
+
+
+
+
77.7
3
Bipolaris sorokiniana
−
−
+
−
+
−
+
+
+
55.5
4
Fusarium monoliformae
+
+
−
+
−
−
+
−
+
55.5
5
Fusarium gramiearum
−
−
+
+
+
+
−
+
+
66.6
6
Fusarium oxysporum
+
−
−
−
+
+
−
−
−
33.3
7
Helminthosporium
−
+
+
+
+
+
+
+
+
88.8
8
Curvularia
+
−
−
+
+
+
+
+
−
66.6
9
Stemphylium
+
+
−
+
+
+
−
−
+
66.6
The pathogens identified from cultivar ‘Fareed-2006’ were A. alternata, A. tenuis, F. monoliformae, F. graminearum, B. sorokiniana, Drechslera, Curvularia, Helminthosporium and Penicillium. The most frequently isolated pathogens were A. alternata, A. tenuis, Fusarium spp and Drechslera (Table 3). Similarly, the isolated pathogens from ‘Sehaar-2006’, include A. alternata, Helminthosporium, Brasilaria, Paecilomyces, Sclers, Curvularia, Stemphylium and B. sorokiniana. The most frequently isolated pathogens include A. alternata, Curvularia and B. sorokiniana (Table 3). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus, The S1-S6 denote samples collected from field, whereas S7-S9 are the samples collected from grain market.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
Fareed-2006
1
Alterneria alternata
+
+
+
+
+
+
−
+
+
88.8
2
Alterneria tenuis
+
+
+
+
+
+
+
+
−
88.8
3
Fusarium monoliformae
+
−
+
+
−
+
+
−
+
66.6
4
Fusarium gramiearum
+
−
+
+
−
+
−
+
+
66.6
5
Bipolaris sorokiniana
+
+
−
−
+
−
+
+
−
55.5
6
Helminthosporium
+
+
−
−
+
−
−
+
−
44.4
7
Curvularia
+
−
+
−
−
−
−
−
−
22.2
8
Drechsleria
+
−
+
+
+
−
−
+
+
66.6
9
Pencillium
+
−
+
−
+
−
−
+
+
55.5
Sehar-2006
1
Alterneria alternata
+
+
+
+
+
+
−
+
+
88.8
2
Helminthosporium
+
+
+
−
−
+
+
−
+
66.6
3
Brasilaria
−
+
−
−
−
−
+
−
+
33.3
4
Paecilomyces
+
−
−
+
+
+
−
+
+
66.6
5
Sclers
+
+
−
−
−
+
−
+
−
44.4
6
Curvularia
+
−
+
+
+
+
+
+
+
88.8
7
Stemphylium
+
−
+
+
+
+
+
+
+
88.8
8
Bipolaris sorokiniana
+
+
+
+
+
+
+
−
−
77.7
The isolated pathogens from ‘Lasaani-2008’ cultivar were A. alternata, A. tenuis, A. sesmicola, F. semitectum, F. monoliformae, and B. sorokiniana, Trincrium, Drechslera and Helminthosporium. The most frequently isolated pathogens in this cultivar included A. spp, F. monoliformae and Helminthosporium (Table 4). From ‘Punjab-11’, the isolated pathogens included A. alternata, A. tenuis, Sclerochia, Drechslera, F. monoliformae, Stemphylium, Helminthosporium, Curvularia and B. sorokiniana. The most frequently isolated fungi included Alternaria spp, F. monoliformae and Helminthosporium (Table 4). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
Lasani-2008
1
Alterneria alternate
+
+
+
+
+
+
+
−
+
88.8
2
Alterneria tenuis
+
+
+
+
+
+
+
+
−
88.8
3
Alterneria sesmicola
+
+
−
+
+
+
+
+
+
77.7
4
Fusarium semitectum
+
+
−
−
+
+
−
+
+
66.6
5
Fusarium monoliformae
−
+
+
+
+
−
+
+
+
77.7
6
Bipolaris sorokiniana
+
−
+
+
−
+
+
−
−
55.5
7
Trincrium
+
+
+
−
+
−
−
−
+
55.5
8
Drechslera
+
+
+
−
−
−
+
−
−
44.4
9
Helminthosporium
+
−
+
+
+
+
+
+
+
88.8
Punjab-2011
1
Alterneria alternata
+
+
+
+
+
+
−
+
+
88.8
2
Sclerochia
+
+
+
−
+
−
+
+
+
77.7
3
Drechsleria
−
+
−
−
+
−
+
−
+
44.4
4
Stemphylium
+
−
+
+
+
−
−
+
−
55.5
5
Helminthosporium
+
+
−
+
+
+
+
+
+
88.8
6
Alterneria tenuis
+
−
+
+
+
+
+
+
+
88.8
7
Fusarium monoliformae
+
−
+
+
+
+
+
+
+
88.8
8
Bipolaris sorokiniana
−
+
+
+
−
+
+
+
−
66.6
9
Curvularia
+
−
+
+
−
+
+
+
+
77.7
From ‘Bhakkar-2002’ wheat cultivar, the isolated fungi included A. alternata, A. tenuis, A. sesmicola, F. monoliformae, F. graminearum, B. sorokiniana, Curvularia. Helminthosporium, Penicillium and Aspergillus. The most frequently isolated fungi were Alternaria spp. and Bipolaris sorokiniana (Table 5). In ‘Galaxy-2013’, the isolated fungi included Fusarium monoliformae, F. nivale, A. alternata, A. tenuis, Phoma, B. sorokiniana, Helminthosporium and Drechslera. The most frequently isolated pathogens included A. alternata, F. monoliformae and B. sorokiniana (Table 5). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus, The S1-S6 denote samples collected from field, whereas S7-S9 are the samples collected from grain market.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
Bhakar-2002
1
Alterneria alternate
+
+
+
+
−
+
+
+
+
88.8
2
Alterneria tenuis
+
−
+
+
−
+
+
+
+
77.7
3
Alterneria sesmicola
+
−
+
+
−
−
+
+
−
55.5
4
Fusarium monoliformae
+
+
−
−
+
−
+
−
+
55.5
5
Fusarium gramiearum
+
+
−
−
+
−
−
+
−
44.4
6
Bipolaris sorokiniana
−
+
+
+
−
+
+
+
+
77.7
7
Curvularia
+
−
−
−
−
+
+
−
+
44.4
8
Helminthosporium
+
−
+
−
+
−
+
−
−
44.4
9
Aspergillus
+
−
+
+
+
−
−
+
+
66.6
10
Pencillium
+
−
+
−
+
−
−
+
+
55.5
Galaxy-2013
1
Fusarium monoliformae
+
+
+
+
−
+
−
+
−
66.6
2
Fusarium nivale
+
+
+
−
−
−
−
−
−
33.3
3
Alternaria alternata
+
+
−
+
+
+
+
+
+
88.8
4
Alternaria tenuis
+
−
−
−
+
+
+
+
+
66.6
5
Phoma
−
−
+
−
+
+
−
−
−
33.3
6
Bipolaris sorokiniana
+
+
+
+
+
−
+
−
+
77.7
7
Helminthosporium
−
−
−
+
−
+
+
−
+
44.4
8
Drechsleria
+
+
+
−
+
+
−
−
+
66.6
Furthermore, the isolated seed borne pathogens from ‘Aas-2011’ included A. alternata, A. tenuis, Phoma, B. sorokiniana, Stemphylium, Phyllosticta, Gonatoboryum, F. monoliformae, Curvularia and Penicillium. The most frequently isolated pathogens were A. alternata, Penicillium, F. monoliformae and B. sorokiniana (Table 6). S = Sample, + = Presence of particular fungus, − = Absence of particular fungus, The S1-S6 denote samples collected from field, whereas S7-S9 are the samples collected from grain market.
Sr. No.
Pathogens isolated
S1
S2
S3
S4
S5
S6
S7
S8
S9
%
1
Alterneria alternate
+
+
+
+
+
+
−
−
+
77.7
2
Alterneria tenuis
+
−
−
+
−
+
+
+
+
66.6
3
Bipolaris
+
+
+
−
+
−
+
+
−
66.6
4
Stemphylium
−
+
+
−
−
+
+
+
−
55.5
5
Phoma
+
−
−
+
−
+
−
−
+
44.4
6
Phyllosticta
+
−
−
+
+
−
−
−
+
44.4
7
Gonatoboryum
+
−
+
+
+
−
+
−
−
55.5
8
Fusarium monoliformae
+
+
+
+
+
+
+
−
+
88.8
9
Curvularia
−
+
+
−
+
+
+
−
+
66.6
10
Pencillium
+
+
−
−
+
+
+
+
+
77.7
4 Discussion
During this study of seed-borne pathogens, different species of mycoflora, i.e., Alterneria alternata, Alterneria tenuis, Fusarium monoliformae, Fusarium graminearum, Bipolaris sorokiniana, Curvularia, Helminthosporium, Aspergillus, Penicillium, Phoma, Stemphylium, Trincrium, Drechslera, Brasilaria, Paecilomyces and Sclers were identified and isolated. This study was in line with the findings of Hussain et al. (2013), who isolated seed-borne mycoflora including A. alternata, B. sorokiniana, Aspergillus flavus and Aspergillus niger from different commercial wheat varieties. Similarly, the genotypes ‘Gold-2016’, ‘TD1’, ‘Shafaq-2011’ and ‘Millat-2011’ were mostly infected by A. alternata (88 %), A. tenuis (77 %), Helminthosporium (70 %), Curvularia (77 %), F. monoliformae (60 %), Aspergillus (44 %) and Penicillium (55 %). Remaining genotypes including ‘Punjab-11’, ‘Sehar-2006’, ‘Lasani-2008’, ‘Fareed-2006’, ‘Aas-2011’, ‘Bhakar-2002’ and ‘Galaxy-2013’ were also infected by wheat pathogens. In general, the results of our research indicate that mixed infections of two or more pathogens were widespread, despite the fact that these infections might be missed by eye examination.
This discovery unquestionably has repercussions for the control of wheat illnesses in the future and may also be applicable to the cultivation of other crops. Fungal infections have disastrous repercussions on both human and animal health as well as global food security. Fungal diseases result in losses for cereal crops connected with decreased production yield and harmed grain quality. These effects result in yearly losses for producers and farmers in the billions of dollars, as well as higher prices for consumers. Global activities concentrate on integrated breeding techniques to enhance resistance, cutting-edge monitoring and detection technologies, and molecular understanding to find disease biomarkers to eliminate fungal infections of cereal crops. However, such studies are less concentrated in Pakistan.
In the earlier studies (Hajihasani et al., 2012; Majumder et al., 2013; Mobasser et al., 2012), a total of fifteen different fungal species were isolated from seeds. These species included Tilletia laevis, T. tritici, Ustilago tritici, F. graminearum, F. culmorum, Microdochium nivale, B. sorokiniana, A. alternata was determined to be the predominant fungus that was isolated from the black pointed seeds in the prior investigation (Fernandez and Conner, 2011). In the same vein, the amount of infection caused by Fusarium spp. was 60 %.
The A. niger and Penicillium spp. have been reported to reduce the seed germination and seed loss during storage (Ijaz et al., 2001; Jamadar and Chandrashekhar, 2015). The A. alternata and Curvularia lunata cause delay in seed germination due to rot of seeds. Aspergillus flavus produce toxic chemicals that results in decrease of shoot and root elongation (Devi and Jesumaharaja, 2020; Hussain et al., 2013).
5 Conclusion
It is concluded that several seed-borne pathogens are responsible for quality and quantity losses in tested wheat genotypes. Most of the isolated pathogens are soil-borne and some have association with seeds. The maximum efforts should be taken to use disease-free seeds to minimize the quality and quantity losses. This study demonstrated that isolated fungal species have significant relationship in term of causing diseases in wheat and can lead to changes in rhizosphere. This high inoculum density and maximum fungal biomass in wheat can act as substantial indicator of maximum disease development. This increased fungal abundance association in seeds of wheat can contribute to maximum fungal saprophytes.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP2023R5) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mycobiota associated with grains of soft wheat (Triticum aestivm L.) cultivars grown in Duhok Province, Kurdistan region. Iraq. J. Agric. Technol.. 2016;12:91-104.
- [Google Scholar]
- Antifungal Potential of Plant Extracts against Seed-borne Fungi Isolated from Barley Seeds (Hordeum vulgare L.) J. Plant Pathol. Microbiol. 2016
- [CrossRef] [Google Scholar]
- Evaluation of selected seed treatment methods for the control of Fusarium graminearum and F. avenaceum on wheat seeds. Int. J. Agric. Technol. 2016
- [Google Scholar]
- Seed-borne mycoflora of stored rice grains and its chemical control. J. Anim Plant Sci. 2011
- [Google Scholar]
- Isolation and identification of endophytic and mycorrhizal fungi from seeds and roots of Dendrobium (Orchidaceae) Mycorrhiza 2012
- [CrossRef] [Google Scholar]
- Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food Microbiol. 2005
- [CrossRef] [Google Scholar]
- Seed-Borne Alternaria helianthi Leaf Blight in Sunflower. In: Advances in Seed Production and Management. Springer; 2020. p. :325-342.
- [Google Scholar]
- Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res.. 2015;173:34-43.
- [CrossRef] [Google Scholar]
- Phylogenetic relationships of some wild wheat species based on the internal transcribed spacer sequences of nrDNA. Curr. Sci. 2009
- [Google Scholar]
- Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol. Plant.. 2016;38:1-9.
- [Google Scholar]
- Prevalence of seed-borne fungi on wheat during storage and its impact on seed germination. Pak. J. Phytopathol. 2011;23:42-47.
- [Google Scholar]
- Incidence and distribution of seed-borne fungi associated with wheat in Markazi Province. Iran. African J. Biotechnol.. 2012;11:6290-6295.
- [Google Scholar]
- Seed Borne Mycoflora of Some Commercial Wheat (Triticum aestivum L.) Cultivars in Punjab, Pakistan. Int. J. Phytopathol. 2013
- [CrossRef] [Google Scholar]
- Technical efficiency of wheat production in rain-fed areas: A case study of Punjab, Pakistan. Pakistan J. Agric. Sci. 2012
- [Google Scholar]
- Seed borne pathogens associated with wheat seed and their role in poor germination. Pakistan J. Phytopathol.. 2001;13:102-106.
- [Google Scholar]
- Effect of chemical and biological seed treatments on germination performance of GCH-7 hybrid castor (Ricinus communis L.) The Bioscan. 2015;10:37-41.
- [Google Scholar]
- A simple method for detection of lipolytic microorganisms in soils. Soil Biol. Biochem. 2005
- [CrossRef] [Google Scholar]
- The efficacy of Cot-based gene enrichment in wheat (Triticum aestivum L.) Genome 2005
- [CrossRef] [Google Scholar]
- Diagnostic methods for detecting fungal pathogens on vegetable seeds. Plant Pathol 2016
- [CrossRef] [Google Scholar]
- McClenny, N., 2007. An Unusual Aspergillus Species at a Major Cancer Center: Implications for the Clinical Laboratory San Francisco State University, San Francisco, CA Course DL-977 1.0 CE/Contact Hour Level: Beginning to Intermediate. MPA, MT.
- Mehboob, S., Rehman, A., Ali, S., Idrees, M., Zaidi, S.H., 2015. Detection of Wheat Seed Mycoflora With Special Referance To Drechslera Sorokiniana. Pakistan J. Phytopathol.
- Wheat seed contamination with seed-borne diseases in cold climatic zone of Iran. Int. J. Plant. Prod. 2012
- [Google Scholar]
- Germination biology of two invasive physalis species and implications for their management in arid and semi-arid regions. Sci. Rep.. 2017;12:1-12.
- [Google Scholar]
- Studies on seed-borne fungi of wheat in seed health testing programme. Arch. Phytopathol. Plant Prot.. 2013;46:389-401.
- [Google Scholar]
- New record of Chaetomium species isolated from soil under pineapple plantation in Thailand. J. Agric. Technol.. 2008;4:91-103.
- [Google Scholar]
- Characterization and pathogenicity of Biplolaris sorokiniana caused spot blotch of wheat in Pakistan. FUUAST J. Biol.. 2014;4:97-100.
- [Google Scholar]
- Abdul Rehman, 2011. Study of most prevalent wheat seed-borne mycoflora and its effect on seed nutritional value. African J. Microbiol. Res. https://doi.org/10.5897/ajmr11.348.
- Hydroxylation of the herbicide isoproturon by fungi isolated from agricultural soil. Appl. Environ. Microbiol.. 2005;71:7927-7932.
- [Google Scholar]
- Isolation and screening of efficiency of phosphate solubilizing microbes. Int. J. Microbiol. Res.. 2011;3:56-58.
- [Google Scholar]
- Study of phosphate solubilization efficiencies of fungi and bacteria isolated from saline belt of Puma river basin. Res. J. Agric. Biol. Sci.. 2007;3:701-703.
- [Google Scholar]
- Influence of seed size and ecological factors on the germination and emergence of field bindweed (Convolvulus arvensis) Planta daninha. 2013;31:39-51.
- [Google Scholar]
- Isolation and identification of fungi from spices and medicinal plants. Res. J. Environ. Earth Sci. 2013
- [CrossRef] [Google Scholar]







