Translate this page into:
Phytochemical analysis and phytotoxic evaluation of Chenopodium glaucum L.
⁎Corresponding authors. drfaizanwazir@gmail.com (Faizan Ullah), saudhort@gmail.com (Shah Saud), shah_fahad80@yahoo.com (Shah Fahad),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plant secondary metabolites such as alkaloids, flavonoids, glycosides, saponins, tannins, and terpenoids with medicinal properties can be investigated as botanical herbicides. Therefore, we have investigated phytochemicals profile and phytotoxicity of aqueous, ethyl acetate, n-hexane and methanol extracts (5 mg/mL and 10 mg/mL) of Chenopodium glaucum L. (C. glaucum) on radish under laboratory conditions. Initial screening of the extracts showed the occurance of several phytochemicals like alkaloids, flavonoids, anthraquinone, cardiac glycosides, saponins, tannins and terpenoids. Alkaloids and tannins content was higher in methanol extract followed by ethyl acetate and n-Hexane extracts. Elemental analysis revealed that the presence of minerals like magnesium 0.725 mg/100 g DW, calcium 2.25 mg/100 g DW, potassium 6.55 mg/100 g DW, sodium 0.375 mg/100 g DW, iron 225 mg/100 g DW, zinc 51 mg/100 g DW, copper 12 mg/100 g DW, and manganese 119 mg/100 g DW was found. In phytotoxicity assay, the extracts at 10 mg/mL showed inhibitory effects on the growth of radish. Maximum decrease in seed germination (%), seed germination index, seedling fresh and dry weight was caused by the methanolic extract at 10 mg/mL. The chlorophyll a content was decreased while chlorophyll b content was increased with increasing concenteration of the extracts. Phenolics content of the seedlings was higher with the application of 10 mg/mL of the extracts. Powder microscopy of C. glaucum showed the presence of calcium oxalate crystal, pieces of trichome, fragments of cork cells, starch granules, phloem fibers and lignified xylem vessels with annular thickenings. Microscopic study of abaxial surface of leaf showed the presence of anisocytic type of stomata, surrounded by subsidiary cells. Stomatal size was measured 25.017 µm and the mean density was 570.66/mm2. Findings of this study concluded that phytochemicals found in C. glaucum extracts were highly phytotoxic on seed germination and seedling growth of radish. It is therefore recommended to isolate and characterize such novel compounds from this plant species for the development of new biodegradable botanical herbicides.
Keywords
Medicinal plants
Secondary metabolites
Botanical herbicdes
Powder microscopy
1 Introduction
Weeds in crops have been reported to minimize crop productivity up to 34 % of the total yield (Jabran et al., 2015). Application of synthetic herbicide is still the most effective way and frequently used to control weeds in cultivated crops (Elshamy et al., 2019; Espig et al., 2022). However, the use of synthetic herbicides have lead to several problems such as development of herbicide resistant species and can damage environment and human health (Landrigan and Benbrook 2015; Mominul-Islam and Kato-Noguchi 2014; Bhandari et al., 2014; Pathak et al., 2022). Extensive use of synthetic herbicides results in the loss of biodiversity and soil fertility (Mahmood et al., 2016; Sangiorgio et al., 2022). Therefore, in order to minimize the effect of synthetic herbicides on environment and crops, several weed management stratagies have been tried and are getting interest. Exploring plants secondry metabolites can be a safe alternative to synthetic herbicides (Araniti et al., 2022). Therefore, reserachers are working on the isolation and charactization of novel compounds from plants with herbicidal propertities (Pouresmaeil et al., 2020).
The secondary metabolites in plants have a diverse type of actions and phtotoxic effects (Lipkus et al. 2008; Araniti et al., 2022). These natural substances are biodegradable and harmLess (Bajwa et al., 2019). Plants are a major source of phytochemicals which have also been reported as phytotoxic in nature (Alvin et al. 2014; Shakya 2016; Franco et al., 2022). Therefore, medicinal plants are needed to be explored as a source of phytotoxic substances.
The chemical compounds responsible for the phytotoxic effects are called allelochemicals, used by plants for their survival under diverse environmental conditions. The allelochemicals can be therefore exploited as an alternatives to synthetic herbicides (Motmainna et al., 2021). Bio-herbicides are advantageous over chemical herbicides because they are non-toxic for the environment, they are highly specific to target weed species and are biodegradable. For searching bio-herbicidal potential of a plant species, phytotoxicity assay is commonly employed (Fukushima et al., 1998).
Radish seed bioassay has been extensively used previously for phytotoxicity evaluation of plants extracts (Terker and camper 2002, Inayatullah et al., 2007, Khan et al., 2011;). Radish seeds are commenly available, easily to germinate and very sensitive to test compounds (Einhelling and Rasmuseen 1978) and are a standard assay in phytotoxicity studies (Patterson 1986).
Correct identification is the main problem in herbal drugs as some plant materials in fresh as well as in dry powder form look too similar that they cannot be distinguished from one another (Springfield, 2005; Muyumba et al., 2021). The real danger in herbal drugs preparation and subscription is their adulteration (Opara, 2004; Monti et al., 2022). Observation of microscopic material of medicinal plants is necessary for identification of powder (WHO, 1998). Therefore, development of standardization is necessary for the identification of the important medicinal plants. Standardization of identification of powder material of medicinal plants is necessary for the prevention of adulteration. It is general observation that the more effective natural drugs are more exploited. The natural drugs are adultered with low quality material easily to meet the growing demand (Chanda et al., 2013).
Chenopodium glaucum belongs to family Chenopodiaceae. The members of this family are found in Australia, New Zealand and Asia. Many members of this family like Salsola and Sueda prefer saline soils. Salsolin and salsolidin used in hypertensive medications are obtained from the members of this family (Akopian et al., 2020). The plant is annual herb and occurs as a weed on road sides, gardens and along cultivated fields. The C. glaucum grows up to 5–20 cm height and is usually glabrous (Laghari et al., 2010; Ahmad et al., 2003). Due to environmental risks associated with synthetic herbicides we carried out these studies to explore phytoxicity of C. glaucum by using radish seed bioassay with perspectives of botanical herbicides.
2 Materials and methods
2.1 Collection of plant material
Arial parts from 100 plants of C. glaucum were collected from District Bannu, Pakistan and shade dried. The C. glaucum was identified by comparing its characteristics with flora of Pakistan and deposited in the herbarium Department of Botany University of Science and Technology Bannu, Pakistan assigning it voucher number Cg-C-1. The coordinates of the collection area are; Latitude: 32.88041 N 32°52′49.31089″ Longitude: 70.58595 E 70° 35′9.03027″ Elevation 342.58 m. All the dried aerial parts were converted to fine powder with a particle size 5 mm. Crude extracts were prepared by soaking 50 g of the plant powder in 1L of distilled water, n-hexane, ethyl acetate and methanol separately. The extracts were filtered separately through Whatman No.1 filter paper and dried by evaporating the solvents at 40 °C with a rotary evaporator (Model, RE301, Japan). The yield of methanolic extract was 9.3 g, aqueous 7 g, ethyl acetate 3.8 g and n-hexane 1.3 g.
3 Phytochemicals analysis
3.1 Qualitative determination of phytochemicals
The qualitative assessment of extracts for diverse groups of phytochemicals was performed according to standard available protocols (Harbone, 1973; Trease and Evans, 1985; Congesta et al., 2005; Ngameni et al., 2013).
3.2 Assessment of alkaloids
First the Mayer’s reagent was prepared for which distilled water (60 mL) was taken to dissolve 335 mg of HgCl2. The 5 g of KI was mixed in 20 mL of distilled H2O. Both solution were mixed in distilled water and volume was made up to 100 mL.
For the qualitative assessment of alkaloids, the 2 mg of each crude extract was separately mixed with concentrated H2SO4. The mixture was further mixed with the Mayer’s reagent. Formation of white precipitation indicated presence of alkaloids in the samples.
3.3 Assessment of flavonoids
The 1 mg of each crude extract was mixed with 1 mL of 2 N NaOH solution. Yellow color formation confirmed flavonoids in the extract samples.
3.4 Assessment of coumarins
Each crude extract (1 mg/mL) was mixed with 1 mL of NaOH (10 %) in a test tube. Yellow color formation is the indication of the presence of coumarins in the test sample.
3.5 Assesment of saponins
The 2 mg of extract was taken (aqueous, ethyl acetate, methanol and n-hexane) and mixed with 2 mL of distilled water by shaking continuously. A soapy layer formations signs the presence of Saponin in the extract samples.
3.6 Assesment of tannins
The 1 mg of each crude extract was mixed with two mL of 5 % ferric chloride. The formation of the dark blue color is the sign of the presence of tannins.
3.7 Assessment of terpenoids
Chloroform (2 mL) and concentrated H2SO4 (2 mL) were mixed with 0.5 mg of each aqueous, ethyl acetate, methanol and n-hexane crude extract. Red brown color appearance in the middle of the two layer is the indication of terpenoids.
3.8 Assesment of anthraquinone
The 1 mg of each crude extract was mixed with 2 mL of diluted 2 % HCL. Development of red color is the indication of anthraquinone.
3.9 Quantitative determination of phytochemicals
3.9.1 Condensed tannins
By using the method of Sun et al. (1998) total content of condensed tannins in various extracts of C. glaucum was estimated. This method measures the content proanthocyanidins in plant samples. For samples preparation the 0.5 μL extract was mixed with 3 mL vanillin solution (4 %) and concentrated HCl (1.5 mL). After 15 min of incubation at normal room temperature (25 ± 1) the absorbance of all the samples was noted at 500 nm using a VIS-Spectrophotometer (VIS-721 Cangzhou, China). The concentration of total condensed tannins was expressed as mg Catechins/g D.W of a plant sample. All the samples were prepared and analyzed in triplicate.
3.10 Alkaloids
Alkaloids content in various extracts of C. glaucum was determined (Harborne 1984). The 200 mL acetic acid (10 %) in ethanol was mixed with 5 g of the extracts separately. The samples were allowed to stand at room temperature for 4 hrs. All the sets of samples were first filtered and then concenterated to one quarter of the total volume in water bath. All the samples were added with concenterated ammonium hydroxide solution until the completion of precipitation was formed. The precipitate was carefully transferred to a separate conical flask and carefully washed with diluted ammonium hydroxide solution. After filtration, the alkaloid’s containing precipitate was collected subsequently dried and weighed.
3.11 Phytotoxicity assay
The 500 mg of aqueous, n-hexane, ethyl acetate and methanol plant extracts were dissolved separately in 50 mL of the respective solvent for making a stock solution of 10 mg/mL. Stock solutions of the extracts (aqueous, methanol, n-hexane, ethyl acetate) were further diluted to make 5 mg/mL solutions. Respective solvents and sterilized distilled water were used negative and as positive control respectively. Phytotoxicity assay was performed according to the method of Atta-ur-Redman (1991) and Turker and Camper (2002). Raphanus sativus L. (Radish) seeds were used for the determination of seed germination indices, seedling fresh weight and dry weight, photosynthetic pigments and total phenolics contents.
The seed surface sterilization solution having 0.1 % HgCl2 was made in a beaker. Rapidly washing the radish seeds with the sterilizing solution for 2 to 3 min followed by 3–4 washings with autoclaved distilled water. The wet seeds were dried with a sterilized blotting paper before using in the assay. Petri plates were sterilized in an autoclave and fitted with two Whatman No.1 filter paper. The 0.5 mL of the 5 mg/mL and 10 mg/mL of the extract was dropped on each filter paper in a Petri plate. The solvent in each Petri plate was evaporated and sprinkled with 5 mL of autoclaved distilled water. Each of the extract treatment had three replicates. For control, 0.5 mL n-hexane, ethyl acetate and methanol added to the plate, vacuum evaporated and then 5 mL autoclaved distilled water added in each Petri plate. Three replicates were prepared for each control. Radish seed (sterilized) 10 in number were cultivated with forceps at suitable distance in each Petri plate. Petri plates were incubated at 25 °C in partial darkness. After the seeds were germinated (100 % germination in control) the seedlings were transferred to a growth chamber (16 hrs dark and 8 hrs illumination, 25 ± 1 °C temperature) for further two weeks and supplied with respective solutions when required. After two weeks all the seedlings were carefully collected and analyzed for different growth traits.
Seed germination (%).
Seed germination index.
3.12 Fresh weight and dry weight of raddish seedling.
The weight of fresh seedlings in all the treatments was measured by using a digital balance. For dry weight, the seedlings were dried in an oven at 72 °C for 72 h and then their weight was determined.
3.13 Determination of photosynthetic pigments
Arnon (1949) and Kirk (1968) method was used for the determination of chlorophyll and carotenoids content of radish seedlings. Leaf extract was prepared by grinding 0.01 g green tissues in 80 % acetone (5 mL). After centrifugation (4000 × g) for 15 min the supernatant obtained was carefully taken in glass test tubes and used for the estimation of leaf pigments content. The absorbance of all the test samples was noted at 645 nm and 663 nm for Chl a and Chl b whereas, the absorbance for carotenoids was recorded at 480 nm using a spectrophotometer (Hitachi's U-5100 Japan).
3.14 Total phenolic contents determination
Quantification of soluble phenolic was made according to the method of Swain and Hillis (1959). A 100 mg of powdered leaves were extracted in methanol, water, ethyl acetate and n-hexane. 50 μL of the supernatant was diluted to a volume of 0.5 mL by adding methanol, water, ethyl acetate and n-hexane respectively. Folin-Ciocalteau reagent (50 % v/v) in amount of 0.25 mL was added in a mixture and kept for 3 min. 7.5 % sodium carbonate solutions (500 µL) was added in the mixture and kept in dark for one hour at 25 °C. The absorbance was taken at 765 nm by using spectrophotometer (Hitachi's U-5100 Japan). Gallic acid in concenteration of 0 to 32 µg/mL was used for plotting a standard curve for comparison of gallic acid from the measured samples.
3.15 Elemental analysis
Procedure outlined by Zafar et al. (2010) was adopted for elemental analysis. The C. glaucum powder (0.25 g) was taken in a 50 mL flask. 6.5 mL of mixed acids solution was added in the flask. The mixed acids solution contained HNO3, H2SO4 and HClO4 in the ratio of 5:1:0.5. The sample was boiled on hot plate (MS-300HS, Korea) until digestion was completed and white fume was formed. The digested material was transferred to another flask and filtration was made with Whatmann No. 42 filter paper. The filtrate was collected in labelled bottles and the mixture was analyzed for elements sodium (Na+), potassium (K+), magnesium (Mg+2), calcium (Ca+2), iron (Fe+2), zinc (Zn+2) and copper (Cu+3) by using atomic absorption spectrophotometer (Shimadzu AA-670 w).
3.16 Microscopic study of C. Glaucum powder
Powder microscopy was carried out according to the protocol of WHO (1998). The dried stem and leaf of C. glaucum were grinded into coarse powder form. The powder material was mounted on a slide by dipping the moistened tip of needle in the powder and then gently tapping on slide. The slide already contained a drop of phloroglucinol in concentrated HCl, chloral hydrate and iodine solution.
3.17 Stomatal density and size
Leaf stomatal density (SD) and stomata size (SS) of C. glaucum were calculated according to the method of Volenikova & Ticha (2001). Clear nail polish was used to prepare a clear cast of leaf lower surface. A strip of clean sticky tape was placed over the lower surface on nail polish area and pressed gently. The sticky tape was pulled off in such a way that the layer of nail polish also come off with it. The sticky tape was put on the slide and observed at 400x. The stomata were count at 3 field of view (FOVs). Stomata density was calculated according to the formula.
Stomata density = Number of stomata/ Area (mm2).
Where area is calculated as.
3.18 Statistical analysis
The data of phytochemicals and phytotoxic assay were analyzed by using analysis of variance technique. Least significant difference test was performed for comparison of treatment means by using Statistix-9 (Steel and Torrie 1997). The standard error value was recorded for replicates of treatments by using excel sheet.
4 Results
4.1 Phytochemicals
Methanol, aqueous, n-hexane and ethyl acetate extract of C. glaucum were analysed for the presence of different phytochemicals such as flavonoids, anthraquinone, alkaloids, cardiac glycosides, tannins, terpenoids and saponins (Table 1). Anthraquinone was found absent in methanol extract, alkaloids and anthraquinone were absent in aqueous extract, anthraquinone and cardiac glycosides were absent in n-hexane extract and anthraquinone and cardiac glycoside were absent in ethyl acetate extact.
Methanol
Aqueous
n-hexane
Ethyl acetate
Alkaloids
+
–
+
+
Flavonoids
+
+
+
+
Anthraquinone
–
–
–
–
Cardiac glycosides
+
+
–
–
Saponin
+
+
+
+
Tannins
+
+
+
+
Terpenoids
+
+
+
+
The results for quantitative analysis of extracts is shown in Table 2. Alkaloids were significantly higher (p < 0.05) in methanol extract (5.15 %), next in n-hexane (3.45 %) and ethyl acetate (3.03 %). Alkaloids were absent in aqueous extract. Significantly higher amount of tannins was present in methanol extract (3.33 mg/g) followed by ethyl acetate extract (2.29 mg/g), aqueous extract (1.98 mg/g) and n-hexane extract (1.78 mg/g).
Extract
Alkaloids (%)
Total condensed tannins (mg/g DW)
Aqueous
0.000 ± 0.00d
1.98 ± 0.37b
Ethyl acetate
3.03 ± 0.05c
2.29 ± 0.17b
Hexane
3.45 ± 0.04b
1.78 ± 0.08b
Methanol
5.15 ± 0.08a
3.33 ± 0.28a
4.2 Elemental analysis
Elemental analysis of C. glaucum is shown in below (Table 3). The elemental analysis showed the presence of calcium 2.25 mg, potassium 6.55 mg, magnesium 0.725 mg, sodium 0.375 mg, iron 225 mg, copper 12 mg, zinc 51 mg, and manganese 119 mg in 100 g.d.w.
Micro elements
(mg/100 g.d.w.)Magnesium
Calcium
Potassium
Sodium
Iron
Zinc
Copper
Manganese
0.725
2.25
6.55
0.375
250
51
12
119
5 Phytotoxicity of C. Glaucum
5.1 Phytotoxicity of C. Glaucum extracts on seed germination indices of radish
A reduction in seed germination (%) and seed germination index was recorded in all the extracts; however, higher concentration of the extracts were significantly more toxic on radish seed germination (Figs. 1 and 2). The 100 percent germination was recorded in control treatments. The treatment of 10 mg/mL of the extracts showed severe decreases in germination (%). The methanol, aqueous, n-hexane and ethyl acetate extracts showed 37 %, 34 %, 25 % and 14 % decrease in seed germination percentage. Like seed germination (%), the seed germination index values were lower in treatments having 10 mg/mL of the extracts. However, n-Hexane and methanol extracts were significantly more toxic on seed germination index of radish.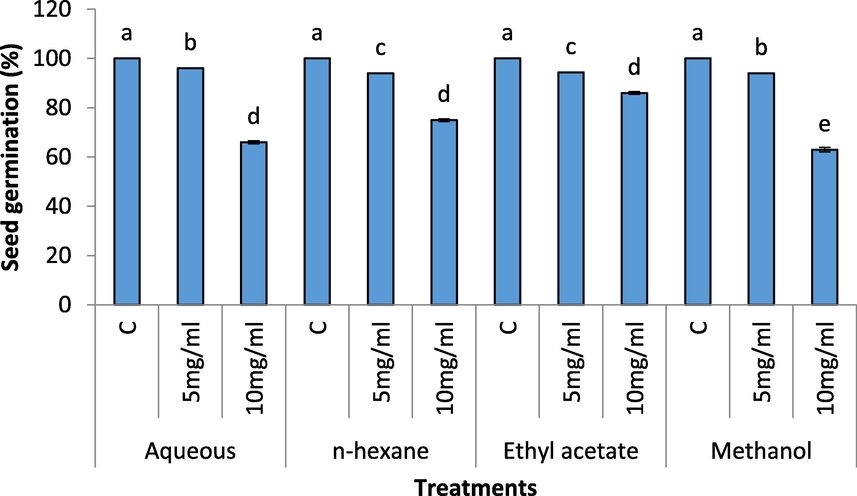
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on seed germination (%) of Raphanus sativus.
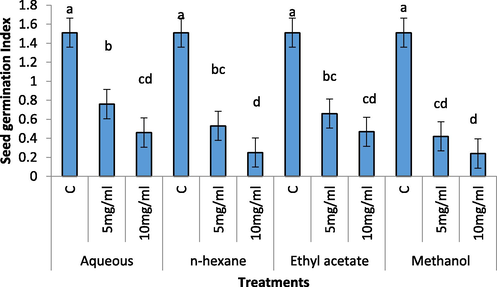
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on seed germination index of Raphanus sativus.
5.2 Phytotoxicity of C. Glaucum extracts on fresh and dry weight of radish seedling
Fresh and dry weights of radish seedling were significantly decreased in treatments having aqueous, ethyl acetate, methanol and n-hexane extracts (5 mg/mL and 10 mg/mL) as compared to its control (Figs. 3 and 4).The most significant decrease was observed in n-hexane and methanolic extracts of C. glaucum (10 mg/mL).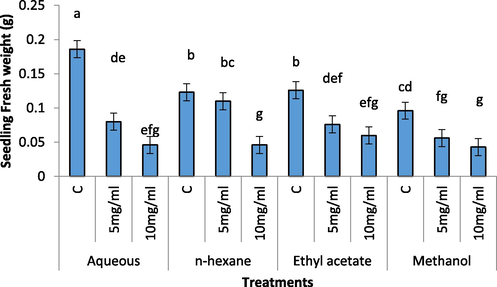
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on fresh weight of Raphanus sativus.
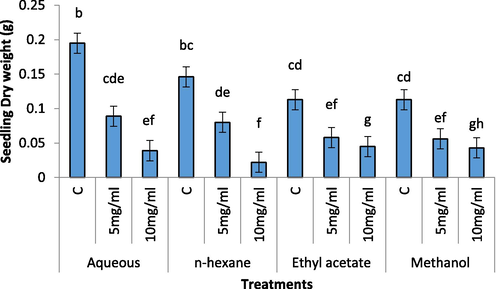
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on dry weight of Raphanus sativus.
5.3 Phytotoxicity of C. Glaucum extracts on photosynthetic pigments of radish
Chlorophyll a content of radish seedling was significantly reduced by aqueous, n-hexane, ethyl acetate and methanolic extract of C. glaucum (Fig. 5). The 10 mg/mL of the extract showed greater reduction in the chl a content but lower concentration (5 mg/mL) also remained significant. The highest reduction in chl a contents observed in 10 mg/mL of n-hexane extract.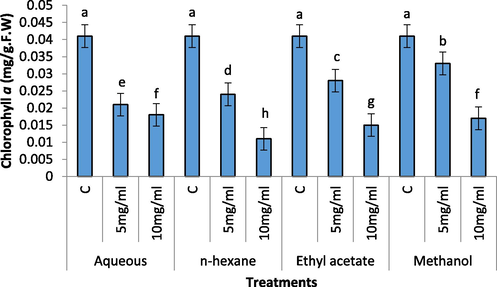
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on Chl a content of Raphanus sativus.
Increase in Chl b content was recorded with increasing concentrations of the extracts (Fig. 6). Aqueous extract (5 mg/mL and 10 mg/l) caused a tremendous increase in chl b content of radish seedling. The 10 mg/mL of the aqueous extract remained more significant as compared to 5 mg/mL. Carotenoids contents of radish seedling significantly increased in 10 mg/mL of aqueous, n-hexane, ethyl acetate and methanolic extract of C. glaucum (Fig. 7). However 5 mg/mL of the extract remained non-significant in aqueous, methanol and ethyl acetate and remained significant in n-hexane extract.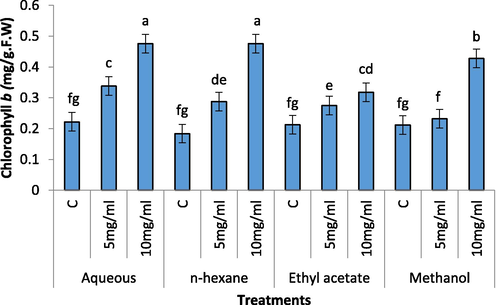
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on Chl b content of Raphanus sativus.
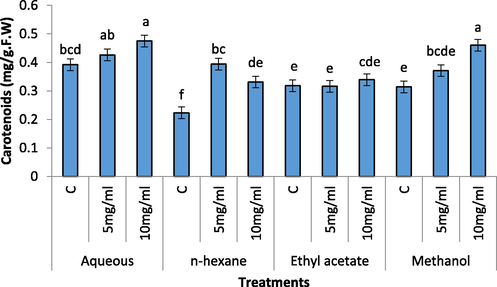
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on Carotenoids content of Raphanus sativus.
5.4 Phytotoxicity of C. Glaucum extracts on total phenolics contents of radish seedling
Increase in phenolics contents of radish seedlings was observed with increasing concentration of the extracts (Fig. 8). Aqueous extract of C. glaucum in both the concentration (5 mg/mL and 10 mg/mL) significantly increased the phenolics contents of radish seedling. The 5 mg/mL of the extract of n-hexane remained insignificant while 10 mg/mL of the extract significantly increased the phenolics contents of radish seedling. Ethyl acetate and methanolic extract in both the concentrations (5 mg/mL and 10 mg/mL) also significantly increased the phenolic contents of radish seedling.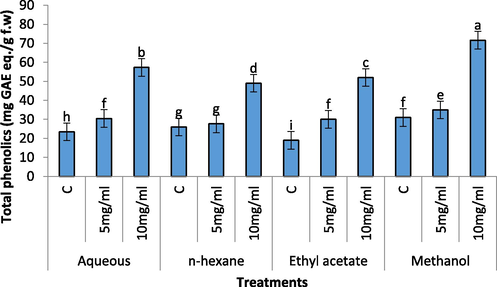
Effect of different concentration of aqueous, n-Hexane, Ethyl acetate and Methanolic extract on total phenolics contents of Raphanus sativus.
5.5 Powder microscopy
Crude powder drugs are adultered with low quality of powder. Correct identification of the powder drug is necessary. Powder microscopy is a useful technique for identification of powder drug. Stem powder microscopy of C. glaucum showed the presence of calcium oxalate crystals. Broken pieces of trichome were also present in the powder. Fragments of cork cells and cortex cells were also present in the powder. Starch granules, phloem fibers and lignified xylem vessels with annular thickening were present as shown in (Fig. 9).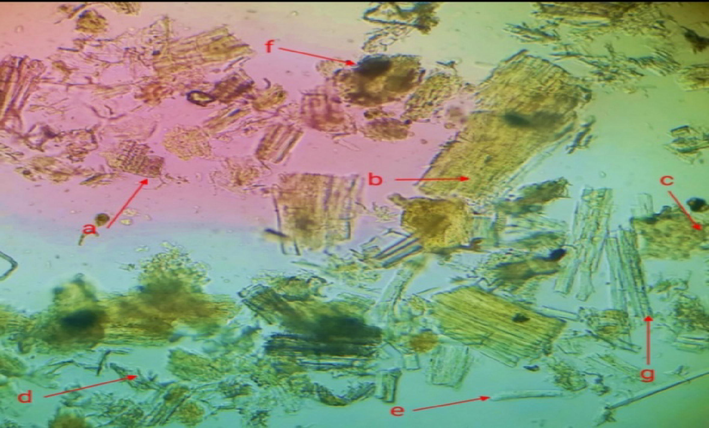
Powder microscopy of C. glaucum. (a) Annular xylem vessels (b) Fragment of cork cell (c) Cortex cells (d) Trichome (e) Calcium oxalate crystals (f) Starch granules (g) Phloem fibers.
5.6 Microscopic study of abaxial surface of C. Glaucum leaf
Microscopic evaluation of leaf abaxial surface is a useful anatomical tool used for accurate identification of a plant species. Microscopic study of abaxial surface of leaf showed the presence of anisocytic type of stomata, surrounded by subsidiary cells (Fig. 10).
Microscopic study of leaf stomatal number and density of C. glaucum. Anisocytic stomata (b)- Subsidiary cells (c)- epidermal cells.
5.7 Stomata density and stomata size of C. Glaucum leaf
The stomatal density of C.glaucum was calculated in three different field of views. Results showed that stomatal density in three different field of views was 560/mm2, 568/mm2 and 584/mm2 with a mean density of 570.66/mm2 (Table 4). Size of stomata was measured 25.017 µm through image j software. FOV means field of view.
Sample name
Magnification
(ocular × objectives)Surface
FOVs
Number of stomata in entire FOV
Stomatal Density
Stomata/mm2
C.glaucum.
400x
Lower
1
70
560
2
71
568
3
73
584
Mean
570.66
6 Discussion
Various groups of phytochemicals such as flavonoids, alkaloids, anthraquinone, cardiac glycosides, tannins, terpenoids and saponins were found in the extracts of C. glaucum. A variety of biological activities like antimicrobial, antioxidant, anti-inflammatory, antiplasmodial, anticancer and phytotoxic activities are reported for these phytochemicals (Malaver et al., 2019; Wang et al., 2022a, 2022b). This study confirmed that methanolic extract was rich in alkaloids and condensed tannins which was also highly phytotoxic on growth of radish. These results are in accordance with those of Hashmi et al., (2021) that tannins and alkaloids were higher in methanolic extracts of Lepidium pinnatifidum.
The results showed that phytotoxic effect of C.glaucum extracts (aqueous, n-hexane, ethyl acetate and methanol) on radish are concentration dependent. Among the various extracts methanolic and ethyl acetate extracts were more phytotoxic then aqueous and n-hexane extracts which can be credited to their phytochemicals composition. Plant species Euphorbia wallichii and Bergenia ciliata enriched in tannins showed greater phytotoxicity in comparison to those with lower concentration of tannins (Ullah et al., 2015). Similarly, Khan et al. (2011) and Lopez-Rodríguez et al. (2022) reported that phytotoxicity of plants was closely related with their tannins and alkaloids content. The reduction in germination percentage and delayed germination by methanolic extract could be due its effects on rate of imbibition, delayed initiation of germination, cell division and elongation (Black, 1989; Ullah et al., 2014; Yan et al., 2022). Germination is hindered as the radicle penetrates the seed coat (Koodkaew et al., 2018; Dordevic et al., 2022).). Toxic substances from the aqueous leaf extract of different plant species inhibited the seed germination of Alfa alfa (Chon et al., 2004). Chon et al., (2003) reported several phytochemicals from Lettuce plant extract as the causative agents of seed germination inhibition. The greatest inhibition of seed germination is observed in methanol extract of Lactuca sativa (Chon et al., 2005). Studies as reported by Pérez et al. (2015) showed that phytotoxicity of plants was significantly correlated with their secondary metabolites content. Moreover, Uddin et al. (2007) reported that primary effects of phytotoxins on target plant was their inhibition of radical elongation.
Our study proved that C. glaucum methanolic and n-hexane extracts were highly phytotoxic on seedling growth of radish. This indicated that phytochemicals occurring in these extracts are phytotoxic in nature and can be exploited for testing as botanical herbicides. In radish phytotoxicity assay seedling fresh and dry weight was reduced by a number of medicinal plants in which Woodfordia fruticosa methanolic extract was most effective when applied at 10 mg/mL concentration (Khan et al., 2011).
The reduction in photosynthetic pigments of radish seedlings may be due to the stress of phytochemicals. The reduction in photosynthetic pigments of plants by phytochemicals have been reported (Huang et al., 2010; Ismail and Siddique, 2011; Han et al., 2012; Wang et al., 2022a). The reduction in Chl a and increase in Chl b may be due to damage of pigments systems by the extracts. In stress condition damage of photosystem I and an increase of Chl b around photosystem II has been mentioned (Kitajima and Hogan, 2003; Wang et al., 2022b). Phenolics compounds also showed increasing trends with increasing concentration of the extracts which may be due to the increased stress induced by the high concentration of the phytochemicals.
While analyzing mineral composition it was found that Fe content of C. glaucum is high (250 mg/100 g) as compared to the value recorded for highly medicinal plant Ipomoea batatas (16 mg/100 g) (Antia et al., 2006). The Zn value is also suitable as compared to early reported values of vegetables (Ibrahim et al., 2001). This indicated that C.glaucum as source of botanical herbicide could be beneficial for crop plants if tested extensively for its selective effects against weeds. Moreover, the various extracts of C. glaucum could be potential source of various kinds of minerals for human consumption.
Standardization and quality control of herbal drugs is necessary because adulteration is found in market herbal medicines. Microscopic analysis is among the cheapest of methods to correctly identify the particular herbal drug in powder form (Akbar et al., 2014; Uza & Dastagir. 2022). Powder microscopy and anatomical features provide useful knowledge about quality control of medicinal powder drugs (Nisar et al., 2021). Therefore, we have established standards for controlling adulteration in this plant powder for future use. Powder microscopy of different and highly medicinal plants like Jasminum sambac and Nigella sativa has been reported (Gowdhami et al., 2015; Rashid et al., 2018).
7 Conclusions
The C. glaucum possessed useful phytochemicals such as flavonoids, alkaloids, terpenoids, tannins, saponins, and cardiac glycosides. Moreover, its various extracts particularly methanolic and n-hexane were highly phytotoxic on radish and could be exploited for testing as botanical herbicides against noxious weeds. The elemental analysis revealed that C. glaucum is rich in zinc and iron increasing its value for human consumption as well. Powder microscopy showed useful structures that can be helpful in correct idendification and quality control of this plant species. Findings of this study indicated the possible use of C. glaucum extracts as a source of natural herbicides in weed management. Moreover, further studies are recommended for the isolation of novel compounds from this plant species as herbicides.
Acknowledgement
The authors extend their appreciation to the Researchers supporting Project number (RSP2023R367), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical evaluation of Chenopodium murale Linn. Asian J. Plant Sci.. 2003;2:1072-1078.
- [Google Scholar]
- Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac. J. Trop. Biomed.. 2014;4(5):410-415.
- [Google Scholar]
- Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol. Res.. 2014;169(7–8):483-495.
- [Google Scholar]
- Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pak. J. Nutr.. 2006;8(5):166-168.
- [Google Scholar]
- Secondary metabolites and eco-friendly techniques for agricultural weed/pest management. Plants.. 2021;10(7):1418-1422.
- [Google Scholar]
- Copper enzymes in isolated Chloroplast Polyphenol-oxidase in Beta vulgaris. L. Plant Physiol.. 1949;24:1-5.
- [Google Scholar]
- A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod.; IND CR.. 2019;139:111526
- [Google Scholar]
- An overview of agrochemicals and their effects on environment in Nepal. Appl. Ecol. Environ. Res.. 2014;2(2):66-73.
- [Google Scholar]
- Seed research-past, present and future. In: Taylorson R.B., ed. Recent Advances in the Development and Germination of Seeds. New York: Plenum; 1989. p. :1-6.
- [Google Scholar]
- In vitro and in vivo methods for anticancer activity evaluation and some Indian medicinal plants possessing anticancer properties: an overview. J. pharmacogn. phytochem.. 2013;2(2):140-152.
- [Google Scholar]
- Herbicidal potential and quantification of causative allelochemicals from several compositae weeds. Weed Res.. 2003;43:444-450.
- [Google Scholar]
- Osmotic and autotoxic effects of leaf extracts on germination and seedling growth of Alfalfa. Agron. J.. 2004;96:1673-1679.
- [Google Scholar]
- Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic.. 2005;106:309-317.
- [Google Scholar]
- Preliminary screening of some folklore medicinal plants from a Preliminary screening of some folklore medicinal plants from Western India for potential antimicrobial activity eastern India for potential antimicrobial activity. Indian J. Pharmacol.. 2005;37(6):408-409.
- [Google Scholar]
- Phytotoxicity and allelopathic potential of Juglans regia L. leaf extract. Front. Plant Sci. 2022:1-12.
- [Google Scholar]
- Synergistic Inhibitory Effects of Vanillic and p-Hydrozbenzoic Cids on Radish and Grain Sorghum. Journal of Chemical Ecology. 1978;4:425-436.
- [CrossRef] [Google Scholar]
- Phytoremediation efficiency of Portulaca oleracea L. naturally growing in some industrial sites, Dakahlia District, Egypt. Chemosphere. 2019;225:678-687.
- [Google Scholar]
- The Drivers of Herbicide Use among Arable Farmers in Canterbury, New Zealand: Toward an Integrated Approach. Soc. Nat. Resour.. 2022;35(3):281-300.
- [Google Scholar]
- Phytochemical Profile and Herbicidal (Phytotoxic), Antioxidants Potential of Essential Oils from Calycolpus goetheanus (Myrtaceae) Specimens, and in Silico Study. Molecules.. 2022;27(15):1-18.
- [Google Scholar]
- Phytotoxicity of three lactones from Nigrospora sacchari. Phytochem. Lett.. 1998;48(4):625-630.
- [Google Scholar]
- Ethnobotany and pharmacognostical studies of Jasminum sambac Linn. Int. Lett. Nat. Sci.. 2015;37:39-45.
- [Google Scholar]
- Autotoxic effects of aqueous extracts of ginger on growth of ginger seedings and on antioxidant enzymes, membrane permeability and lipid peroxidation in leaves. Allelopathy Journal.. 2012;30(2):259-269.
- [Google Scholar]
- Harbone J., ed. Phytochemical Methods: a Guide to Modern Techniques of Plant Analysis. London: Chapman & Hall; 1973.
- Phytochemical Methods (11th edition). New York, NY, USA: Chapman & Hall; 1984.
- Qualitative and Quantitative Analysis of Phytochemicals in Lepidium Pinnatifidum Ledeb. Sch Int J Tradit Complement Med.. 2021;4(5):67-75.
- [Google Scholar]
- Allelopathic effects of cassava (Manihot esculenta crantz) on radish (Raphanus sativus L.) and ryegrass (Lolium perenne L.) Allelopathy J.. 2010;25(1):155-162.
- [Google Scholar]
- Elemental analysis of the leaves of Vernonia amygdalina and its biological evaluation in rats. Niger J Nat Prod Med.. 2001;5:13-16.
- [Google Scholar]
- The inhibitory effect of grasshopper’s cyperus (Cyperus iria L.) on the seedling growth of five Malaysian rice varieties. Trop. Life Sci. Res.. 2011;22(1):81.
- [Google Scholar]
- Allelopathy for weed control in agricultural systems. J. Crop Prot.. 2015;72:57-65.
- [Google Scholar]
- Phytotoxic effects of selected medicinal plants Collected from Margalla Hills. Islamabad Pakistan. J. Med. Plant Res.. 2011;5(18):4671-4675.
- [Google Scholar]
- Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ.. 2003;26(6):857-865.
- [Google Scholar]
- Characterization of phytochemical profile and phytotoxic activity of Mimosa pigra L. Agric. Nat. Resour.. 2018;52(2):162-168.
- [Google Scholar]
- Determination of free phenolic acids and antioxidant activity of Methanolic extracts obtained from fruits and leaves of Chenopodium album. Food Chem.. 2010;126:1850-1855.
- [Google Scholar]
- Structural diversity of organic chemistry. A scaffold analysis of the CAS Registry. J. Org. Chem. Res.. 2008;73(12):4443-4451.
- [Google Scholar]
- Phytotoxic activity of Ulex europaeus, an invasive plant on Chilean ecosystems: separation and identification of potential allelochemicals. Nat. Prod. Res. 2022:1-7.
- [Google Scholar]
- Plant Complexes and their Isolated Compounds for Oxidative Stress and Inflammation: from Basic Research to Clinical Evidence. Oxidative Medicine and Cellular Longevity 2021
- [CrossRef] [Google Scholar]
- Hydrolysable tannins and biological activities of Meriania hernandoi and Meriania nobilis (Melastomataceae) Molecules.. 2019;24(4):746.
- [Google Scholar]
- Phytotoxic activity of Ocimum tenuiflorum extracts on germination and seedling growth of different plant species. Sci. World J.. 2014;9:1569-1588.
- [Google Scholar]
- Adulteration of low-delta-9-tetrahydrocannabinol products with synthetic cannabinoids: Results from drug checking services. Drug Test Anal.. 2022;4(6):1026-1039.
- [Google Scholar]
- Assessment of allelopathic compounds to develop new natural herbicides: A review. Allelopathy J.. 2021;52(1):21-40.
- [CrossRef] [Google Scholar]
- Quality control of herbal drugs and preparations: The methods of analysis, their relevance and applications. Talanta. 2021;4:1-11.
- [Google Scholar]
- 9 - Flavonoids and Related Compounds from the Medicinal Plants of Africa. In: Kuete V., ed. Medicinal Plant Research in Africa: Pharmacology and Chemistry. Oxford: Elsevier; 2013. p. :301-350.
- [Google Scholar]
- The efficacy and safety of Chinese herbal medicines. Br. J. Nutr.. 2004;91(2):171-173.
- [Google Scholar]
- Allelopathy. In: Camper N.D., ed. Research Methods in Weed Science (3rd edition). Champaign, IL: Southern Weed Science Society; 1986. p. :125-126.
- [Google Scholar]
- Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. in Microbiol. 2022:1-29.
- [Google Scholar]
- Triterpenoid saponins from the aerial parts of Trifolium argutum Sol. and their phytotoxic evaluation. Phytochem. Lett.. 2015;13:165-170.
- [Google Scholar]
- Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Industrial Crops and Products.. 2020;155:112785
- [Google Scholar]
- Microscopic investigations and pharmacognostic techniques used for the standardization of herbal drug Nigella sativa L. Microsc Res Tech.. 2018;81(12):1443-1450.
- [Google Scholar]
- The unseen effect of pesticides: The impact on phytobiota structure and functions. Front. Agron. 2022:1-12.
- [Google Scholar]
- Quality assessment of South African herbal medicines by means of HPLC fingerprinting. J. Ethnopharmacol.. 2005;101(1–3):75-83.
- [Google Scholar]
- Principles and Procedures of Statistics, A Biometrical Approach (3rd Edition,). New York: McGraw Hill, Inc.Book Co.; 1997. p. :352-358.
- Critical factors of vanillin assay for catechins and proanthocyanidins.J. Agric. Food Chem.. 1998;46:4267-4274.
- [Google Scholar]
- The phenolic constituents of Prunus domestica. The quantitative analysis of phenolic constituents. J. Sci. Food Agric.. 1959;10(1):63-68.
- [Google Scholar]
- Pharmacognosy (12th ed.). D.C.: London; ELBS Publication; 1985.
- Biological activity of common mullein, a medicinal plant. J. Ethnopharmcol.. 2002;82(2):117-125.
- [Google Scholar]
- Inhibitory effects of Albizia lebbeck leaf extracts on germination and growth behavior of some popular agricultural crops. J. For. Res.. 2007;18(2):128-132.
- [Google Scholar]
- Phytotoxicity evaluation and phytochemical analysis of three medicinally important plants from Pakistan. Toxicol. Ind. Health.. 2015;31(5):389-395.
- [Google Scholar]
- Phytotoxic effects of safflower yellow exposure on seed germination and early seedling growth of canola (Brassica napus L.) Pak. J. Bot.. 2014;46(5):1741-1746.
- [Google Scholar]
- Microscopic and pharmacognostic standardization of Astragalus scorpiurus bunge. Microsc. Res. Tech.. 2022;85(1):324-338.
- [Google Scholar]
- Study on phytotoxicity evaluation and physiological properties of nicosulfuron on sugar beet (Beta vulgaris L.) Front. Plant Sci. 2022:1-13.
- [Google Scholar]
- Phytochemicals, biological activity, and industrial application of lotus seedpod (Receptaculum nelumbinis): A review. Front. nutr.. 2022;85(1):324-338.
- [Google Scholar]
- Quality control methods for medicinal plant materials. World Health 1998 Organization
- [Google Scholar]
- Phytotoxic Effects of Allelochemical Acacetin on Seed Germination and Seedling Growth of Selected Vegetables and Its Potential Physiological Mechanism. J. Agron. 2022;12(5):1038-1050.
- [Google Scholar]
- Elemental analysis of some medicinal plants used in traditional medicine by atomic absorption spectrophotometer (AAS) J. Med. Plant Res.. 2010;4(19):1987-1990.
- [Google Scholar]







