Translate this page into:
Gut microbiota-centered risk factors and altered immunometabolism in the pathogenesis and prophylaxis of Clostridium difficile infection
⁎Corresponding author. priyankardey28@gmail.com (Priyankar Dey) priyankar.dey@thapar.edu (Priyankar Dey)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aim
Clostridium difficile (C. diff.) infection (CDI) is the major contributor of nosocomial infection globally. The current review aims to provide a comprehensive understanding of the mechanisms behind the critical role of intestinal 3Ms (i.e., microbiota, metabolome, and metabolism) in the pathogenesis and prophylaxis of recurrent CDI.
Material and methods
Primarily centering around the mechanisms related to gut microbiota-dependent risk factors, we have discussed why gut microbial diversity and abundance are critical determinants of infection risk. Additionally, emphasis has been given on the intestine-specific molecular mechanisms related to the modulation of the global metabolome and signaling processes which trigger disease susceptibility.
Key findings
Patients with CDI harbor altered gut microbial phenotype compared to healthy individuals and asymptomatic careers. Clinical and experimental (germ-free mice, mono-strain interactions, longitudinal trails) evidences indicate that alterations of the gut microbiota due to antibiotic exposure, advanced age, immunocompromised conditions and prolonged hospitalization could lead to gastrointestinal colonization of C. diff. Further, the gut microbiota mediated modulation of the mucosal signaling (farnesoid X receptor, aryl hydrocarbon receptor, conserved nuclear receptors, immunological signaling) and the luminal metabolomic signatures play critical role in the disease susceptibility and progression. The use of live biotherapeutics and fecal microbiota transplantation are currently the most suitable prophylactic strategy against recurrent CDI.
Significance
The current review highlights the host-microbiota interaction which dictates the intestinal signaling responsible for the pathogenesis of recurrent CDI.
Keywords
Clostridium difficile
Mucosal inflammation
Opportunistic infection
Commensal bacteria
Risk factor
Fecal microbiota transplantation
1 Introduction
Loss of gut commensals, increased population of pathobionts, and decreased overall microbial diversity and richness are generally considered hallmarks of dysbiosis and are associated with the pathogenesis of chronic diseases. Nevertheless, clinical reports suggest that gut commensals and symbionts that are generally considered as non-harmful or beneficial microbes, under altered physiological conditions could trigger disease causation (Gupta and Dey, 2023; Gupta and Dey, 2023). The mechanisms through which gut commensals could trigger opportunistic infections include colonization fitness, nutritional adaptation, pathoadaptive mutations, and evasion of the host immune response. Thus, the chances of opportunistic infections are condition specific and is dictated by an array of host and microbe specific factors (Tewari and Dey, 2024). In line, while several clinical reports of infections caused by probiotic bacteria are available, GI colonization of Helicobacter pylori (H. pylori) is plausible in individuals without any pathological symptoms.
The most well-studied example of the role of gut bacteria in infectious diseases is Clostridioides difficile infection (CDI). In CDI, antibiotics disrupt the normal gut bacterial community, thereby allowing C. diff. to proliferate and to trigger severe diarrhea and colitis. CDI is difficult to treat owing to bacterial toxin production, resistance to medicines, and compromised immune responses. However, new approaches, such as fecal microbiome transfer (FMT), have emerged as a result of advances in our understanding of the human GI bacterial community, raising the hope that C. diff. infections can be successfully treated even in patients with recurrent infections. The normal microflora also competes with C. diff. for nutrients and attachment sites in the gut, limiting its ability to colonize and cause diseases. In healthy individuals, the gut microbiota is diverse and dynamic with a balance between commensals and pathobionts. However, antibiotic use can disrupt this balance, leading to a reduction in community diversity and an increase in the abundance of pathobionts and pathogens such as C. diff.
This review provides an all-inclusive narration of the diverse functions of the intestinal bacterial population in the pathogenesis and prevention of CDI. We also highlight the association of the gut microbiota with the risk factors for CDI and how GI immunometabolic mediators, which are known to reciprocally interact with the microbiota, influence CDI. Collectively, the current review provides an updated bird’s eye view of the role of gut microbiota in the pathogenesis and prophylaxis of CDI.
2 General concepts of Clostridium difficile infection
The bacterial genus Clostridium (phylum Firmicutes) is an anaerobic, fermentative, gram-positive bacterium that forms endospores. Initially known as Bacillus difficilis, it was renamed C. diff. in 1970 after being properly characterized and establishing its infectious role. A vast part of the genome consists of genes involved in tissue colonization, antibiotic resistance, pathogenicity, and surface antigens. Additionally, specific genes have been shown to be responsible for the adaptive mechanisms that allow C. diff. to endure and develop under harsh conditions in the GI tract. C. diff. was recognized as an infectious pathogen, despite being commonly reported in the gut microbiota of newborns owing to the presence of certain toxins (Rousseau, 2012). Although C. diff. is capable of generating both enterotoxin toxin A (TcdA) and cytotoxic toxin B (TcdB), the strain that exclusively produces TcdB is more common worldwide.
The prevalence of C. diff. was found in environmental samples, fecal matter, and hospital surfaces (Fig. 1). Oral uptake of live C. diff. or spores through tainted food or water, or direct transfer from active carriers in medical settings is the main method of disease transmission. Because C. diff. are highly resistant to severe environmental factors, such as bleach and antimicrobials, C. diff. endospores are the most probable transmission forms that endure under aerobic environmental conditions. Vegetative cells are destroyed in the gastric environment after oral ingestion, but resistant spores are able to survive and germinate under suitable circumstances in the proximal intestine. When an appropriate composition of bile acids (e.g., taurocholate, cholate, deoxycholate, glycolate), short-chain fatty acid (SCFA) (e.g., butyrate and succinate), and altered immune reactions (e.g., impaired adaptive and humoral immune response) are present in the GI tract, viable cells of the bacterium multiply rapidly and form colonies in the duodenum. Thereafter, TcdR gene is translated, causing C. diff. Toxin production and subsequent GI pathologies.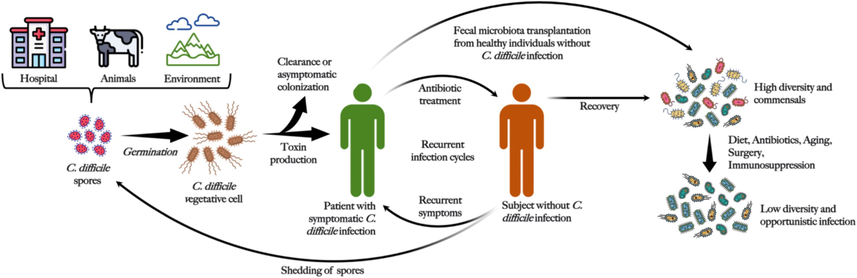
Pathogenesis of C. diff. infection.
The pathogen C. diff. causes opportunistic infections in a favorable intestinal environment (Fig. 2). Age, underlying comorbidities, non-surgical GI surgical procedures, use of nasogastric cannulas, anti-ulcer medications, extended hospitalization, and extensive use of antibiotics have all been identified as risk factors for CDI.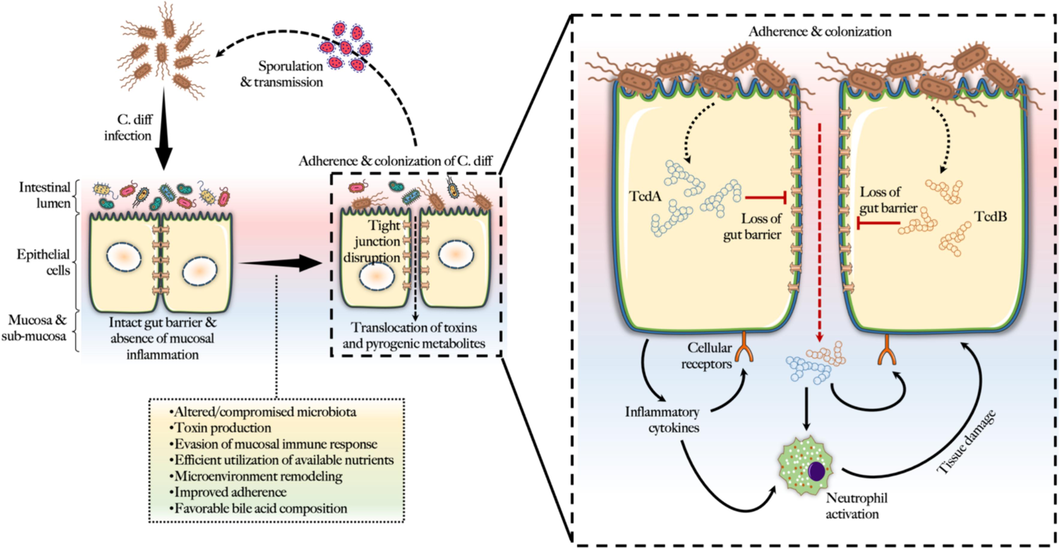
Mechanisms of gastrointestinal colonization and toxicity by C. diff.
3 Role of gut microbiota in risk factors associated with CDI
A number of risk factors other than the use of antibiotics contribute to CDI severity and susceptibility to CDI. The main risk factors associated with CDI development were as follows (Fig. 3).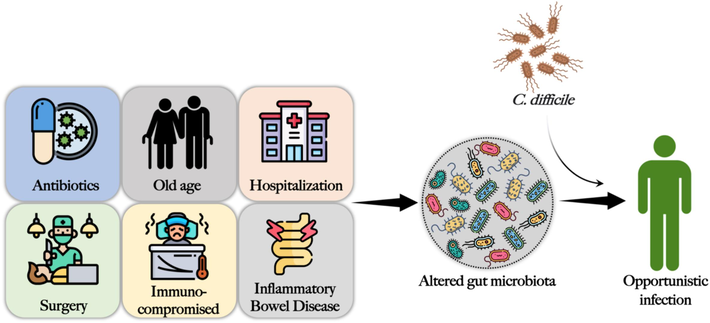
Risk factors of C. diff. infection that alters the gut microbiota to create an intestinal microenvironment that supports opportunistic colonization and infection by C. diff.
3.1 Exposure to antibiotics
Antibiotic use alters normal gut flora, allowing C. diff. to grow and release toxins that can cause illnesses (Dey, 2024; Kaur and Dey, 2022). Broad-spectrum antibiotics, lengthy courses, and repeated antibiotic exposure carry the greatest risks. In one study, the FDA Adverse Event Report System (FAERS) was used to assess the relationship between CDI and significant antibiotic classes in recent years (Teng, 2019). A total of 5,187 of the 2,042,801 reports examined in this study were CDI. The inclusion criteria revealed that lincosamides, including clindamycin, had the greatest proportion of CDI reports associated, accounting for 10.4 % of all lincosamide treatments. Early age antibiotic exposure is related to both the onset of gastrointestinal problems and IBD later in life. Therefore, antibiotic-treatment-mediated depletion of commensal bacteria likely creates a mucosal microenvironment that supports opportunistic infection caused by C. diff. Patients taking proton pump inhibitors had a 65 percent higher risk of CDI. Shannon's diversity and microbial richness were lower in 211 people who used proton inhibitor medications, accounting for a 20 % depletion of overall bacterial taxa. Antibiotic-associated dysbiosis may sometimes result from overgrowth of C. diff. is also known as Clostridiodes difficile–associated diarrhea (CDAD) (Kesavelu and Jog, 2023). It may manifest as deadly pseudomembranous colitis and chronic recurring infections. Collectively, regardless of the country and population, chronic antibiotic treatments have been associated with opportunistic clinical infections and associated clinical manifestations have been extensively reported.
3.2 Advanced age
CDI susceptibility is increased by age-related changes in the gut microbiota, weakened immunity, and a greater incidence of comorbidities. Additionally, the risk is increased for older persons because they regularly visit healthcare facilities where C. diff. transmission is more prevalent. One study assessed the risk variables, particularly advanced age, that may be associated with the development of severe CDI (Patel et al., 2016). Using a regression model, it was shown that advanced age (odds ratio, 2.43; p < 0.005) was associated with severe CDI. Veterans were more likely than non-veterans to be in the critical care unit when diagnosed with severe CDI (p = 0.004). Additionally, a statistically significant relationship existed between the administration of three or more antibiotics and severe CDI (48 % severe vs. 34 % mild-moderate). According to data from the US Emerging Infections Program (EIP), older people made up 57 % of the predicted CDI cases and the prevalence of CDI increased considerably with age. According to EIP research, older people (18–44-y) had a more than 13-fold higher incidence of CDI than their younger counterparts (1–17-y). A weakened immune system with age is implicated in increased CDI risk in older individuals. For instance, in a mouse model, it was demonstrated that, during severe CDI, aging impairs the intestinal innate immune response and is linked to altered cytokine levels and granulocyte mobilization, which are essential for infection resistance and clearance of C. diff. (Dey, 2020; Dey, 2019). Aging is associated with loss of bacterial diversity and reduced populations of gut commensals. Thus, with the progressive loss of commensals and reduced immune protection with aging, there is an increased risk of CDI in older individuals. Increased usage of antibiotics in aged individuals may alter the usual intestinal flora and make patients more prone to infection. Numerous studies have shown that elderly patients, particularly those with CDI, have a lower gut microbial diversity. The term ‘microbe-aging’ refers to the age-related dysbiosis that occurs in humans with a decrease in Clostridiales and Bifidobacterium, an enrichment of Proteobacteria, and an overrepresentation of pathobionts such Enterobacteriaceae.
Researchers investigated the function of gut microbiota in regulating the differences in innate immune responses to CDI in older (18-mo) and young (3-wk) mice (Shin, 2018). During early infection (day 2, before the peak C. diff. burden), they observed decreased levels of pro-inflammatory cytokines, less recruitment of neutrophils, and reduced tissue damage in the cecum of older animals, but they noted no alterations by day 5, when the illness was at its worst. Intriguingly, older mice gained members of the gut microbiota of young mice when their cages were switched, and as a result, they showed more robust early innate immune responses and better survival following CDI. The opposite did not occur; young mice did not exhibit increased CDI disease or acquired characteristics of the aged mouse microbiota, indicating either that young mouse microbiota members are more invasive or that the microbiota or aged mice have less capacity to resist colonization and, therefore, are susceptible to invading pathogenic infections. This evidence suggests that, with older age, changes in the gut microbiota and a diminishing immune response can create an intestinal microenvironment that can support CDI.
3.3 Healthcare-associated exposure
Patients with multiple comorbidities, extended hospital stays, and frequent antibiotic usage are seen in hospitals, long-term care institutions, and outpatient clinics where CDI is commonly reported. The individuals were exposed to C. diff. spores via repeated contact with healthcare facilities, which aids transmission. One case-control study evaluated whether healthcare exposures prior to hospitalization were associated with an increased risk of hospital-onset CDI and how the risk of infection varied with time between exposure and hospitalization (Miller, 2021). The data showed that prior to admission, hospitalized individuals with CDI had considerably greater healthcare exposure. Medical visits, use of antibiotics, and family exposure were linked to a higher risk of CDI during hospitalization. With time, the correlation between exposure and hospitalization reduced. The outcomes were the same for all CDI cases. The abundance of gut commensal Bifidobacterium is known to limit CDI and is depleted during hospitalization. For instance, one study investigated gut dysbiosis in critically ill individuals and assessed how well it could predict in-hospital death (Wei, 2021). The data showed an inverse association between mortality rate and abundance of Bifidobacterium. Interestingly, recent data showed (Mahnic, 2020) that in hospitalized gastroenterological patients, the diverse phenotypes of microbiota dysbiosis are disease-independent and distinguished by the predominance of either Enterobacteriaceae or Enterococcus, both of which are associated with GI chronic pathologies, while several members of these genera demonstrate resistance to antibiotics and opportunistic infections. Specifically, by analyzing the gut microbial profiles of 121 hospitalized gastroenteritis patients, common features of dysbiosis, such as depletion of Faecalibacterium and Roseburia, and an increase in Enterobacteriaceae or Enterococcus, were identified. Collectively, under hospitalized conditions and with an already underperforming immune system, individuals are highly prone to CDI infections because under such conditions, the gut microbial population is significantly altered, facilitating opportunistic infections.
3.4 Procedures and surgery related to the gastrointestinal tract
The gut microbiota is altered by procedures, including colonoscopy, ileostomy, and stomach acid suppression treatment (such as proton pump inhibitors), which fosters C. diff. colonization. CDI is associated with secondary infections, operational delays, and emergencies. Recent study suggested that the abundance, function, and health effects of gut commensals may be altered significantly as a result of gastrointestinal surgery (Fang, 2021). Neoplastic or inflammatory bowel illness may require elective colorectal surgery. In these cases, microbiota imbalance is referred to as a preoperative condition and is associated with both preoperative and postoperative problems. Altered microbiota is a key risk factor for opportunistic CDI, and dysbiosis has been reported in several types of surgical procedures including cardiovascular, gastrointestinal and bariatric surgery. Specifically, alterations in common microbes associated with CDI. For example, the microbiome was examined in European and Asian populations undergoing GI surgery and a decrease in Firmicutes, Bacteroides, Bifidobacterium, Atopobium, Prevotella, and Escherichia (Vakili, 2020). Others show that a higher concentration of facultative anaerobes and nitrate reducers (e.g., Proteobacteria, Pseudomonas, Enterobacter, Staphylococcus, Klebsiella, Enterococcus) were present in surgical sites within a week after surgery (Gibiino, 2022). An increase in Bifidobacterium and a reduction in Enterobacteriaceae, Staphylococcus, and Pseudomonas during the first 2-wk after surgery showed a similar tendency towards fewer post-surgical infection problems, according to another research (Maekawa, 2020). Researchers have revealed that intestinal surgical procedures (e.g., ileocolonic resections and colectomy) decrease the diversity of gut microbiota in IBD patients among studies that examined IBD, a condition closely related to CDI.
3.5 Immunocompromised state
The connection between intestinal dysbiosis and immunocompromised conditions is well established. In particular, intestinal dysbiosis increases the risk of infection in immunocompromised individuals. The microbial equilibrium is disturbed when the gut microbiota is altered, resulting in a hyperactive immune system that is more prone to infection. The following examples illustrate how gut dysbiosis may result in an immunocompromised state.
Infection: Individuals with pre-existing infections could be vulnerable to opportunistic infections, such as CDI due to an already diminished immune response and disruption of the gut commensal population. One of the best examples comes from the fact that patients treated with metronidazole, amoxicillin, proton blockers, and bismuth subsalicylate to eradicate the primary infection, Helicobacter pillory could become susceptible to CDI. Similarly, H. pylori infection also alters the gut microbiota, and these microbial features (e.g., lower microbial diversity and richness, increased pathobionts, and reduced commensals) are common with microbial features associated with CDI. Nevertheless, secondary CDI is attributed to antibiotic-mediated unintentional disruption of the normal gut microflora during the treatment of primary infection.
Autoimmune disease: By producing inflammatory cytokines in the gut and facilitating their systemic translocation, gut dysbiosis can aggravate tissue injury by influencing the immune response. One group of researchers investigated hospitalized immunocompromised patients with CDI for humoral immune response against C. diff. toxins (Alonso, 2021). In this study, 98 participants, divided into intracerebral hemorrhage (ICH) and non-ICH groups, had similar baseline antitoxin A and B antibody levels. By day 3, ICH group had lower antitoxin A IgG, antitoxin A IgA, and antitoxin B IgA levels. By days 10–14, ICH participants had reduced antitoxin A IgG concentrations, and on day 3, lower antitoxin B IgA was evident in their stool samples compared to non-ICH subjects. These data collectively suggest that, when compared to non-ICHs, ICHs with CDI showed reduced levels of C. diff. antitoxin antibodies in serum and stool throughout early CDI treatment, reflecting a diminished host humoral immune response. Using samples from 248 patients with severe-complicated and recurrent CDI, it was demonstrated that, among other risk factors, patients with autoimmune disorders, having a weakened immune response, were at high risk of CDI (Tijerina-Rodríguez, 2022). Individuals with impaired immune systems, such as those with HIV infection, who have undergone solid organ transplantation, or are receiving chemotherapy, are more vulnerable to CDI. Immune system vulnerabilities make it difficult to manage C. diff. Using 149 immunocompromised patients, the risk of CDI during an immunocompromised state was evaluated. The data showed that in immunocompromised patients, CDI risk was considerably higher. Hemodialysis, antibiotic treatment, and prior hospitalization are also associated with recurrent CDI. In fact, immunocompromised patients who receive vancomycin for CDI are at risk of recurrence, likely due to antibiotic-mediated depletion of the gut microbiota.
4 Gut microbial phenotypes in CDI patients
Patients with CDI often exhibit significant alterations in their gut microbial profiles. For example, a study utilizing metagenome sequencing to analyze the gut microbiome of 26 CDI patients with CDI found that C. diff. abundance could reach up to 2.8 % (Gregory et al., 2021). Patients with intestinal blooms of the bacterium have been reported to have a decreased abundance of Bifidobacterium. Based on the CDI patient’s microbial profile of the CDI patient, one microbial group had a higher Enterococcus abundance but lower microbial richness. However, patients in the separate group had a higher abundance of Lactobacillus and Bacteroides. Another study examining the community of gut bacteria in people with toxic CDI found a considerable decline in bacterial diversity (Martinez, 2022). In TcdB+ subjects, relative to controls, the mean fraction of Proteobacteria at the phylum level was greater, whereas that of Firmicutes was lower. Additionally, CDI patients with CDI have larger proportions of Enterococcaceae and Porphyromonadaceae. However, the proportions of Ruminococcaceae, Lachnospiraceae, Prevotellaceae, Phascolarctobacterium, Haemophilus, Prevotella, Butyricimonas, Lachnospira, Faecalibacterium, Catenibacterium, Coprococcus and Anaerostipes were lower in TcdB+ patients than in controls. Veillonella, Klebsiella, Enterococcus, and Akkermansia populations increased during the same period. By computationally analyzing 16S rRNA data of 225 fecal matter samples from 93 CDI patients with CDI, Henson reported that Enterobacteriaceae likely provide a favorable gut environment for C. diff. Germination and toxin production. Specifically, an Enterobacteriaceae-rich microbial cluster in post-diagnosis samples was associated with recurring cases and demonstrated reduced secondary bile acid production, but enhanced aromatic amino acid degradation. This pattern also appeared in pre-FMT samples from donors and patients with recurrent CDI, showing that similar microbial traits were present prior to FMT.
CDI and its associated gut microbial phenotypes have also been investigated using longitudinal approaches. For instance, a study from Belgium investigated the occurrence of C. diff. over a 4-mo period in 23 senior residents in nursing homes and examined the total microbial diversity in feces using 16S rRNA (Rodriguez, 2016). The data showed that each patient exhibited a distinct, stable bacterial profile throughout the trial. There were noticeable changes in the prevalence of certain bacterial families, such Lachnospiraceae and Verrucomicrobiaceae, among patients with CDI. Those who tested positive for C. difficile also showed a consistent decline in A. muciniphila. Indeed, recent preclinical data has shown that treatment with A. muciniphila. can ameliorate CDI by modulating the gut microbiota and metabolome. Among the observations from other longitudinal studies, one study reported that the fecal communities in patients with recurrent CDI were very variable in bacterial composition and were distinguished by significantly less diversity and richness than those from control participants and patients with an initial episode of infection (Chang, 2008).
5 Intestinal metabolomic signatures in CDI
Both host and microbiota-derived intestinal metabolites, are increasingly gaining importance owing to their diverse bioactivities and for serving as disease predictive markers. Toxin-producing C. diff. induces specific metabolite production in gut commensals that are not linked to the patient’s condition. Specifically, the metabolite profile of patients with CDI (toxin+ vs. toxin-) showed no relationship with age, sex, patient physiology, antibiotic treatment, duration of the condition, or medical history. It has been speculated that C. diff. can adjust its metabolism according to the host to achieve successful colonization by competing against gut commensals. Indeed, a significant alteration in the gut metabolomic profile was reported 24–30-h after CDI, indicating changes in the global metabolome due to CDI (Fletcher, 2018). During fecal metabolomic analysis, it was found that 38 % of the metabolites were common between symptomless and symptomatic CDI patients (Theriot and Fletcher, 2019). Major volatile metabolites in C. diff. included straight and branched carboxylic acids; notably, p-cresol. In addition, CDI reduced the amount of secondary bile acids, sugars, free lipids, and dipeptides but increased the levels of primary bile acids and sugar alcohols. This indicates that CDI influences the gut microbial community since the majority of these metabolites are associated with gut commensals. Under antibiotic therapy that eliminates gut microbes, C. diff. utilizes various available sugars (e.g., fructose and raffinose) to proliferate and utilize bile acid taurocholate for germination. By increasing the levels of lipids and peptides and lowering free amino acids and sugars, Firmicutes and Bacteroidetes can influence C. diff. These metabolites are essential for the growth and germination of C. diff.
Elevated concentrations of sphingomyelins, sphingolipids, and phospholipids have been predicted to be initial biomarkers of gut inflammation and severe CDI. CDI vulnerability of CDI is linked to greater amounts of primary bile acid (promoting C. diff. spore germination), and amino acids (helps in C. diff. growth). Robinson et al. reported lower non-canonical and unsaturated bile acid levels in CDI patients. According to them, 2-hydroxy-4-methylpentanoic acid, 4-methyl pentanoic acid, cholenoic acid, isoleucine, eicosatrienoic acid, ribitol, tyrosol, glyceryl glycoside, and fructose are the major metabolites linked to CDI. N-acetylglucosamine, sialic acids, and N-acetylneuraminic acid (gut mucins) have been observed to make GF mice more susceptible to CDI. In addition, it was observed that bacterial strains that were unable to consume sialic acid, demonstrated 4-times less infectivity than C. diff. variants capable of utilizing sialic acid (Jandhyala, 2015). In previous studies, C. diff. induces gut commensals to generate indole, which helps C. diff. growth, and CDI subjects were found to have increased concentrations of indole in fecal samples (Baunwall, 2020). Based on the concept that bacterial metabolic processes can influence toxin production in CDI, researchers investigated the volatile metabolomic signatures of C. diff. using the culturomic approach (Biwer, 2022). In addition to several sulfur-containing metabolites, aromatic compounds such as 2-methyl-1-propanol and 2-methyl-1-propanethiol were identified. Changes in the quantities of cysteine and methionine in the growth medium had an impact on the overall volatile profiles, indicating that cysteine functions in the formation of sulfur volatiles and provides a backbone for disulfide synthesis. Methionine may help in short-chain disulfide formation, supporting the typical odor of C. diff. However, certain metabolites can also limit CDI and associated mucosal damage. For instance, it has been experimentally proven that retinol has the potential to reduce apoptosis, enhance cell migration and proliferation, and reduce gut barrier defects caused by TcdA (Maciel, 2007). Collectively, these results indicate that GI metabolomic shifts are associated with altered gut microbial profiles in CDI, which likely influences bacterial nutrient utilization and altered host and microbial metabolic processes. Therefore, fecal metabolomic profile could be a predictor of long-term CDI risk, pathogenesis, treatment success, and infection recurrence.
6 Intestine-specific signaling in CDI: Potential role of gut microbiota
6.1 Bile acid signaling
Proliferation of gut commensals can be stimulated by bile acids. The production of pathogenic microorganisms may be inhibited by bile acids, as observed in the case of C. diff. that is inhibited by the secondary bile acid, lithocholic acid. One type of immune cell that exerts a humoral immune response against C. diff., and helps decrease mucosal inflammation and the burden of CDI, is called a regulatory T cell, are stimulated by bile acids. Comparison of stool samples from controls with recurrent CDI (rCDI) and first-time CDI (fCDI) patients revealed greater secondary bile acids in controls (Allegretti, 2016). Compared to controls and fCDI, primary bile acids were increased in rCDI. Bile acid ratios successfully identified rCDI and fCDI using random forest regression (84.2 % accuracy). The best predictor was the ratio of glycoursodeoxycholate to deoxycholate in stool. Indeed, gut microbial functional data predicted that bacterial bile salt hydrolase gene abundance differed considerably across groups, likely due to an altered microbial profile in CDI. Changes in bile acid profile have also been reported in individuals undergoing FMT. For instance, bile acid analysis of samples from five donors, seven patients, and six post-FMT recipients revealed that post-transplant patients’ fecal secondary bile acid profiles became more donor-like (Brown, 2018). FMT consistently increased and diversified fecal secondary bile acid levels, potentially due to the ligand activity of chenodeoxycholic acid (CDCA) and cholic acid (CA), impacting the overall bile acid levels. Bile acid deconjugation was unaffected, but 7α-dehydroxylation and epimerization were depleted, possibly owing to Clostridium targeting antibiotics and elemental nutrition. FMT restored secondary bile acids and decreased primary CDCA and CA levels.
In addition to influencing the gut microbiota, bile acids are known to influence C. diff. in various ways. Bile acids are essential for C. diff. spores to germinate. Indeed, experimental data show that within 24-h of the host consuming spores, CDI causes an influx of bile acids into the stomach (Wexler, 2021). In response, the host lowers the gene expression for bile acid production. These bile acids promote the development of C. diff., as shown by the delayed colonization and decreased germination observed in mice treated with the bile acid sequestrant cholestyramine. These results implied that C. diff. may promote germination after infection and that changing the flow via bile acid pathways might control C. diff. outgrowths in individuals prone to CDI (Wexler, 2021). Other studies have shown that specific microbes that produce secondary bile acids (e.g., C. scindens) defend against C. diff. infection irrespective of the production of secondary bile acids (Aguirre, 2021). Attempts to re-establish colonization resistance (such as FMT or precision bacterial treatment) based on re-establishing secondary bile acid production could therefore be crucial in CDI treatment. It is more crucial to rebuild organisms that produce 5-aminovalerate or absorb proline and glycine, which are associated with bile acid signaling.
C. diff. Spore formation and survival in the colon are dependent on bile acids. Specific gut bacteria (such as Lactobacillus, Bifidobacteria, Enterococcus) metabolize primary to secondary bile acids, which then signal through Farnesoid X receptor/ Takeda G-protein-coupled receptor 5 (FXR/TGR5) to influence various intestinal processes. In rodents fed a high-fat diet, treatment with the bile acid analog obeticholic acid can reduce the severity of CDI. Supplementation with obeticholic acid has been shown to lower primary bile acid production and to reduce C. diff. proliferation, and enhances treatment outcomes. However, bile acids can trigger the sprouting of bacterial spores, and a gut microbial community abundant in Clostridium spp. can increase the luminal level of bile acids, causing diarrhea and mucosal inflammation (Weingarden, 2016). Given that ursodeoxycholic acid medication heals infection in individuals with repeated gut inflammation, normalizing bile acid degradation may be a crucial component for curing CDI (Malhi and Camilleri, 2017). Additionally, research involving CDI-affected animals has demonstrated that ursodiol therapy modifies bile acid metabolism separately from alterations in the gut flora. Finally, tryptophan-derived antibiotics produced by gut microorganisms with bile acid 7-dehydroxylating capabilities can inhibit C. diff. growth (Kang, 2019). By limiting C. diff. Cell proliferation and secondary bile acids improve the efficacy of antibacterial therapies, demonstrating a relationship between specific bile acids and infection. The protective mechanism of C. diff. is excluded is the metabolism of bile acids through a 7α-dehydroxylation pathway present in certain members of healthy microbiota. C. diff. Vegetative development is severely inhibited by these 7α-dehydroxylated secondary bile acids, and the bacteria responsible for this metabolism are eradicated by antibiotic therapy. It has been shown that gut microbes that 7α-dehydroxylate primary bile acids provide bile acid-independent protection against CDI (Aguirre, 2021). Monoassociating C. diff. spores in either wild-type or Cyp8b1-/- (cholic acid-deficient) mutant mice, researchers demonstrated that Stickland metabolism by gut commensals consumes resources important for C. diff. proliferation, indicate that 7α-dehydroxylation (i.e., secondary bile acid production) is unnecessary for protection against C. diff. infection. Collectively, it is suspected that a deeper reciprocal interaction exists between gut commensals and bile acid metabolism, which in turn influences CDI pathogenesis and protection from it.
6.2 Endocannabinoid signaling
Gut microbiota can influence disease outcomes by affecting the intestinal endocannabinoid (CB) system. Changes in CB signaling have been shown to accompany pathological perturbations of gut microbial composition from eubiosis to dysbiosis, which is brought on by high-fat diet-induced obesity or continuous treatment with antibiotics. CB1 and peroxisome proliferator-activated receptors (PPAR) were upregulated, whereas GPR18 and GPR55 were downregulated in the intestines of GF animals, demonstrating dramatic changes in CB signaling in the absence of gut microbiota (Manca, 2020). It is worth noting that within only 7-d, these changes were undone in adult male mice that had undergone a successful FMT operation, emphasizing the fact that gut microbiota influences CB1-dependnet intestinal processes. Gut microbes can also influence CB signaling by producing metabolites (e.g., commendamide) that bind to CB receptors (GPR132). In recent years, it has been shown that the CB system is another intestine-specific signaling mechanism that cooperates with gut microorganisms to control host immunometabolic activities and preserve gut barrier integrity. By interacting with the CB1 receptor and lessening damage brought on by C. diff toxin TcdA, cannabidiol (CBD), a CB1 receptor agonist, has been demonstrated to prevent apoptosis and attenuate leakiness in Caco-2 cells (Gigli, 2017). Previous data suggest that CBD can repair gut integrity and shield enterocytes from inflammatory damage. It is well established that TcdA inhibits Rho GTP, altering mucosal homeostasis, and impairing barrier function. Therefore, patients with CDI may benefit from CBD's inhibitory effects of CBD on the TcdA-mediated downregulation of Rho GTP. Clinical data from almost 60,000 CDI patients who were hospitalized revealed a connection between extensive cannabis use and a decreased prevalence of CDI (Adejumo and Bukong, 2020). N-palmitoylethanolamide (PEA) is an endocannabinoid-mimicking fatty molecule with immunoregulatory characteristics is N-palmitoylethanolamide (PEA). A recent study investigated the efficacy of a genetically engineered probiotic, pNAPE-LP (based on Lactobacillus paracasei), in mice with CDI colitis (Esposito, 2021). A probiotic was prepared to create PEA, which has immunomodulatory properties and restores the integrity of the epithelial barrier. When given pNAPE-LP, mice with colitis demonstrated substantial improvements in histological damage, macrophage count, myeloperoxidase levels, and tight junction protein expression. These findings indicate that pNAPE-LP, which inhibits inflammation and improves gut health, most likely via interaction with the endocannabinoid system, may be a potential treatment for CDI. The participation of the CB system in CDI was also shown by a prospective, long-term investigation of 53 non-immunocompromised people with CDI (Dawkins, 2022). The data revealed that individuals with CDI recurrence of CDI had decreased concentrations of endocannabinoids and anti-inflammatory substances, particularly behenoyl ethanolamide and lignoceroyl ethanolamide. CBs maintain gut homeostasis by regulating the immune system and gut motility. They have also been found to boost the amount of the helpful bacteria Akkermansia muciniphila, which is more prevalent in individuals without recurrent CDI. Collectively, these results point to a crucial function of CBs in recurrent CDI linked to changes in the gut microbiota.
6.3 Aryl hydrocarbon receptor
Gut microbiota can influence aryl hydrocarbon receptor (AhR)-dependent signaling in several ways. Pseudo-endogenous AHR ligands (e.g., tryptophan and indole metabolites) are generated by gut commensals. For instance, the probiotic bacterium Lactobacillus bulgaricus OLL1181 may prevent mucosal inflammation in mice by activating the AhR pathway in the intestines, in addition to providing ailments from C. diff. colonization and mucosal injury. Intestinal AhR controls metabolic processes by acting as a metabolite sensor. Additionally, AhR is essential for attenuating the mucosal adverse immune response and leaky gut. The gut microbial profile differs in animals with missing functioning AhR ligands, which inhibit AhR-dependent signaling. Researchers have shown that administration of indole-3-carbinol (I3C), an AhR precursor ligand, reduces CDI (Julliard, 2017). Before and throughout CDI, C57BL/6, AHR+/-, and AHR-/- mice received diets containing or without I3C as part of the investigation. The findings showed that compared to mice fed a diet high in I3C, those fed a diet lacking I3C showed greater CDI and death rates. In addition, relative to AhR homozygous littermates, I3C supplementation had no discernible effect on CDI in AhR null mice. Without any adverse immune response or microbial translocation, mice receiving I3C supplements showed enhanced populations of regulatory T cells and neutrophil reactions. In a different investigation, the same team demonstrated that I3C supplementation in mice reduced antibiotic-triggered dysbiosis and enhanced the intestinal immune response against CDI associated inflammation, protecting against CDI (Julliard, et al., 2015). Others have reported that to boost the activation of AhR in mice and humans, IL-22 modifies the makeup and function of the gut microbiota (Mar 2023). Specifically, it was demonstrated that mice exposed to IL-22 have altered microbiota, including an abundance of bacteria with the ability to break down the amino acid l-tryptophan, an agonist of AhR. Bacterial indole compounds were elevated in feces, which was associated with enhanced AhR activity. Exogenous IL-22 in UC patients boosted AhR activity and indole concentrations above those of placebo, despite UC patients' lower levels of indole derivatives and AhR activity (Mar 2023). These data are important because by regulating the metabolic activity of the gut microbiota, IL-22-mediated host glycosylation inhibits CDI. Other studies have shown that IFNγ-expressing neutrophils and CDI-associated mucosal immune responses are limited by tryptophan catabolism, indicating that diminished AhR activity can regulate the mucosal immune response in CDI.
6.4 Nuclear receptor superfamily
The retinoid X receptor (RXR) family plays an important role in the control of gut inflammation. Evidence suggests that retinol protects C. diff. toxin TcdA-induced apoptosis, barrier dysfunction, and enhanced cell migration and proliferation. RXR-dependent transcriptional mechanisms that control genes linked to barrier protection. The gut microbiota affects the intestinal pregnane X receptor (PXR), which is triggered by ligands and controls genes that regulate xenobiotic metabolism and enzymes involved in drug detoxification and transport. PXR is crucial for the host intestinal response to C. diff. toxins; hence, inhibition PXR signaling may be a successful method to reduce inflammatory damage in CDI. Pregnenolone 16-carbonitrile, a PXR agonist, was used to reduce the severity of mucosal inflammation induced by C. diff. toxin in mice with functional PXR, in contrast to Nr1i2-/- PXR-deficient mice, which have an increased innate inflammatory response and decreased survival (Erickson, 2020). Targeting PXR-dependent signaling in mice also reduced the inflammation induced by TcdA in colonocytes (Esposito, 2016). Collectively, these data indicate that the RXR and PXR signaling pathways significantly affect mucosal immunity and may offer potential therapeutic targets for CDI. PPAR primarily functions as a metabolic regulator. Earlier studies showed that PPAR-γ plays a key role in CDI including the immune response, mucosal tissue damage, and disease severity. Using a mouse model of CDI, researchers recently discovered a reduction in the expression of PPAR-γ and tight junction protein occludens in colonic tissues (Lai, 2022). In PPAR-γ deficient animals, intestinal permeability and bacterial dispersion were found to be much greater in these mice than in wild-type mice during CDI. Furthermore, the characteristics of CDI, including weight loss, inflammation, and intestinal integrity, were enhanced by the administration of the PPARγ agonist pioglitazone. These findings suggest that PPAR-γ might be a therapeutic target in CDI owing to its role in modulating colonic inflammation and integrity (Lai, 2022). Finally, another nuclear class of receptor, vitamin D receptor, plays a critical role in intestinal metabolic processes and is associated with pathological consequences along the gut-liver axis. Mouse KO for the vitamin D receptor not only shows altered gut microbiota, but the serum bile acid and lipid profile also correlates with the gut microbial profile (Sun, 2018). As vitamin D is known to have immunomodulatory effects, one study investigated the role of vitamin D in CDI (Sahay and Ananthakrishnan, 2014). Data from 68-mateched case-control pairs showed that patients with CDI had low serum levels of vitamin D compared to controls. This finding indicates that individuals lacking functional vitamin D receptors are likely to be more susceptible to CDI. Vitamin D receptors have been shown to protect against mucosal inflammation and limit gut microbial dysbiosis, which are directly associated with CDI risk.
7 Gut microbiota and C. Diff. interactions: Experimental evidences
Clinical data have shown that healthy individuals typically do not have C. diff. in their intestines. However, asymptomatic patients have some presence of C. diff., whereas symptomatic patients have an increased abundance (Zhang, 2015). The subsequent section discusses how C. diff. colonization affects the gut microbiota and how some good gut bacteria, such as probiotics, can experimentally demonstrated to help eradicate C. diff. or less, the negative effects associated with CDI. These findings reveal how pathobionts (as C. diff.) and gut commensals interact, as will be explored further below (Fig. 4).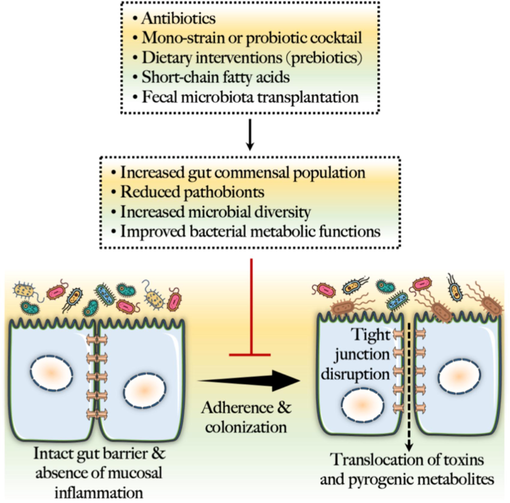
Microbiome-centered prophylactic strategies against C. diff. infection.
Investigations using antibiotic challenges have shown that typical anaerobic microflora protect against aerobic bacteria (Dethlefsen and Relman, 2011). Even though C. diff. does not belong to the normal gut flora, patients receiving antibiotics have higher rates of opportunistic colonization. Animal studies have demonstrated that the use of antibiotics can reduce the colonization resistance of opportunistic diseases. The loss of intestinal microbiota, which is equivalent to an increase in the colonization of opportunistic microorganisms, such K. pneumonia, E. coli, P. aeruginosa, Salmonella sp., and Citrobacter sp., is associated with a decrease in colonization resistance (Yadav and Jha, 2019). Changes in the gut microbial community caused by antibiotic therapy may affect the capacity of microbes to degrade bile acids, which could facilitate increased colonization of C. diff. Bile acids are necessary C. diff. spore germination and antimicrobial properties. To create secondary bile acids (e.g., ursodeoxycholate, deoxycholate, and lithocholate), gut bacteria deconjugate and hydroxylate primary bile acids (e.g., conjugated taurocholate and unconjugated cholate). However, microbial bile acid metabolism is disturbed in individuals receiving long-term antibiotic therapy, which promotes C. diff. overgrowth, and higher germination. Based on the data connecting CDI with dysbiosis and drug-induced sensitivity, it has been hypothesized that C. diff. Colonization might change intestinal metabolites, inhibiting the re-colonization of normal gut microbiota following antibiotic therapy. Researchers have found that tryptophan-derived metabolite indole levels in the feces of CDI patients with CDI were greater. Despite the fact that C. diff. itself does not produce indole-like compounds, it may stimulate additional intestinal organisms to do so, which would prevent the growth of commensals and allow C. diff. to induce opportunistic infections.
GF mice are regarded as the gold standard for researching variables connected to the gut microbiota in human health and illnesses, despite immature immune responses and many physiological abnormalities. Numerous studies have examined the C. diff. colonization patterns in GF mice. Previous research has demonstrated C. diff. injected GF mice exhibited inflammation in the lamina propria, diarrhea, and elevated cecal endotoxin levels, although their death rates were low (<2%) (Feuerstadt, 2022). This shows that the existence of microbiota or the interaction of bacteria with microbiota is necessary for greater CDI pathogenicity. According to previous studies, specific microbes can improve C. diff. colonization and severity of illness Since previous research has indicated that Lachnospiraceae populates animals with moderate CDI, they examined the impact of E. coli and Lachnospira on C. diff. colonization. However, animals with severe CDI exhibit overgrowth of E. coli (Kamada, 2013). Before administration C. diff, several Lachnospira and Escherichia strains were individually colonized in mice lacking gut microbes. These findings demonstrated that Lachnospira but not E. coli pre-colonized animals, displayed significant clinical symptoms and mucosal damage. In particular, infection with the VPI-10463 strain in mice pre-colonized with E. coli was lethal within 2-d, whereas animals pre-colonized with Lachnospira did not exhibit similar effects.
Owing to their important function as gut symbionts that regulate the intestinal immunological response, Bifidobacteria have grown in popularity. Accordingly, Bifidobacteria could be used to effectively treat CDI. For instance, the abilities of several Bifidobacterial spp. to inhibit C. diff. using a mixed culture strategy was examined, and the toxicity profiles when inulin, Synergy, and Actilight were used as substrates (Valdés-Varela, 2016). According to previous studies, the growth and toxicity of C. diff. was decreased by the fructooligosaccharides employed by B. longum and B. breve but not by inulin. It is noteworthy that in co-culture environments, only B. animalis increased. Additionally, B. longum and C. diff. co-culture or B. longum cell-free media supernatant lowered C. diff. growth, and toxin levels (Pal, 2023). Additionally, treatment with B. longum attenuated intestinal damage and C. diff. toxicity. Taken together, these findings suggest that inter-microbial interactions can affect CDI resistance or susceptibility and that knowledge of these interactions could aid in the development of effective CDI therapies using gut commensals.
In contrast to methods that utilize antibiotics to investigate the impact of microbiota depletion on CDI, it has been shown that adding probiotics or microbial consortia can attenuate CDI. For example, researchers have examined the curative and preventing powers of L. reuteri (Milner, 2021). Antibiotics were administered orally to the mice before intraperitoneal injection of clindamycin to initiate CDI exposure. L. reuteri was added to the basal diet, and C. diff. was administered orally to the mice. Collectively, these results show that L. reuteri decreased the intensity and incidence of infection in preventative and therapeutic contexts. Another study demonstrated that L. acidophilus therapy increased the survival of infected mice while also reducing the gene expression crucial for pathogen pathogenicity (Silva, 2020). The effectiveness of a multi-strain probiotic and synbiotic composition against CDI has been proven. Prior to CDI, antibiotic-treated C57BL/6 mice were administered a cocktail of nutrients including resistant starch, galactooligosaccharides, isomaltooligosaccharides, L. plantarum, B. breve, L. paracasei, and B. lactis. These mice showed decreased levels of fecal toxins, attenuated CDI, and improved colonization by Bifidobacterium and Lactobacillus. Others have shown that a probiotic cocktail made of the bacteria L. plantarum, L. reuteri, L. gasseri, L. murinus, and B. infantis and B. adolescentis limits C. diff. colonization (Pal, 2023). After receiving probiotic therapy, antibiotic-treated mice with CDI exhibited less C. diff. colonization, and more diverse gut microbes. Prevalence of C. diff. was inversely correlated with Butyricicoccus, Rikenellaceae, Ruminococcus and positively correlated with Akkermansia, Bacteroides, and Blautia. In mice receiving probiotic treatment, secondary bile acids, which have antimicrobial effects, and primary bile acids, which normally encourage C. diff. germination was decreased.
8 Gut microbial modulation as a treatment for CDI
8.1 CDI and dietary interventions: Role of gut microbiome
A key component of CDI is the altered intestinal microflora, which is frequently correlated with Western diets. Diet is the main factor that interacts with microbes and influences the overall abundance, diversity, and richness of gut microbes. For instance, a diet rich in fat and protein exacerbated antibiotic-induced CDI in rats, whereas a diet high in carbohydrates prevented it. Another study reported that after 12-d of infection, mice were infected with C. diff. and supplemented with a diet with a high microbiota-accessible carbohydrates (MACs) content can reduce the extent of CDI and foster a gut microbial community with an anti-inflammatory phenotype (Hryckowian, 2018). After switching from breast milk to cow’s milk, colonized newborns demonstrated a rapid shift in the gut microbiome, leading to the elimination of C. diff. (Davis, 2016). Specifically, there was a fluctuating level of colonization with toxic and non-toxic C. diff. strains, with toxins being found, but no diarrhea in the neonate. Within 5-d of switching to cow's milk, the microbiota composition underwent a significant and long-lasting change, which coincided with a decrease in C. diff. at weaning. Along with a progressive rise in fecal pH, there was an increase in certain bacterial species, including Bacteroides and Blautia, and a decline in Bifidobacterium and Lactobacillus. Many pathogenic cellular processes require micronutrients and specific nutritional components, and their availability in the gut can contribute to CDI susceptibility by modulating the gut microbes. Other studies have indicated that dietary components such as proline, glycine, hydroxyproline, sorbitol, trehalose, and cellobiose can be utilized by C. diff. to achieve successful colonization through a mechanism involving reduced abundance of colonization-resistant gut commensals. The results of specific dietary intervention studies on CDI are summarized in Table 1.
Dietary component
Study conclusions
Pectin (Ramos Meyers et al., 2022)
Attenuates intestinal permeability and increased microbiome diversity while reducing inflammation markers in mice with CDI. SCFA levels increased as a result of pectin treatment, and the microbial composition improved with a greater proportion of Lachnospiraceae and a reduced population of Enterobacteriaceae. The defense mechanisms of pectin against CDI have been linked to the AhR pathway
Vitamin D (Ananthakrishnan, 2014)
Mothers' milk with vitamin D fortification reduced the amount of C. diff. in their children, and this impact was unrelated to changes in the newborns' microbiome. In particular, the microbiota diversity of infants nursed with milk enriched with vitamin D and those who did not did not vary.
Microbiota-accessible carbohydrates (MACs) (Hryckowian, 2018)
A diet high in MACs can reduce C. diff. loads whereas MACs lack can prolong CDI. Dietary changes are correlated with altered microbial composition, its metabolic activity, and inflammation caused by C. diff.
Xanthan gum (Schnizlein, 2020)
Increased levels of SCFAs and fiber-degrading microbial taxa provide protection against C. diff. colonization during antibiotic therapy. The preservation of the microbiota's variety and absolute abundance, which prevented C. diff. colonization, is what caused xanthan gum's protective benefits.
Epigallocatechin-3-Gallate (Wu, 2022)
Improves survival rates, reduces inflammation, and restores the intestinal barrier after CDI in mice. It also modifies the intestinal microbial community, reducing the abundance of Enterococcaceae and Enterobacteriaceae, increasing SCFA, and improving amino acid metabolism.
Curcumin (Mody et al., 2020)
C. diff proliferation was slowed down independent of any inhibitory effects on the growth of gut commensals. Compared to antibiotic fidaxomicin, curcumin was more effective in preventing the synthesis of C. diff toxin but less successful at preventing spore formation.
8.2 Fecal microbiota transfer (FMT) in CDI
The standard management for CDI generally involves treatment with vancomycin and fidaxomicin or surgery in serious cases. However, FMT has emerged as a potential approach to reduce the severity of CDI because of the significance of gut bacteria in maintaining immunometabolic homeostasis (Fig. 5). An earlier comprehensive review and meta-analysis that examined the efficiency of FMT in recurring and stubborn cases of CDI, including 30 case series and seven RCTs, found a connection between the two. In summary, FMT with a clinical resolution rate of 92 % was more successful than vancomycin in treating recurrent and refractory cases of CDI. Any discernible difference between fresh and frozen FMT samples was absent, and lower GI delivery of FMT was more efficient than upper GI delivery. After the failure of the first course of FMT, administering further courses had an incremental impact. While recipient preparation and FMT amounts varied, donor screening remained stable. Serious negative incidents are infrequent. Thus, it was determined that FMT, independent of the preparation technique and route of administration, is a successful therapy for recurrent and challenging CDI.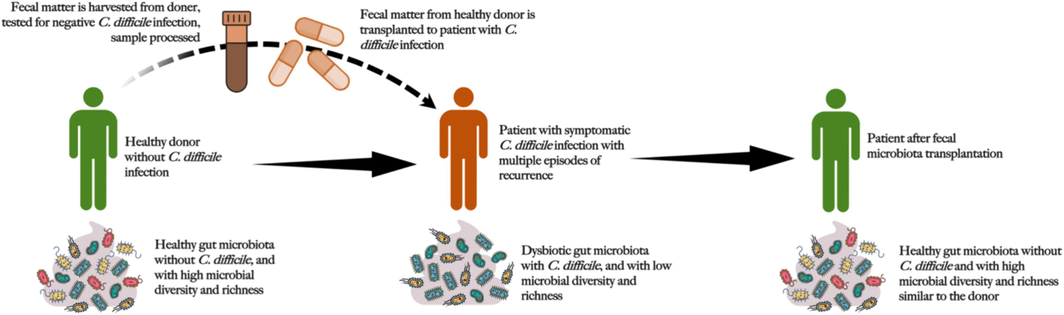
Schematic representation of the process of fecal microbiota transplantation.
Animal research has provided significant evidence that FMT is a viable strategy against CDI. Researchers have shown that full recovery from recurrent CDI requires the restoration of normal gut microbes. After administration of the broad-spectrum antibiotic cefoperazone and later vancomycin post-clinical symptoms, the animals were infected with bacterial spores. Vancomycin decreased the levels of bacterial toxins and colonization; however, CDI reappeared after 7-d. The Porphyromonadaceae family of gut microorganisms has been linked to effective recovery, and FMT following vancomycin therapy reduced these side effects. In contrast, samples collected from non-FMT mice after antibiotic treatment and infection included Lactobacillus or C. diff. Members of Bacteroidetes returned to normal 24-h after FMT. By 3-wk, the FMT receiving mice was comparable to that of the donor microbiota, and the abundance of Bacteroides, Porphyromonadaceae, and Barnesiella was similar. Another team of researchers, using mice, demonstrated the immunoregulatory potential of FMT (Buffie and Pamer, 2013). Under CDI, Rag1 heterozygote mice recovered from infection, but Rag1-/- mice did not recover. It was further shown that the ability of FMT to function relies on the specific immunological profile of the host. It has also been shown that animals experiencing ineffective FMT have intestinal immune hyper-reactivity, poor gut microbiota renewal, and altered metabolism of bile acids. These lines of evidence are important since CDI is closely associated with mucosal inflammation, and FMT-mediated favorable immunoregulation could be helpful for patients.
After repeated vancomycin treatment cycles, the first effective FMT for CDI was observed in a 65-y-aged lady with recurrent relapsing CDI-associated enterocolitis in 1983. Freshly collected fecal matter was collected from her spouse and used to create an enema that improved bowel function in the patient. In another study, subjects with recurrent CDI were randomized to receive one of three regimens: vancomycin therapy plus bowel lavage and donor face infusion, vancomycin therapy plus intestinal lavage, or only treatment with vancomycin (Chiu and Miller, 2019). The cohort receiving fecal infusion showed the highest extent of CDI resolution, and the microbial diversity in their guts was improved to the level of healthy donors. The numbers of pathogenic Proteobacteria decreased; however, the abundance of commensal Bacteroides and Clostridia was elevated. Collectively, these investigations show that α-diversity among pre-FMT patients is minimal and that successful engraftment of donor species often occurs, resulting in remission from CDI. Notably, one study contended that species might influence the effectiveness of engraftment. In this study, pre-FMT samples had a bloom of Proteobacteria, whereas commensal Bacteroidetes and Firmicutes were reduced (e.g., Bacteroidaceae, Clostridiaceae, Eubacteriaceae, Lachnospiraceae, Prevotellaceae, and Ruminococcaceae). Furthermore, the gut microbiota in post-FMT samples matched that of a healthy person; species from the Proteobacteria phylum were reduced, but those from the Bacteroidaceae, Clostridiaceae, Eubacteriaceae, Lachnospiraceae, Prevotellaceae, and Ruminococcaceae families were restored (Gonzales-Luna et al., 2023).
FMT is a successful treatment for CDI however, it is poorly regulated and its mechanism is unknown. Primary bile acids are beneficial for C. diff. spore germination. Hence, changes in bile acid metabolism and FXR-dependent signaling may increase the effectiveness of FMT. An increase in bacterial genera linked to bile acid metabolism, such as Faecalibacteria, Bacteroides, Ruminococcus, Eubacterium, and Blautia, and a decrease in bacteria that were unable to metabolize bile acids, such as Escherichia, Klebsiella, and Veillonella, were found in the gut microbial analysis of 116 recurrent CDI who received FMT (Khoruts et al., 2021). Lower amounts of primary bile acids (cholic acid and chenodeoxycholic acid) and higher secondary bile acids (deoxycholic acid and lithocholic acid) may have been due to successful FMT and favorable regulation of the FXR-pathway. Owing to the key role of bile acids in CDI, one longitudinal study explored the effects of FMT on the composition of gut bile acids in CDI affected children with CDI (n = 8), with and without IBD (Chen, 2023). The results showed that prior to FMT, recipients' primary and secondary bile acid contents were greater than those of donors. After FMT, secondary bile acids gradually increased while primary bile acids decreased, while it had been shown that gut bacterial diversity was recovered in all infants rapidly after FMT, bile acid normalization to donor levels took place only by 6-mo. In children with IBD, secondary bile acids persisted at donor levels, even if the microbiota diversity was restored to pre-FMT levels after 6-mo.
Generally, in cases of FMT for CDI, clinical results are typically assessed after 8-wk. However, a recent study showed that restoration of the gut microbiota can occur much earlier after FMT. In contrast to non-responders, FMT-treated patients with persistent resolution showed greater microbial diversity, higher levels of Ruminococcaceae and Lachnospiraceae, lower levels of Enterobacteriaceae, integration of donor microbiota, and resolution of gut microbiota dysbiosis just 7-d after FMT. Only FMT patients experienced these microbiome modifications, demonstrating the unique therapeutic response mechanism of FMT in contrast to antibiotics. As discussed earlier, the risk of CDI is higher in IBD patients. A recent trial investigated the efficacy of FMT against recurrent CDI in IBD patients (Ianiro, 2021). In brief, FMT through colonoscopy was used in this 8-wk trial comprising 18 IBD patients with rCDI, with the main objective being to obtain negative C. diff. toxin findings. Eight of these individuals had consecutive FMT as a result of unsuccessful treatment. At 8-wk, 17 of 18 patients had zero toxin levels, and 83 % reported improvements in their IBD symptoms. Since there were no significant side effects, FMT seems to be a very effective and secure method for treating rCDI in IBD patients, with the potential to improve their IBD symptoms. Collectively, FMT is revolutionizing CDI treatment owing to several factors, including better performance and fewer health impacts than conventional antibiotics. Finally, to understand the gut microbial changes associated with FMT in CDI patients with CDI, a longitudinal investigation was undertaken in recurrent CDI patients with UC (Mintz, 2018). In brief, FMT resulted in substantial alterations in microbiota composition. In the CDI and CDI+UC groups, FMT caused an increase in microaerophilic Proteobacteria and Firmicutes/Bacilli, whereas then populations of anaerobic Bacteroidetes and butyrate-producing Firmicutes sub-phyla (Ruminococcaceae and Lachnospiraceae) were decreased. With bigger alterations in CDI-related receivers, FMT had a substantial impact on 81 genus-level OTUs, including higher abundance of certain bacteria including Escherichia-Shigella and Fusobacteria in all cohorts. Following FMT, butyrate-producing OTUs were recovered in CDI-related FMT recipients.
8.3 Use of live biotherapeutics (LBP) in CDI
FMT has improved recurrent CDI outcomes; however, questions regarding the product's safety and standardization still exist. LBP based on microbiota is becoming increasingly popular as a contender to replace FMT for the treatment of recurrent CDI. LBP is classified as a biological product that is not a vaccine but includes living organisms and may be used to treat and prevent human diseases. They are designed to lower disease recurrence after antibiotic therapy in patients with one or more recurrent episodes of CDI. The recent FDA's approval of gut microbiota-based live-jslm (FMBL-jslm; brand name Rebyota) in November 2022 and the subsequent approval of gut microbiota spores, live-brpk (FMSL-brpk; brand name VOWST) in April 2023 marked the beginning of a new class of microbiota-based medications, while others, such as VE303 and MET2, are still being studied for CDI.
The microbiota suspension in live jslm–REBYOTA (RBL), which comprises 150 mL of microbial suspension, contains approximately 107 organisms per mL from healthy stool donors. At week 8, the phase II trial participants given live jslm saw a treatment success rate of 78.9 % compared to 30.7 % in the controls (Orenstein, 2022). The few side effects that occurred were generally mild-to-moderate gastrointestinal complaints, and RBL was well tolerated. Treatment responders were free of CDI in 97 %, 95 %, and 91 % of the patients after 6, 12, and 24-mo, respectively, showing long-term clinical effectiveness. Rebiosis, or restoration of the microbiota, has also been shown to happen within seven days after RBL delivery and to remain stable for up to 24-mo (Orenstein, 2023). Another FDA-approved LBP is the VOWST, which has shown remarkable efficacy in clinical trials. For instance, at week 8 of the phase 1 trial, VOWST exhibited a favorable safety profile and clinically resolved CDI in 96.7 % of the participants (Khanna, 2016). Both the safety profile and long-term efficacy of the VOWST were subsequently established in phase II and III trials performed in larger cohorts of CDI patients. Another product, VE303, is composed of eight distinct commensal strains of Clostridia. In addition to mitigating recurrent CDI, oral intake of VE303 colonizes the gut and modifies the immune system to support a balanced population of gut bacteria and prevent C. diff. colonization. In 14-d, VE303 was linked to a greater conversion of SCFA and primary and secondary bile acids (Nagarakanti and Orenstein, 2023). In a randomized, double-blind, placebo-controlled, phase II trial, participants were randomly allocated to receive either a high or low dose of VE303 or a placebo in the form of capsules every day for 2-wk (Louie, 2023). A total of 79 adults who had one or more episodes of CDI during the previous 6-mo or who had primary CDI with a high probability of recurrence were included in the study. The 8-wk CDI recurrence rate for high-dose VE303 was 13.8 % (86.2 % effective), for low-dose VE303 was 37 % (63 % effective), and for placebo was 45.5 % (54.5 % effective). Finally, the efficacy and safety of RBX2660 (a live biotherapeutic prepared from human feces containing a variety of microbes) were tested in 180 individuals (vs. 87 placebo) (Khanna, 2022). Compared to placebo, RBX2660 had a higher treatment success rate of 70.6 %, a remarkable therapeutic effect of 13.1 %, and a high likelihood of superiority of 99.1 %. In both groups, > 90 % of the patients who experienced early success continued to respond. Despite mild GI issues, RBX2660 was well tolerated by patients. Collectively, LBP-based strategies are becoming popular over conventional FMT while demonstrating similar efficacy in terms of improving the gut microbial profile in patients with recurrent CDI.
9 Conclusion and future prospects
This in-depth review explores the connection between CDI and the gut microbiota, emphasizing the possibility of treating and preventing CDI using microbiome-based strategies. There are few practical ways to control the intestinal flora to induce favorable intestinal immunity because of the intricate and dynamic interactions between gut microorganisms, host immune systems, and illnesses. Despite significant advancements in therapeutic approaches, CDI management of CDI continues to pose a challenge for clinicians worldwide. This is principally true when it comes to preventing recurrent infections, as opposed to rare cases of fulminant colitis, which have a high mortality risk. CDI patients are often associated with antibiotic therapies, immunosuppressive medications, or unhealthy eating patterns that not only disrupt the normal microbiota but also render them more vulnerable to CDI. In particular, the dietary regimen is the major environmental influence that governs the composition and diversity of microbiota, and thus, can significantly influence disease susceptibility or inhibition.
The FDA has approved FMT as one of the many microbiota-based therapeutic approaches to treat recurring infections that are irresponsive to conventional antibiotic therapy. The FDA has also issued guidance stating that it would exercise enforcement discretion regarding using FMT for recurrent CDI, allowing physicians to use FMT without an Investigational New Drug (IND) application if certain conditions are met. Recently, the FDA issued another guidance that established new requirements for the use of FMT for CDI, including donor screening and testing for pathogens. Nevertheless, the FDA has not approved FMT for the treatment of any other conditions beyond persistent and recurring CDI, and the use of FMT for other conditions is considered investigational and requires IND application. Although FMT, essentially a microbiota-based strategy, is currently the best treatment for chronic infections, ensuring desired therapeutic outcomes, the FMT approach remains a concern owing to the potential risks of unintentional transfer of pathogens into the recipient's system. Additionally, there is a lot of variation in how feces are processed and administered for FMT in different centers worldwide, making standardized microbial replacement therapy an appealing alternative. FMT's potential of FMT as a treatment for conditions such as IBD, NASH, obesity, multidrug-resistant infections, and neuropsychiatric illnesses is being investigated in ongoing clinical trials, despite the need for more research on the extended safety of the therapy. FMT has had positive results in the treatment of a number of ailments, including CDI and IBD, but its safety in high-risk patients, such as those who are immunocompromised or have underlying diseases, has not been established.
Finally, beyond gut-microbiota-based therapeutics for CDI, several issues related to C. diff. and gut microbiota interactions and the interaction between C. diff. The immunometabolic processes in the host remain underexplored. These interactions and associations need to be understood through experimental approaches using specific experimental models (e.g., GF animals, monoassociation studies, culturomic approaches, and multiomics techniques), and the understanding needs to be translated into clinical settings. For example, diverse gut microbial signatures have been associated with CDI. However, the precise make-up of the gut microbiota increases a person’s susceptibility to CDI and particular microbial species or communities that act as barriers to C. diff. colonization. Although a stable and diversified gut microbiota may protect against CDI, the exact mechanisms by which this protection occurs are not well understood. Interaction of certain bacteria or metabolites with C. diff. to prevent its development or colonization have been studied by researchers. CDI recurrence remains a major concern. Studies have focused on determining why some patients continue to have CDI after receiving initial therapy and how the gut microflora influences recurrence. Bacteriophages are viruses that attack bacteria and have the potential to influence the composition of gut microbiota. There has been a continuing investigation into the connections between C. diff. and bacteriophages and how they can be used therapeutically. Finally, there is ongoing research on the function of the host immune response in CDI and its interaction with the gut flora. How the immune system detects and reacts with C. diff. infections under the influence of diverse microbial species remains underexplored. While the available evidence undoubtedly indicates the essential role of gut microbiota in the pathogenesis and prophylaxis of CDI, understanding these phenomena would facilitate the development of universally efficacious microbiome-based prophylactic strategies against CDI.
Author contributions
RR, NB, KA, IA, and DKY performed the literature review; NB compiled the data; AS and PD wrote the manuscript; and PD conceptualized and supervised the project.
Funding
Scientific Research Deanship at University of Hail − Saudi Arabia (MDR-22 032).
CRediT authorship contribution statement
Amir Saeed: Writing – review & editing, Writing – original draft, Funding acquisition, Data curation, Conceptualization. Nehal Batra: Formal analysis, Data curation, Conceptualization. Raja Rezgui: Conceptualization. Khalid Alshaghdali: Data curation, Conceptualization. Ibrahim Alkhalaf: Formal analysis, Data curation, Conceptualization. Dharmendra Kumar Yadav: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Priyankar Dey: Writing – review & editing, Writing – original draft, Data curation, Conceptualization.
Acknowledgments
Not applicable.
References
- Cannabis use and risk of Clostridioides difficile infection: analysis of 59,824 hospitalizations. Anaerobe. 2020;61:102095
- [Google Scholar]
- Bile acid-independent protection against Clostridioides difficile infection. PLoS Pathog.. 2021;17(10):e1010015.
- [Google Scholar]
- Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment. Pharmacol. Ther.. 2016;43(11):1142-1153.
- [Google Scholar]
- Humoral immune response to Clostridioides difficile toxins A and B in hospitalized immunocompromised patients with C difficile infection. In: Open Forum Infectious Diseases. Oxford University Press US; 2021.
- [Google Scholar]
- Higher plasma vitamin D is associated with reduced risk of Clostridium difficile infection in patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther.. 2014;39(10):1136-1142.
- [Google Scholar]
- Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;29:100642
- [Google Scholar]
- Thiol metabolism and volatile metabolome of clostridioides difficile. Front. Microbiol.. 2022;13:864587
- [Google Scholar]
- Changes in microbiota composition, bile and fatty acid metabolism, in successful faecal microbiota transplantation for Clostridioides difficile infection. BMC Gastroenterol.. 2018;18(1):1-15.
- [Google Scholar]
- Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol.. 2013;13(11):790-801.
- [Google Scholar]
- Decreased diversity of the fecal microbiome in recurrent Clostridium difficile—associated diarrhea. J. Infect. Dis.. 2008;197(3):435-438.
- [Google Scholar]
- Longitudinal bile acid composition changes following faecal microbiota transplantation for clostridioides difficile infection in children with and without underlying inflammatory bowel disease. J. Crohns Colitis 2023:jjad057.
- [Google Scholar]
- Rapid change of fecal microbiome and disappearance of Clostridium difficile in a colonized infant after transition from breast milk to cow milk. Microbiome. 2016;4(1):53.
- [Google Scholar]
- Gut metabolites predict Clostridioides difficile recurrence. Microbiome. 2022;10(1):1-18.
- [Google Scholar]
- Dethlefsen, L. and D.A. Relman, Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proceedings of the National Academy of Sciences, 2011. 108(Supplement 1): p. 4554-4561.
- Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res.. 2019;147:104367
- [Google Scholar]
- Targeting gut barrier dysfunction with phytotherapies: effective strategy against chronic diseases. Pharmacol. Res.. 2020;161(11):105135
- [Google Scholar]
- Good girl goes bad: understanding how gut commensals cause disease. Microb. Pathog. 2024106617
- [Google Scholar]
- The xenobiotic sensing pregnane X receptor regulates tissue damage and inflammation triggered by C difficile toxins. FASEB J.: Official Pub. Federation Am. Soc. Exp. Bio.. 2020;34(2):2198-2212.
- [Google Scholar]
- Rifaximin improves Clostridium difficile toxin A-induced toxicity in Caco-2 cells by the PXR-dependent TLR4/MyD88/NF-κB pathway. Front. Pharmacol.. 2016;7:120.
- [Google Scholar]
- A palmitoylethanolamide producing lactobacillus paracasei improves clostridium difficile toxin a-induced colitis. Front. Pharmacol.. 2021;12:639728
- [Google Scholar]
- Gastrointestinal surgery for inflammatory bowel disease persistently lowers microbiome and metabolome diversity. Inflamm. Bowel Dis.. 2021;27(5):603-616.
- [Google Scholar]
- SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N. Engl. J. Med.. 2022;386(3):220-229.
- [Google Scholar]
- Shifts in the Gut Metabolome and Clostridium difficile Transcriptome throughout Colonization and Infection in a Mouse Model. mSphere. 2018;3(2)
- [Google Scholar]
- Dysbiosis and gastrointestinal surgery: current insights and future research. Biomedicines. 2022;10(10)
- [Google Scholar]
- Cannabidiol restores intestinal barrier dysfunction and inhibits the apoptotic process induced by Clostridium difficile toxin A in Caco-2 cells. United Eur. Gastroenterol J.. 2017;5(8):1108-1115.
- [Google Scholar]
- Gut microbiota changes associated with Clostridioides difficile infection and its various treatment strategies. Gut Microbes. 2023;15(1):2223345.
- [Google Scholar]
- A short chain fatty acid–centric view of Clostridioides difficile pathogenesis. PLoS Pathog.. 2021;17(10):e1009959.
- [Google Scholar]
- Gupta, U. and P. Dey, Rise of the guardians: Gut microbial maneuvers in bacterial infections. Life Sciences, 2023: p. 121993.
- The oral microbial odyssey influencing chronic metabolic disease. Arch. Physiol. Biochem. 2023:1-17.
- [Google Scholar]
- Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat. Microbiol.. 2018;3(6):662-669.
- [Google Scholar]
- Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: experience of a large-volume European FMT center. Gut Microbes. 2021;13(1):1994834.
- [Google Scholar]
- Amelioration of clostridium difficile infection in mice by dietary supplementation with indole-3-carbinol. Ann. Surg.. 2017;265(6):1183-1191.
- [Google Scholar]
- Julliard, W., et al., Supplementation of the aryl hydrocarbon receptor ligand indole-3-carbinol protects mice from Clostridium difficult associated disease.(MUC5P. 762). 2015, Am Assoc Immnol.
- Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol.. 2013;14(7):685-690.
- [Google Scholar]
- Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit Clostridium difficile: role of secondary bile acids. Cell Chem. Biol.. 2019;26(1) pp. 27–34. e4
- [Google Scholar]
- Bacterial exopolysaccharides as emerging bioactive macromolecules: from fundamentals to applications. Res. Microbio. 2022104024
- [Google Scholar]
- Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis.. 2023;10 20499361231154443
- [Google Scholar]
- A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent clostridium difficile infection. J Infect Dis. 2016;214(2):173-181.
- [Google Scholar]
- Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a bayesian primary analysis for the prevention of recurrent clostridioides difficile infection. Drugs. 2022;82(15):1527-1538.
- [Google Scholar]
- Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat. Rev. Gastroenterol. Hepatol.. 2021;18(1):67-80.
- [Google Scholar]
- Peroxisome proliferator-activated receptor-γ as the gatekeeper of tight junction in Clostridioides difficile infection. Front. Microbiol.. 2022;13:986457
- [Google Scholar]
- VE303, a defined bacterial consortium, for prevention of recurrent clostridioides difficile infection: a randomized clinical trial. J. Am. Med. Assoc.. 2023;329(16):1356-1366.
- [Google Scholar]
- Role of retinol in protecting epithelial cell damage induced by Clostridium difficile toxin A. Toxicon. 2007;50(8):1027-1040.
- [Google Scholar]
- Association between postoperative changes in the gut microbiota and pseudopsia after cardiac surgery: prospective observational study. BMC Surg.. 2020;20(1):1-10.
- [Google Scholar]
- Distinct types of gut microbiota dysbiosis in hospitalized gastroenterological patients are disease non-related and characterized with the predominance of either Enterobacteriaceae or Enterococcus. Front. Microbiol.. 2020;11:120.
- [Google Scholar]
- Modulating bile acid pathways and TGR5 receptors for treating liver and GI diseases. Curr. Opin. Pharmacol.. 2017;37:80-86.
- [Google Scholar]
- Alterations of brain endocannabinoidome signaling in germ-free mice. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2020;1865(12):158786
- [Google Scholar]
- IL-22 alters gut microbiota composition and function to increase aryl hydrocarbon receptor activity in mice and humans. Microbiome. 2023;11(1):47.
- [Google Scholar]
- Gut microbiota composition associated with clostridioides difficile colonization and infection. Pathogens. 2022;11(7):781.
- [Google Scholar]
- Risk for clostridioides difficile infection among hospitalized patients associated with multiple healthcare exposures prior to admission. J Infect Dis. 2021;224(4):684-694.
- [Google Scholar]
- Utilizing probiotics for the prevention and treatment of gastrointestinal diseases. Front. Microbiol.. 2021;12:689958
- [Google Scholar]
- Longitudinal microbiome analysis of single donor fecal microbiota transplantation in patients with recurrent Clostridium difficile infection and/or ulcerative colitis. PLoS One. 2018;13(1):e0190997.
- [Google Scholar]
- Curcumin: a natural derivative with antibacterial activity against Clostridium difficile. J Glob Antimicrob Resist. 2020;21:154-161.
- [Google Scholar]
- Treating clostridioides difficile: could microbiota-based live biotherapeutic products provide the answer? Infect Drug Resist. 2023;16:3137-3143.
- [Google Scholar]
- Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect. Dis.. 2022;22(1):245.
- [Google Scholar]
- The role of microbiome-based therapeutics in clostridioides difficile infection: durable, long-term results of RBX2660. Infect. Dis. Ther.. 2023;12(1):1-7.
- [Google Scholar]
- Probiotics: insights and new opportunities for Clostridioides difficile intervention. Crit. Rev. Microbiol.. 2023;49(3):414-434.
- [Google Scholar]
- Evaluation of advanced age as a risk factor for severe Clostridium difficile infection. J. Clin. Gerontol. Geriatrics. 2016;7(1):12-16.
- [Google Scholar]
- Short chain fatty acid metabolism in relation to gut microbiota and genetic variability. Nutrients. 2022;14(24):5361.
- [Google Scholar]
- Longitudinal survey of Clostridium difficile presence and gut microbiota composition in a Belgian nursing home. BMC Microbiol.. 2016;16:1-12.
- [Google Scholar]
- Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin. Infect. Dis.. 2012;55(9):1209-1215.
- [Google Scholar]
- Vitamin D deficiency is associated with community-acquired clostridium difficile infection: a case-control study. BMC Infect. Dis.. 2014;14:661.
- [Google Scholar]
- Dietary xanthan gum alters antibiotic efficacy against the murine gut microbiota and attenuates Clostridioides difficile colonization. Msphere. 2020;5(1) e00708-19
- [Google Scholar]
- Innate immune response and outcome of clostridium difficile infection are dependent on fecal bacterial composition in the aged host. J Infect Dis. 2018;217(2):188-197.
- [Google Scholar]
- Probiotics as an alternative antimicrobial therapy: current reality and future directions. J. Funct. Foods. 2020;73:104080
- [Google Scholar]
- Dietary vitamin D, vitamin D receptor, and microbiome. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21(6):471-474.
- [Google Scholar]
- Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA adverse event reporting system. Int. J. Med. Sci.. 2019;16(5):630-635.
- [Google Scholar]
- Tewari, N. and P. Dey, Navigating Commensal Dysbiosis: Gastrointestinal Host-Pathogen Interplay in Orchestrating Opportunistic Infections. Microbiological Research, 2024: p. 127832.
- Human fecal metabolomic profiling could inform Clostridioides difficile infection diagnosis and treatment. J. Clin. Invest.. 2019;129(9):3539-3541.
- [Google Scholar]
- Clinical characteristics associated with the severity of Clostridium [Clostridioides] difficile infection in a tertiary teaching hospital from Mexico. Biomed. J.. 2022;45(1):200-205.
- [Google Scholar]
- Intestinal Microbiota in Elderly Inpatients with Clostridioides difficile Infection. Infect. Drug. Resist.. 2020;13:2723-2731.
- [Google Scholar]
- Effect of bifidobacterium upon clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front. Microbiol.. 2016;7:738.
- [Google Scholar]
- Dysbiosis of intestinal microbiota in critically ill patients and risk of in-hospital mortality. Am. J. Transl. Res.. 2021;13(3):1548-1557.
- [Google Scholar]
- Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control clostridium difficile germination and growth. PLoS One. 2016;11(1):e0147210.
- [Google Scholar]
- Clostridioides difficile infection induces a rapid influx of bile acids into the gut during colonization of the host. Cell Rep.. 2021;36(10):109683
- [Google Scholar]
- Epigallocatechin-3-Gallate improves intestinal gut microbiota homeostasis and ameliorates clostridioides difficile infection. Nutrients. 2022;14(18)
- [Google Scholar]
- Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol.. 2019;10(1):1-11.
- [Google Scholar]
- Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe. 2015;34:1-7.
- [Google Scholar]







