Translate this page into:
Green synthesis of Moringa oleifera leaf nanoparticles and an assessment of their therapeutic potential
⁎Corresponding authors at: King Abdullah Institute for Nanotechnology, King Saud University, Riyadh 11451, Saudi Arabia bvirk@ksu.edu.sa (Promy Virk), mawad@ksu.edu.sa (Manal A. Awad), mfelkhadragy@pnu.edu.sa (Manal F. El-Khadragy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The study highlights the bio fabrication of stable Moringa oleifera leaf nanoparticles fostering a ‘green’ approach in synthesis sans the use of chemical surfactants/reducing agents. The phytochemical screening of the synthesized nanoparticles showed an enhanced phenolic and flavonoid content that demonstrated a potent antioxidative activity. The nanoparticles exhibited profound anti-diabetic and antiproliferative activity against human cancer lines. Taken together the findings broaden the horizons on the prospective use of these nanoparticles as a nutraceutical/drug in the field of biomedicine.
Abstract
The present study centered around the prospect of bio fabricating nanoparticles using Moringa oleifera leaf and investigating their therapeutic potential with an assessment of the antioxidant status. Synthesis of stable ‘green’ nanoparticles of Moringa leaf extract is successfully reported with an extensive characterization. The average particle size and polydispersity index (PDI) of the nanoparticles obtained by Dynamic light scattering (DLS) analysis were 141.6 nm and a polydispersity index (PDI) of 0.32 respectively. The UV–visible spectroscopy (UV–vis) revealed a strong red absorbance peak at 664 nm,characteristic of chlorophyll. Electron micrographs confirmed that particles were in the nano range and were spherical with minimal agglomeration. The constituent functional groups were assessed by Fourier transformed infrared spectroscopy (FTIR). The X-ray diffractograms of reflected both the amorphous and crystalline domains.Furthermore, the phytochemical screening showed a higher phenolic/ flavonoid content of the Moringa nanoparticles (NMo) in comparison to the bulk Moringa leaf extract(Mo). These results corresponded to a potent antioxidant status of the NMo which was assessed by three complementary antioxidant assays. The therapeutic potential of the nanoparticles was evaluated based on in vitro anti-diabetic assays and cytotoxicity assay on human cancer cell lines (MCF-7 and HepG-2). The key findings suggest a more profound therapeutic efficacy of NMo than the Moringa leaf extract.
Keywords
Moringa oleifera
Green synthesis
Antioxidant
Phytochemical profile
Anti-diabetic
Anticancer

1 Introduction
Moringa oleifera, also known as drumstick tree is a member of the Moringaceae family and is also called the “Miracle Tree” (Mahato et al., 2022). Being a native to north-western India it has been well recognized both in the Ayurveda and Unani systems of medicine (Milla et al., 2021) and in ethanomedicine as well. Its diverse therapeutic benefits attributed to the constituent bioactive phytochemicals in its leaves, pods, and seeds have garnered attention in pharmacology (Palizban et al., 2015; Mahato et al., 2022). It is one of the most widely used functional foods in the current times being nutrient dense that adds to its therapeutic value. Additionally, Moringa is an important versatile tree species for agroforestry as it survives under a variety of meteorological conditions (Mridha, 2015)and is thus cultivated in many tropical and sub-tropical countries including Saudi Arabia (Alaklabi, 2015). The leaves and the pods have been a part of the Indian diet since ancient times. The pharmacological benefits of the leaves are extensively reported in previous literature (Irfan et al., 2021). During the past decade several experimental studies have evaluated the phytochemical profile of the Moringa leaves emphasizing on their antioxidant potency. The appreciable amount of ascorbic acid, vitamins A, B, α -tocopherol, β-carotene, β-sitosterol, protein, and specifically essential amino acids present in Moringa leaves and pods attribute to is status of an ideal nutraceutical (Ali et al.2018). The dried leaves of Moringa are also endowed with polyphenols such as flavonoids and phenolic acids. The key concept of scientific development in the current times is human wellbeing and health. The buzz word is ‘green’ which in green technology refers to the use of plant products/phytocompounds. An offshoot of green technology is green nanotechnology, a key technology of the century. This includes a nanotechnological approach in phyto formulation of pharmacologically important bioactive constituents in herbs (Verma et al., 2019). The fundamental problem of some these bioactive compounds being insoluble and prone to rapid degradation in the biological milieu is effectively solved which enhances their bioavailability and therapeutic efficacy. Literature is replete with studies on plant based, both metallic and non-metallic nanoparticles making the bio-based nano synthesis, environmentally safe, facile and cost effective (Verma et al.,2018). Furthermore, recent studies (Matinese et al., 2017;Moodely et al., 2018;Amina et al., 2019; Venkatachalam et al., 2021) have reported the use of M.oliefera extracts as capping and reducing agent in the biosynthesis of metallic nanoparticles. Nevertheless, the nanoformulation of the Moringa leaf extract in its pure form without the use of chemical surfactants and metals has not been reported.
Considering this premise, the current study included the novel synthesis of nanoparticles sans any chemical surfactants or stabilizers followed by in vitro bioassays to evaluate their anti-diabetic and anti-carcinogenic potency.
2 Materials and methods
2.1 Synthesis of moringa nanoparticles
Moringa leaves were dried and 400 mg dried leaf powder was then added to 20 mL of methanol. Thereafter, this solution was sprayed at a rate of 0.2 mL/min for 5 min into 50 mL of boiling water under ultrasonic conditions(power of 750 W and a frequency of 20 kHz). After sonicating for 5 min the contents were stirred at room temperature at 200–800 rpm for approximately 20 min. The solution was concentrated and then freeze-dried.
2.2 Characterization of the nanaoparticles
An extensive characterization of nanoparticles included the following techniques; UV–visible spectrophotometer(Perkin Elmer, Japan) within a wavelength range of 200–800 nm, Dynamic light scattering (DLS) technique by Zeta sizer (ZEN 3600, MALVERN, United Kingdom), Fourier-transform infrared spectroscopy (FTIR) (Perkin-Elmer FTIR-1600, USA)within the range of 500–4000 cm−1 and a resolution of 4 cm−1. X-ray diffraction (PAN analytical X PRT PRO, D-8, Advanced Brucker instrument (Netherlands) and Transmission electron microscopy (TEM) (JEM-1011, JEOL, Japan).
2.3 Phytochemical screening
Phytochemical examinations were carried out for the Moringa leaf extract and the nanoparticles as per the standard tests. Total phenol content was quantitated using the Folin Ciocalteu reagent with gallic acid as the standard (Siddiqui et al., 2017). Colorimetric assays were used to quantitate the total flavonoid (Fattahi et al., 2014). Total flavonoids were expressed as catechin and rutin in milligrams equivalents per gram of sample (mg/g) in the two assays.
2.4 Determination of in vitro antioxidant activity
The antioxidative status of the Moringa leaf extract and its nanoparticles was measured by three complementary tests.
2.4.1 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) radical scavenging activity
ABTS radical-scavenging activity of the Moringa leaf extract and its nanoparticles was evaluated in accordance with the protocol given by Re et al. with some modifications(1999). Trolox was used as a standard for the assay and the optical density(OD) was recorded at 734 nm. Results were expressed in terms of g trolox equivalent per g of dry weight (g eq. trolox/g).
2.4.2 α, α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging activity
This test was in accordance with the standard protocol by Adebiyi et al. (2017). The OD of the solutions was recorded at 515 nm. The DPPH radical scavenging activity was illustrated as percentage inhibition and computed as follows:
DPPH scavenging activity(%)=(A0-A1)/A0 × 100, where A0 is the OD of the control and A1 is the OD of the sample.
2.4.3 Ferric reducing antioxidant power (FRAP) assay
A modified form of the FRAP assay as previously reported by Benzie and Strain(1996) was used in the study. Trolox was used as a standard for the assay and the absorbance of the samples was measured at 593 nm. The FRAP results were expressed in terms of g trolox equivalent per g of dry weight.
(g eq.trolox/ g).
2.5 Cytotoxicity assay
The human cancer cell lines (MCF-7;human breast cancer and HepG-2;human hepatocellular carcinoma) were procured from VACSERA Tissue Culture Unit(Giza,Egypt).
Cytotoxicity was evaluated by the viability assay as per the standard protocol (Riss et al.,2013).Cell control without the tested compounds used to compare the treated cells. The optical density (SunRise, TECAN, Inc, USA) assessed the number of viable cells and their percentage was computed as [(ODt/ODc)]x100% where ODt is the mean optical density of wells treated with the tested sample and ODc is the mean optical density of control cells. The dose response curve estimated the IC50 (50 % inhibitory concentration) using the GraphPad Prism software (San Diego, CA. USA).
2.6 In-vitro antidiabetic assays
α-glucosidase inhibitory activity of Moringa leaves and the nanoparticles was evaluated following the standard method with minor modifications (Shai et al. 2011). Further, α-amylase inhibitory activity was determined in accordance with the standard protocol (Heidari et al., 2005). Acarbose at various concentrations 1000 to 7.81 μg/mL) was the standard for both the assays. Inhibitory activity (%) = (1 − As/Ac) × 100) was calculated, and expressed as mean ± standard deviation and IC50 values were quantified using GraphPad.
3 Results
3.1 Characterization of Moringa oleifera nanoparticles
UV–vis spectroscopy was scanned in the range of 200–800 nm. The absorption spectra of moringa leaf extract (Mo) (as a control) and synthesized nano Moringa (NMo) showed a strong red absorbance peak at 664 nm which is distinctive of chlorophyll common to both nano Moringa and Moringa leaf extract (Fig. 1). The formed nanoparticles demonstrate mean diameter of 141.6 nm with a polydispersity index (PDI) of 0.32 (Fig. 2). The TEM micrographs (Fig. 3 A and B), illustrate spherical nanoparticles with diverse sizes. In addition, the nanoparticles (NMo) were covered by a layer with minimal agglomeration. Fig. 4, shows the FTIR spectra for the bulk Moringa leaves and the nanoparticles.The absorption peaks at 3401.36 cm−1, 3401.10 cm−1, 2925.92 cm−1 and 2925.88 cm−1 respectively, were elongated and sharpened, this could be due to the O—H, C—H stretching off –C⚌O and/or –CH3 groups. At 1655.04 cm−1 and 1654.52, respectively, an elongation was observed due to the C⚌O group of the carboxylic acids being stretched. It was also observed that the peak at 1059.93 cm−1 was shifted to 1062.07 cm−1 and the peak at 1239.69 cm−1 to 1236.79 cm−1 which could be due to the stretching of C—O. Peak at 1319.02 cm−1 also was elongated due to the bending in the N⚌O (Fig. 4).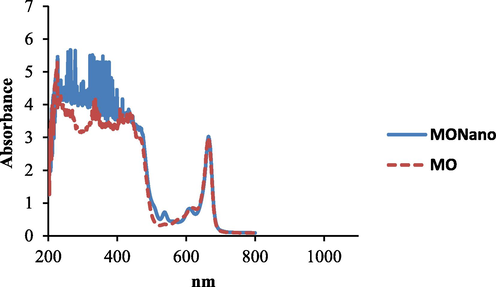
UV–vis spectra of synthesized nanoparticles moringa and moringa leaf extract.
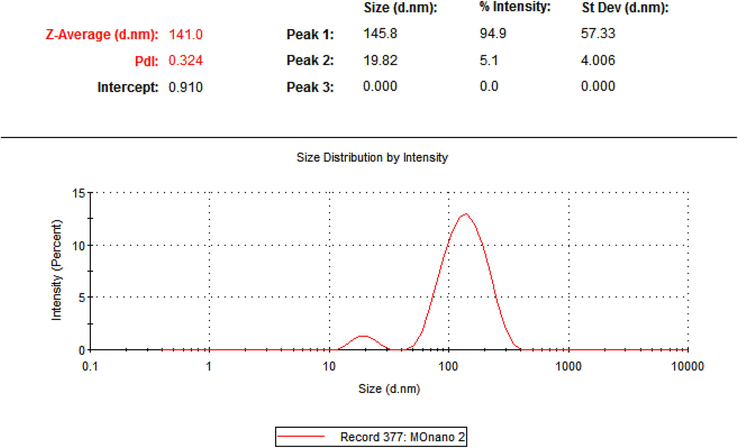
The DLS graph showing the average particle size of the nano particles with size distribution.
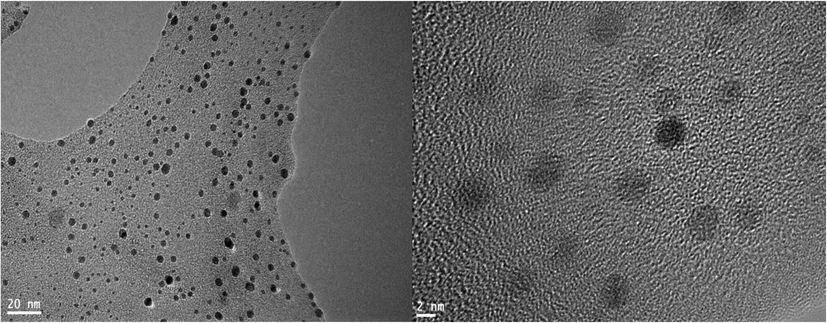
TEM micrographs showing the Moringa leaf nanoparticles.
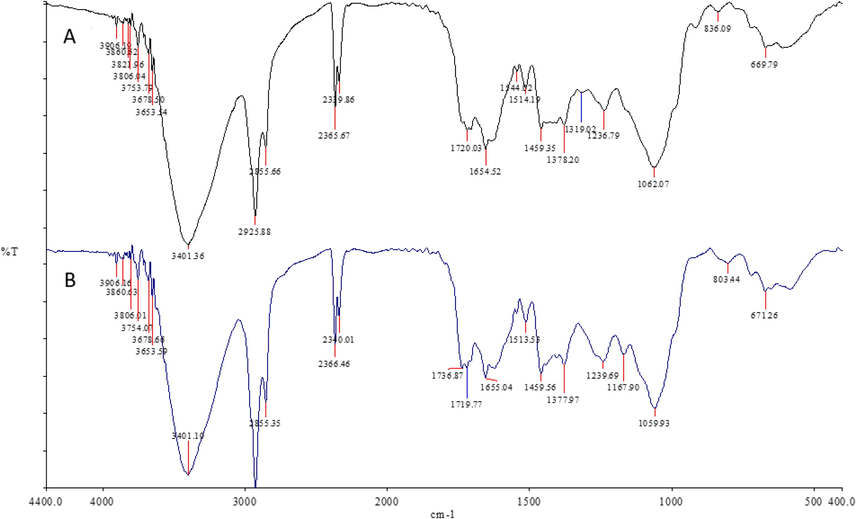
FTIR spectra of (A) Moringa nanoparticles and (B) Moringa leaf extract.
To assess the crystalline nature of the Mo and, nano-moringa NMo powder, the XRD analysis was conducted. The X-ray diffractograms of Mo and NMo showed broad five low intensity distinct diffraction characteristic peaks at 2 Theta (2θ) values of 21°, 37 ͦ,43 ͦ,77 ͦ, and 810°, reflecting both the amorphous and crystalline domain (Fig. 5).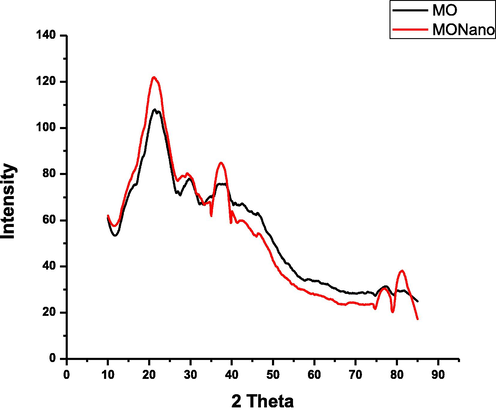
X-ray diffractograms Moringa nanoparticles and Moringa leaf extract.
3.2 Phytochemical profile and in vitro antioxidative activity
The analysis shows that the phytochemical content; total phenols, total flavonoids was higher in the NMo than the leaf extract (Mo) (Table 1). Furthermore, the ability of the Mo and the NMo to scavenge the reactive oxygen species (ROS) was evaluated by three complementary in vitro antioxidant assays. In the DPPH assay, the NMo showed a notably higher radical scavenging activity with a percent inhibition of 80.630 ± 0.545 in comparison to the leaf extract (Mo)(37.611 ± 0.472). A higher antioxidant activity was observed in the ABTS assay for NMo; 57.750 ± 2.385 g equiv.Trolox/g sample as compared to the Mo; 4.983 ± 0.101 g equiv.Trolox/g sample. The results of the FRAP assay showed a higher value for NMo(8.989 ± 0.671 equiv.Trolox/g sample) as compared to Mo (1.989 ± 0.123 g equiv.Trolox/g sample).The values observed for the Mo and nanoparticles NMo were consistent within all three assays (Table 1). Values are means of four replicates. Results are given as mean ± SD.
T.Phenols (mg Gallic acid /g sample)
T. Flavonoids (mg Catachin /g sample)
T. Flavonoids (mg Rutin /g sample)
DPPH (%)inhibition
ABTS (g Trolox/g sample)
FRAP (g Trolox/g sample)
Mo
149.702 ± 1.510
0.303 ± 0.119
3.809 ± 1.319
37.611 ± 0.472
4.983 ± 0.101
1.989 ± 0.123
NMo
455.621 ± 2.008
6.777 ± 0.207
57.130 ± 2.285
80.630 ± 0.545
57.750 ± 2.385
8.989 ± 0.671
3.3 Bioassays
3.3.1 Cytotoxicity
As illustrated in Figs. 6 and 7, a cell survival significantly declined in both MCF-7 and HepG-2 cancer cell lines when treated with Mo and NMo. For MCF-7, an IC50 of 168 ± 27.04 µg/ml was observed for Mo (Fig. 6A). The inhibitory activity of MoN was significantly higher when compared to Moringa leaf powder, with an IC50 of 65.6 ± 13.97 µg/ml (Fig. 6B). Additionally, for the HepG-2 cell line, an IC50 of 113.5 ± 6.59 µg/ml was observed for Moringa leaf powder (Mo). The inhibitory activity of NMo showed a significant increase in comparison to Mo, with an IC50 of 53.4 ± 1.93 µg/ml (Fig. 7 A and B). Taken together, the results the study strongly suggest a “switching off” of cancer cell survival mechanisms under the Moringa leaf treatments, being more profound with the nanoformulation.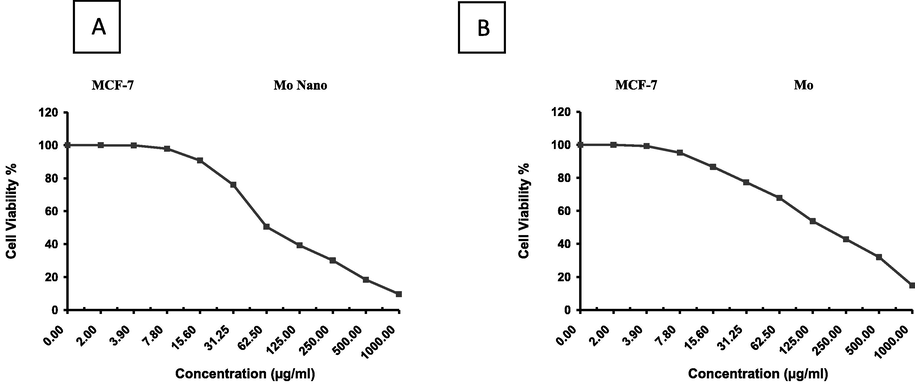
Effect of variable concentrations of nanoparticles of (A)Moringa leaf extract(NMo) and (B)Moringa leaf extract(Mo) on the cell viability (%) of MCF-7 cell line.
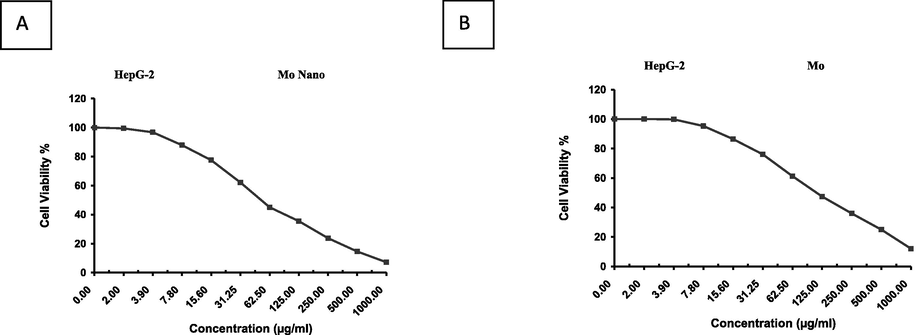
Effect of variable concentrations of nanoparticles of (A)Moringa leaf extract(NMo) and (B) Moringa leaf extract(Mo) on the cell viability (%) of HepG-2 cell line.
3.3.2 Anti-diabetic activity
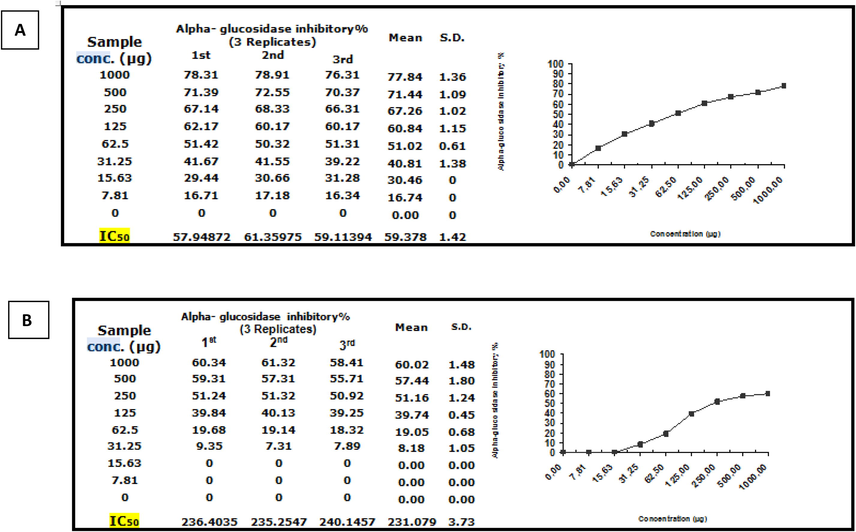
Inhibitory potency of Moringa leaf extract (Mo) against α-amylase activity was observed with an IC50 value of 719.92 ± 4.41 µg. While the nano formulation,(NMo) showed significantly more lower IC50 value of 115.9 ± 1.11 µg/ml Fig.8A and B. Further, inhibitory potency of Moringa leaf extract against α-glucosidase activity was observed with an IC50 value of 231.08 ± 3.73 µg/ml. While the nano Moringa (Mo N), was significantly more efficacious as is evident from the IC50 value, 59.38 ± 1.42 (Fig. 9A and B).Thus, based on the results of both the complementary anti-diabetic assays Moringa nanoparticles (NMo) exhibited a more potent anti-diabetic activity.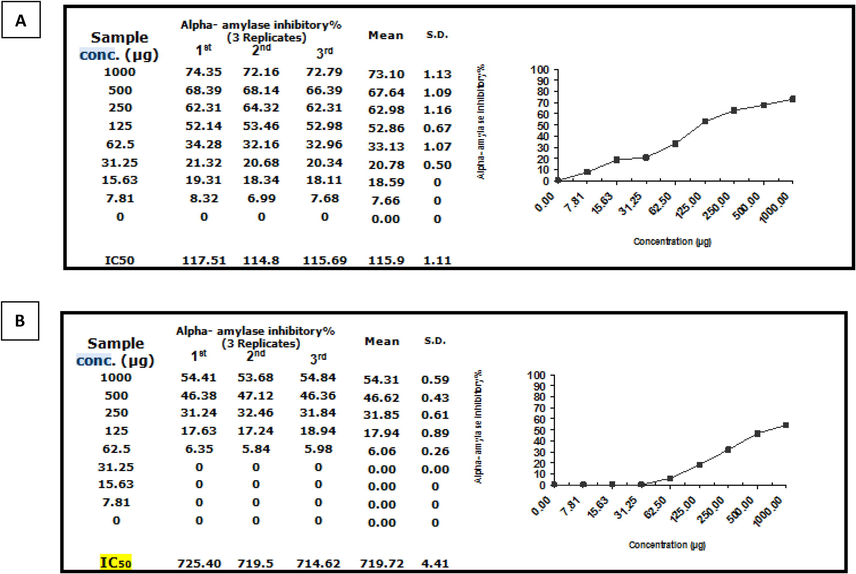
α-glucosidase inhibitory activity(%) of (A)Moringa nanoparticles and (B)Moringa leaf extract.
α-amylase inhibitory activity(%) of (A)Moringa nanoparticles and (B)Moringa leaf extract.
4 Discussion
Green nanotechnology has become popular over the past decade and has the potential of being successfully commercialized as an industry with strong green credentials. The present study focused on fostering the concept with the ‘green’ synthesis of non-metallic nanoparticles of M. oliefera leaves. Nanoparticles of Moringa leaves which were spherical in shape,141.6 nm in size with a (PDI) 0.32 were successfully synthesized. It has been reported that a homogenous distribution is demonstrated at values of 0.3 and below and are considered acceptable(Danaei et al., 2018). A close observation of this agglomeration shows that the NPs were enveloped in a faint thin layer of constituent biomolecules in the Moringa leaf; protein or cellulose. This is in concurrence with the study by Mallikarjuna et al. (2011). The absorption spectra Mo and NMo showed a strong absorbance peak at 664 nm which is the distinctive for chlorophyll, a feature of the leaves used. The spectrum for the Moringa nanoparticles showed smaller absorbance peaks possibly due to nanosization and modulation in the concentration of some of the bio components (Aleixandre-Tudo and Toit, 2018). The broader pattern of the FTIR spectra of both the Mo and the nanoparticles was similar reflecting related constituent functional groups which were not altered on being nano scaled. However, a slight shift in the spectra was evident with the presence of distinguishable peaks for the nanoparticles. These findings are in consensus with previous reports (Saleem et al., 2020; Prasetyaningrum et al., 2022). In comparison to other previous studies, the variability observed in the results, could be possibly due to the different source of M. oleifera leaves used. Overall, series of sharp peaks are characteristic of crystalline materials while a broad pattern is illustrated by amorphous substances (Khatri et al., 2018). The extraordinarily small diffraction patterns of NMo suggested a small crystallite size (part of crystalline cellulose and hemicellulose). Furthermore a higher protein content of the NMo (Gopalakrishnan et al., 2016), contributes to the prevalence of amorphous nature of native proteins, corresponding to the α-helix and β-sheet secondary conformations. Furthermore, the presence of lipids with polyunsaturated fatty acids results in high background base and broader pattern due to their amorphous nature. Similar results were reported in the literature previously (Sharma, 2019; Ferreira et al., 2021).
There have been previous studies that reported the phytochemical profile of Moringa leaves emphasizing on the various constituent pharmacologically active phytocomponents in the leaves with (Bhalla et al., 2021; Reminus and Cornelius, 2019).The current study is among the very few studies where the phytochemical profile coupled with the potency to scavenge free radicals has been reported for Mo and its nanoparticles. The antioxidant status of a phytocompounds is of vital importance and adds to the therapeutic potential. The results clearly showed a higher total content of phenols and flavonoids in the NMo compared to the aqueous Mo. Further, based on the results of the in vitro antioxidant assays, NMo reflected higher free radical scavenging ability in comparison to the aqueous extract of Mo. This mirrors the results on the phytochemical screening and could be attributed to the higher amount of the polyphenolic components in the nanoparticles contributing towards higher scavenging potency. Consistent with this, a study reported that the incorporation of Ag-NPs into crude extract of moringa leaves increased the antioxidative ability, total polyphenolic content, and free radical scavenging activity in addition to enhanced cytotoxic effect on colon cancer cells (Shousha et al., 2019; Mohammad et al., 2022). This could be best explained by an enhanced bioavailability of the active phytocompounds. Oxidative stress has been incriminated in the pathogenesis of several human disorders including diabetes mellitus (DM) and cancer. The anti-diabetic effect of M. oleifera leaves has been reported as its potential ability to lower blood sugar levels after ingestion attributed to the presence of polyphenols;quercetin-3-glycoside, rutin, kaempferol and glycosides (Al- Malki and El Rabey, 2015). In addition, Villarruel-López et al. (2018) reported that was blood glucose levels and enterobacteria counts were improved in diabetic rats administered with M. oleifera powdered leaves. These reports clearly support the anti-diabetic activity of the Mo and its nanoparticles reported in the current study. The obtained results of both in vitro anti-diabetic assays showed that the Moringa nanoparticles were more efficacious as illustrated by lower IC50values. Additionally, the antioxidant status of the Moringa leaves was demonstrated as the leaf extract (Mo) and the nanoparticles (NMo) proved to be effective in the cytotoxicity assay in both cancer cell lines (MCF-7; HepG-2) used. Nevertheless, the higher content of phenols and flavonoids in the nanoparticles (NMo) attributed to a more profound inhibitory activity in comparison to the leaf extract (Mo). A docking analysis of M. oleifera leaves showed several constituent ligands demonstrated good docking scores with BRCA-1 (Balogun et al., 2021).
5 Conclusions
The study included the synthesis, characterization and an assessment of the phytochemical profile, antioxidant status of the nanoparticles with the response to the bioassays. The mode of synthesis of nanoparticles kept the ‘green’ credential as the cornerstone which was facile, environmentally benign and safe.The nanoparticles were therapeutically more potent than the leaf extract owing to the increased bioavailability of the phytocompounds on nanosization. Nevertheless, further in-depth studies and in vivo testing of the nano formulation as a nutraceutical is imperative to open a new horizon in nanomedicine.
Acknowledgment
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R23), Princess Nourah bint Abdulrahman University Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adebiyi, O.E., Olayemi, F.O., Ning-Hua, T., Guang-Zhi, Z.,2017. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia, Beni-Suef Univ. J. Basic Appl. Sci.6(1),10-14.https://doi.org/10.1016/j.bjbas.2016.12.003.
- Al- Malki, A.L., El Rabey, H.A.,2O15. The antidiabetic effect of low doses of Moringa oleifera Lam. Seeds on streptozotocin induced diabetes and diabetic nephropaty in male rats. Bio.Med. Res. Int. 2015,381040. doi: 10.1155/2015/381040.
- Genetic diversity of Moringa peregrine species in Saudi Arabia with ITS sequences. Saudi J. Biol. Sci.. 2015;22:186-190.
- [Google Scholar]
- Aleixandre-Tudo, J. L.,Toit, W. D.,2018. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In R. L. Solís-Oviedo, & Á. de la Cruz Pech-Canul (Eds.), Frontiers and New Trends in the Science of Fermented Food and Beverages. Intech Open. https://doi.org/10.5772/intechopen.79550.
- Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot.. 2018;119:383-389.
- [CrossRef] [Google Scholar]
- Amina, M., Al Musayeib, N.M., Alarfaj, N.A., El-Tohamy, M.F., Orabi, H.E., Bukhari, S.I., Mahmoud, A.Z.,2019. “Exploiting the Potential of Moringa oleifera Oil/Polyvinyl Chloride Polymeric Bionanocomposite Film Enriched with Silver Nanoparticles for Antimicrobial Activity”. Intl. J. Polym. Sci. 2019, 5678149, 11 pp. https://doi.org/10.1155/2019/5678149.
- Anticancer Potential of Moringa oleifera on BRCA-1 Gene. Systems Biology. Bioinform. Biol.. 2021;15:11779322211010703
- [CrossRef] [Google Scholar]
- Phytochemical analysis of Moringa Oleifera leaves extracts by GC-MS and free radical scavenging potency for industrial applications. Saudi J Biol Sci. 2021;28(12):6915-6928.
- [CrossRef] [Google Scholar]
- Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57.
- [Google Scholar]
- Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on Hela and BT-474 cell lines. Int. J. Mol. Cell Med.. 2014;3(2):102-107. PMID: 25035860
- [Google Scholar]
- Characterization of cassava starch/soy protein isolate blends obtained by extrusion and thermocompression. Ind. Crops Prod.. 2021;160:113092
- [Google Scholar]
- Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness. 2016;5:49-56.
- [Google Scholar]
- Heidari,R., Zareae,S., Heidarizadeh,M., 2005. Extraction, Purification, and Inhibitory Effect of Alpha - Amylase Inhibitor from Wheat ( Triticum aestivum Var. Zarrin ). Pak J Nutr. 4,2.10.3923/pjn.2005.101.105
- Moringa oleifera gum based silver and zinc oxide nanoparticles: green synthesis, characterization and their antibacterial potential against MRSA. Biomater. Res.. 2021;25:17.
- [CrossRef] [Google Scholar]
- Preparation and characterization of pyrimethamine solid dispersions and an evaluation of the physical nature of pyrimethamine in solid dispersions. J. Drug Deliv. Sci. Technol.. 2018;45:110-123.
- [Google Scholar]
- Ethnopharmacological properties and Nutraceutical potential of Moringa oleifera. Phytomed. Plus. 2022;2(1):100168
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using ocimum leaf extract and their characterization. Dig. J. Nanomater. Bios.. 2011;6:181-186.
- [Google Scholar]
- Matinise,N., Fuku,X.G., Kaviyarasu,K., Mayedwa,N., Maaza,M.,2017.ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci.406,339-347.https://doi.org/10.1016/j.apsusc.2017.01.219.
- Milla, P.G., Peñalver, R., Nieto, G.,2021. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants (Basel).10(2),318. doi: 10.3390/plants10020318
- Mohammed, A.B.A, Mohamed, A., El-Naggar, N.E., Mahrous, H., Nasr, G.M., Abdella, A., Ahmed, R.H., Irmak, S., Elsayed, M.S.A., Selim, S., Elkelish, A., Alkhalifah, D.H.M., Hozzein, W.N., Ali, A.S.,2022. "Antioxidant and Antibacterial Activities of Silver Nanoparticles Biosynthesized by Moringa oleifera through Response Surface Methodology". J. Nanomater., 2022, Article ID 9984308, 15 pp,doi.org/10.1155/2022/9984308
- Green synthesis of silver nanoparticles from Moringa oleifera leaf extracts and its antimicrobial potential. Adv. Nat. Sci: Nanosci. Nanotechnol.. 2018;9:015011
- [Google Scholar]
- Prospects of Moringa cultivation in Saudi Arabia. J. Appl. Environ. Biol. Sci.. 2015;5(3):39-46.
- [Google Scholar]
- Quantitative analysis of the nutritional components in leaves and seeds of the Persian Moringa peregrina (Forssk.) Fiori. Pharm. Res.. 2015;7(3):242-248.
- [Google Scholar]
- Sequential microwave-ultrasound assisted extraction of flavonoid from Moringa oleifera: product characteristic, antioxidant and antibacterial activity. Indones. J. Chem.. 2022;22(2):303-316.
- [Google Scholar]
- Phytochemical Analysis Of Moringa Oleifera (Leaves And Flowers) And The Functional Group. J.World.Sci.. 2019;7(6):41-51.
- [Google Scholar]
- HPLC analysis, cytotoxicity, and safety study of Moringa oleifera Lam. (wild type) leaf extract. J. Food Biochem.. 2020;44(10):e13400.
- [Google Scholar]
- Inhibitory effects of five medicinal plants on rat alpha-glucosidase: comparison with their effects on yeast alpha-glucosidase. J. Med. Plant. Res.. 2011;5:2863-2867.
- [Google Scholar]
- A review on characterization of solid dispersion. Int. J. Eng. Appl. Sci. Technol.. 2019;4:127-128.
- [Google Scholar]
- Evaluation of the biological activity of Moringa oleifera leaves extract after incorporating silver nanoparticles, in vitro study. Bull. Natl. Res. Cent.. 2019;43:212.
- [CrossRef] [Google Scholar]
- Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth) J. Taibah. Univ. Med. Sci.. 2017;12(4):360-363.
- [CrossRef] [Google Scholar]
- Venkatachalam,A., Jesuraj,J.P., Sivaperuman,K.,2021. “Moringa oleifera Leaf Extract-Mediated Green Synthesis of Nanostructured Alkaline Earth Oxide (MgO) and Its Physicochemical Properties”, J.Chem., 2021, 4301504, 22 pp, https://doi.org/10.1155/2021/4301504.
- Green nanotechnology: advancement in phytoformulation research. Medicines. 2019;6:39.
- [CrossRef] [Google Scholar]
- Effect of Moringa oleifera consumption on diabetic rats. BMC Complement Altern. Med.. 2018;18(1):127.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102576.
Appendix A
Supplementary material
The following are the Supplementary data to this article:






